Kefir Peptides Prevent Estrogen Deficiency-Induced Bone Loss and Modulate the Structure of the Gut Microbiota in Ovariectomized Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Kefir Peptides (KPs) Preparation
2.2. Animal Experiments
2.3. Microcomputed Tomography (μ-CT)
2.4. Nanoindentation
2.5. Scanning Electron Microscopy
2.6. Gut Microbiota
2.7. Statistical Analysis
3. Results
3.1. Body Weight and Affected Organ Weight
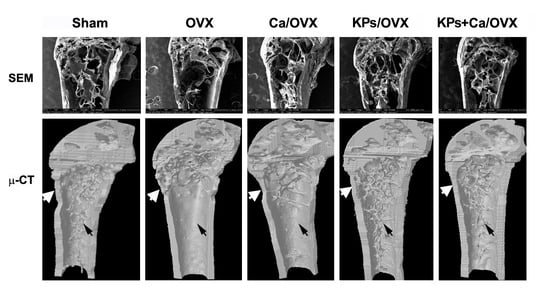
3.2. Effects of KPs on BMD and Bone Structure
3.3. Effect of KPs on the Mechanical Indices of the Cortical Bones
3.4. Effect of KPs on the Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and Economic Burden of Osteoporosis-Related Fractures in the United States, 2005–2025. J. Bone Miner. Res. 2006, 22, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Fardellone, P. The effect of milk consumption on bone and fracture incidence, an update. Aging Clin. Exp. Res. 2019, 31, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Bourrie, B.C.T.; Willing, B.P.; Cotter, P.D. The Microbiota and Health Promoting Characteristics of the Fermented Beverage Kefir. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzel-Seydim, Z.B.; Kök-Taş, T.; Greene, A.K.; Seydim, A.C. Review: Functional Properties of Kefir. Crit. Rev. Food Sci. Nutr. 2011, 51, 261–268. [Google Scholar] [CrossRef]
- Ebner, J.; Arslan, A.A.; Fedorova, M.; Hoffmann, R.; Küçükçetin, A.; Pischetsrieder, M. Peptide profiling of bovine kefir reveals 236 unique peptides released from caseins during its production by starter culture or kefir grains. J. Proteom. 2015, 117, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.C.; Citerne, F.; Tian, T.; Silva, V.L.M.; Kalanetra, K.M.; Frese, S.A.; Robinson, R.C.; Mills, D.A.; Barile, D. Peptidomic analysis reveals proteolytic activity of kefir microorganisms on bovine milk proteins. Food Chem. 2016, 197, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Behera, J.; Ison, J.; Tyagi, S.C.; Tyagi, N. The role of gut microbiota in bone homeostasis. Bone 2020, 135, 115317. [Google Scholar] [CrossRef]
- Dar, H.Y.; Shukla, P.; Mishra, P.K.; Anupam, R.; Mondal, R.K.; Tomar, G.B.; Sharma, V.; Srivastava, R.K. Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. Bone Rep. 2018, 8, 46–56. [Google Scholar] [CrossRef]
- Malik, T.A.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency–associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef] [Green Version]
- Parvaneh, M.; Karimi, G.; Jamaluddin, R.; Ng, M.H.; Zuriati, I.; Muhammad, S.I. Lactobacillus helveticus (ATCC 27558) upregulates Runx2 and Bmp2 and modulates bone mineral density in ovariectomy-induced bone loss rats. Clin. Interv. Aging 2018, 13, 1555–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Motyl, K.J.; Irwin, R.; MacDougald, O.A.; Britton, R.A.; McCabe, L.R. Loss of Bone and Wnt10b Expression in Male Type 1 Diabetic Mice Is Blocked by the Probiotic Lactobacillus reuteri. Endocrinology 2015, 156, 3169–3182. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, A.M.; Yu, M.; Darby, T.M.; Vaccaro, C.; Li, J.-Y.; Owens, J.A.; Hsu, E.; Adams, J.; Weitzmann, M.N.; Jones, R.M.; et al. The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-Mediated Regulation of WNT10B Expression. Immunity 2018, 49, 1116–1131.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-L.; Tung, Y.-T.; Chuang, C.-H.; Tu, M.-Y.; Tsai, T.-C.; Chang, S.-Y.; Chen, C.-M. Kefir improves bone mass and microarchitecture in an ovariectomized rat model of postmenopausal osteoporosis. Osteoporos. Int. 2014, 26, 589–599. [Google Scholar] [CrossRef]
- Tu, M.-Y.; Chen, H.-L.; Tung, Y.-T.; Kao, C.-C.; Hu, F.-C.; Chen, C.-M. Short-Term Effects of Kefir-Fermented Milk Consumption on Bone Mineral Density and Bone Metabolism in a Randomized Clinical Trial of Osteoporotic Patients. PLoS ONE 2015, 10, e0144231. [Google Scholar] [CrossRef] [PubMed]
- Aslam, H.; Marx, W.; Rocks, T.; Loughman, A.; Chandrasekaran, V.; Ruusunen, A.; Dawson, S.L.; West, M.; Mullarkey, E.; Pasco, J.A.; et al. The effects of dairy and dairy derivatives on the gut microbiota: A systematic literature review. Gut Microbes 2020, 12, 1799533. [Google Scholar] [CrossRef]
- Bellikci-Koyu, E.; Sarer-Yurekli, B.P.; Akyon, Y.; Kose, F.A.; Karagözlü, C.; Özgen, A.G.; Brinkmann, A.; Nitsche, A.; Ergünay, K.; Yilmaz, E.; et al. Effects of Regular Kefir Consumption on Gut Microbiota in Patients with Metabolic Syndrome: A Parallel-Group, Randomized, Controlled Study. Nutrients 2019, 11, 2089. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-L.; Hung, K.-F.; Yen, C.-C.; Laio, C.-H.; Wang, J.-L.; Lan, Y.-W.; Chong, K.-Y.; Fan, H.-C.; Chen, C.-M. Kefir peptides alleviate particulate matter <4 mum (PM4.0)-induced pulmonary inflammation by inhibiting the NF-kappaB pathway using luciferase transgenic mice. Sci. Rep. 2019, 9, 11529. [Google Scholar] [CrossRef]
- Chen, H.-L.; Tung, Y.-T.; Tsai, C.-L.; Lai, C.-W.; Lai, Z.-L.; Tsai, H.-C.; Lin, Y.-L.; Wang, C.-H.; Chen, C.-M. Kefir improves fatty liver syndrome by inhibiting the lipogenesis pathway in leptin-deficient ob/ob knockout mice. Int. J. Obes. 2013, 38, 1172–1179. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Chen, H.-L.; Wu, H.-S.; Ho, M.-H.; Chong, K.-Y.; Chen, C.-M. Kefir Peptides Prevent Hyperlipidemia and Obesity in High-Fat-Diet-Induced Obese Rats via Lipid Metabolism Modulation. Mol. Nutr. Food Res. 2018, 62, 1700505. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nat. Cell Biol. 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Choi, J.-W.; Kang, H.W.; Lim, W.-C.; Kim, M.-K.; Lee, I.-Y.; Cho, H.-Y. Kefir prevented excess fat accumulation in diet-induced obese mice. Biosci. Biotechnol. Biochem. 2017, 81, 958–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.L.; Tsai, T.C.; Tsai, Y.C.; Liao, J.W.; Yen, C.C.; Chen, C.M. Kefir peptides prevent high-fructose corn syrup-induced non-alcoholic fatty liver disease in a murine model by modulation of inflammation and the JAK2 signaling pathway. Nutr. Diabetes 2016, 6, e237. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.-N.; Choi, J.-W.; Lim, W.-C.; Kim, M.-K.; Lee, I.-Y.; Cho, H.-Y. Kefir inhibits 3T3-L1 adipocyte differentiation through down-regulation of adipogenic transcription factor expression. J. Sci. Food Agric. 2012, 93, 485–490. [Google Scholar] [CrossRef]
- Santini, G.; Bonazza, F.; Pucciarelli, S.; Polidori, P.; Ricciutelli, M.; Klimanova, Y.; Silvi, S.; Polzonetti, V.; Vincenzetti, S. Proteomic characterization of kefir milk by two-dimensional electrophoresis followed by mass spectrometry. J. Mass Spectrom. 2020, e4635. [Google Scholar] [CrossRef]
- Amorim, F.G.; Coitinho, L.B.; Dias, A.T.; Friques, A.G.F.; Monteiro, B.L.; De Rezende, L.C.D.; Pereira, T.D.M.C.; Campagnaro, B.P.; De Pauw, E.; Vasquez, E.C.; et al. Identification of new bioactive peptides from Kefir milk through proteopeptidomics: Bioprospection of antihypertensive molecules. Food Chem. 2019, 282, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-González, J.J.; Amil-Ruiz, F.; Zazzu, S.; Sánchez-Lucas, R.; Fuentes-Almagro, C.A.; Rodríguez-Ortega, M.J. Proteomic analysis of goat milk kefir: Profiling the fermentation-time dependent protein digestion and identification of potential peptides with biological activity. Food Chem. 2019, 295, 456–465. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, M.S.; Oh, H.-G.; Lee, H.-Y.; Park, J.-W.; Lee, B.-G.; Park, S.-H.; Moon, D.-I.; Shin, E.-H.; Oh, E.-K.; et al. Treatment of eggshell with casein phosphopeptide reduces the severity of ovariectomy-induced bone loss. Lab. Anim. Res. 2013, 29, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Sun, S.; Guo, B.; Miao, B.; Luo, Z.; Xia, Z.; Ying, D.; Liu, F.; Guo, B.; Tang, J.; et al. Bioactive peptide isolated from casein phosphopeptides promotes calcium uptake in vitro and in vivo. Food Funct. 2018, 9, 2251–2260. [Google Scholar] [CrossRef]
- Meisel, H.; Olieman, C. Estimation of calcium-binding constants of casein phosphopeptides by capillary zone electrophoresis. Anal. Chim. Acta 1998, 372, 291–297. [Google Scholar] [CrossRef]
- Teucher, B.; Majsak-Newman, G.; Dainty, J.R.; McDonagh, D.; Fitzgerald, R.J.; Fairweather-Tait, S.J. Calcium absorption is not increased by caseinophosphopeptides. Am. J. Clin. Nutr. 2006, 84, 162–166. [Google Scholar] [CrossRef]
- Narva, M.; Kärkkäinen, M.; Poussa, T.; Lamberg-Allardt, C.; Korpela, R. Caseinphosphopeptides in milk and fermented milk do not affect calcium metabolism acutely in postmenopausal women. J. Am. Coll. Nutr. 2003, 22, 88–93. [Google Scholar] [CrossRef]
- Pandey, M.; Kapila, S.; Kapila, R.; Trivedi, R.; Karvande, A. Evaluation of the osteoprotective potential of whey derived-antioxidative (YVEEL) and angiotensin-converting enzyme inhibitory (YLLF) bioactive peptides in ovariectomised rats. Food Funct. 2018, 9, 4791–4801. [Google Scholar] [CrossRef]
- Pandey, M.; Kapila, R.; Kapila, S. Osteoanabolic activity of whey-derived anti-oxidative (MHIRL and YVEEL) and angiotensin-converting enzyme inhibitory (YLLF, ALPMHIR, IPA and WLAHK) bioactive peptides. Peptides 2018, 99, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mada, S.B.; Reddi, S.; Kumar, N.; Kumar, R.; Kapila, S.; Kapila, R.; Trivedi, R.; Karvande, A.; Ahmad, N. Antioxidative peptide from milk exhibits antiosteopenic effects through inhibition of oxidative damage and bone-resorbing cytokines in ovariectomized rats. Nutrients 2017, 43, 21–31. [Google Scholar] [CrossRef]
- Mada, S.B.; Reddi, S.; Kumar, N.; Kapila, S.; Kapila, R. Protective effects of casein-derived peptide VLPVPQK against hydrogen peroxide–induced dysfunction and cellular oxidative damage in rat osteoblastic cells. Hum. Exp. Toxicol. 2017, 36, 967–980. [Google Scholar] [CrossRef]
- Weitzmann, M.N. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef] [Green Version]
- D’Amelio, P.; Grimaldi, A.; Di Bella, S.; Brianza, S.Z.M.; Cristofaro, M.A.; Tamone, C.; Giribaldi, G.; Ulliers, D.; Pescarmona, G.P.; Isaia, G. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: A key mechanism in osteoporosis. Bone 2008, 43, 92–100. [Google Scholar] [CrossRef]
- Taxel, P.; Kaneko, H.; Lee, S.-K.; Aguila, H.L.; Raisz, L.G.; Lorenzo, J.A. Estradiol rapidly inhibits osteoclastogenesis and RANKL expression in bone marrow cultures in postmenopausal women: A pilot study. Osteoporos. Int. 2007, 19, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Madak-Erdogan, Z. Estrogen and Microbiota Crosstalk: Should We Pay Attention? Trends Endocrinol. Metab. 2016, 27, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Kaliannan, K.; Robertson, R.C.; Murphy, K.; Stanton, C.; Kang, C.; Wang, B.; Hao, L.; Bhan, A.K.; Kang, J.X. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 2018, 6, 1–22. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Xiang, L.; Wang, Z.; Xiao, G.G.; Hu, J. Effect of Curcumin on the Diversity of Gut Microbiota in Ovariectomized Rats. Nutrients 2017, 9, 1146. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Han, Y.; Li, F.; Gu, X.; Su, D.; Yu, W.; Li, Z.; Xiao, J. Neuropeptide Y1 Receptor Antagonist Alters Gut Microbiota and Alleviates the Ovariectomy-Induced Osteoporosis in Rats. Calcif. Tissue Int. 2019, 106, 444–454. [Google Scholar] [CrossRef]
- Chung, Y.W.; Gwak, H.-J.; Moon, S.; Rho, M.; Ryu, J.-H. Functional dynamics of bacterial species in the mouse gut microbiome revealed by metagenomic and metatranscriptomic analyses. PLoS ONE 2020, 15, e0227886. [Google Scholar] [CrossRef] [Green Version]
- Ning, K.; Lu, K.; Chen, Q.; Guo, Z.; Du, X.; Riaz, F.; Feng, L.; Fu, Y.; Yin, C.; Zhang, F.; et al. Epigallocatechin Gallate Protects Mice against Methionine–Choline-Deficient-Diet-Induced Nonalcoholic Steatohepatitis by Improving Gut Microbiota To Attenuate Hepatic Injury and Regulate Metabolism. ACS Omega 2020, 5, 20800–20809. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Wu, H.; Wu, S.; Lu, N.; Wang, Y.-T.; Liu, H.-N.; Dong, L.; Liu, T.-T.; Shen, X. Parasutterella, in association with irritable bowel syndrome and intestinal chronic inflammation. J. Gastroenterol. Hepatol. 2018, 33, 1844–1852. [Google Scholar] [CrossRef]
- Jian, X.; Zhu, Y.; Ouyang, J.; Wang, Y.; Lei, Q.; Xia, J.; Guan, Y.; Zhang, J.; Guo, J.; He, Y.; et al. Alterations of gut microbiome accelerate multiple myeloma progression by increasing the relative abundances of nitrogen-recycling bacteria. Microbiome 2020, 8, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Alhinai, E.A.; Walton, G.E.; Commane, D.M. The Role of the Gut Microbiota in Colorectal Cancer Causation. Int. J. Mol. Sci. 2019, 20, 5295. [Google Scholar] [CrossRef] [Green Version]
- Naderpoor, N.; Mousa, A.; Gomez-Arango, L.F.; Barrett, H.L.; Nitert, M.D.; De Courten, B. Faecal Microbiota Are Related to Insulin Sensitivity and Secretion in Overweight or Obese Adults. J. Clin. Med. 2019, 8, 452. [Google Scholar] [CrossRef] [Green Version]
- D’Amelio, P.; Sassi, F. Gut Microbiota, Immune System, and Bone. Calcif. Tissue Int. 2018, 102, 415–425. [Google Scholar] [CrossRef]
- Besten, G.D.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Bloemen, J.G.; Damink, S.W.O.; Venema, K.; Buurman, W.A.; Jalan, R.; DeJong, C.H. Short chain fatty acids exchange: Is the cirrhotic, dysfunctional liver still able to clear them? Clin. Nutr. 2010, 29, 365–369. [Google Scholar] [CrossRef]
- Ma, S.; Qin, J.; Hao, Y.; Shi, Y.; Fu, L. Structural and functional changes of gut microbiota in ovariectomized rats and their correlations with altered bone mass. Aging 2020, 12, 10736–10753. [Google Scholar] [CrossRef]





| α-Diversity Index | β-Diversity Index | ||||||
|---|---|---|---|---|---|---|---|
| Group | Observed-OTU | Ace | Chao1 | Shannon | Simpson | Unweighted uniFrac | Weighted uniFrac |
| Sham | 160 | 177.0 | 180.6 | 4.938 | 0.932 | 0.199 | 0.038 |
| OVX | 217 | 233.1 | 232.9 | 5.657 | 0.965 | 0.080 | 0.034 |
| KPs/OVX | 230 | 244.1 | 248.3 | 5.790 | 0.968 | 0.327 | 0.042 |
| Comparison | p value of α-diversity | p value of β-diversity | |||||
| Sham vs OVX | 0.004 | 0.002 | 0.006 | 0.055 | 0.062 | 0.441 | 0.645 |
| Sham vs KPs/OVX | 0.001 | 0.001 | 0.002 | 0.028 | 0.049 | 0.395 | 0.761 |
| OVX vs KPs/OVX | 0.480 | 0.514 | 0.363 | 0.850 | 0.979 | 0.077 | 0.299 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, M.-Y.; Han, K.-Y.; Chang, G.R.-L.; Lai, G.-D.; Chang, K.-Y.; Chen, C.-F.; Lai, J.-C.; Lai, C.-Y.; Chen, H.-L.; Chen, C.-M. Kefir Peptides Prevent Estrogen Deficiency-Induced Bone Loss and Modulate the Structure of the Gut Microbiota in Ovariectomized Mice. Nutrients 2020, 12, 3432. https://doi.org/10.3390/nu12113432
Tu M-Y, Han K-Y, Chang GR-L, Lai G-D, Chang K-Y, Chen C-F, Lai J-C, Lai C-Y, Chen H-L, Chen C-M. Kefir Peptides Prevent Estrogen Deficiency-Induced Bone Loss and Modulate the Structure of the Gut Microbiota in Ovariectomized Mice. Nutrients. 2020; 12(11):3432. https://doi.org/10.3390/nu12113432
Chicago/Turabian StyleTu, Min-Yu, Kuei-Yang Han, Gary Ro-Lin Chang, Guan-Da Lai, Ku-Yi Chang, Chien-Fu Chen, Jen-Chieh Lai, Chung-Yu Lai, Hsiao-Ling Chen, and Chuan-Mu Chen. 2020. "Kefir Peptides Prevent Estrogen Deficiency-Induced Bone Loss and Modulate the Structure of the Gut Microbiota in Ovariectomized Mice" Nutrients 12, no. 11: 3432. https://doi.org/10.3390/nu12113432
APA StyleTu, M. -Y., Han, K. -Y., Chang, G. R. -L., Lai, G. -D., Chang, K. -Y., Chen, C. -F., Lai, J. -C., Lai, C. -Y., Chen, H. -L., & Chen, C. -M. (2020). Kefir Peptides Prevent Estrogen Deficiency-Induced Bone Loss and Modulate the Structure of the Gut Microbiota in Ovariectomized Mice. Nutrients, 12(11), 3432. https://doi.org/10.3390/nu12113432