Food Intervention with Folate Reduces TNF-α and Interleukin Levels in Overweight and Obese Women with the MTHFR C677T Polymorphism: A Randomized Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Characterization
2.2. Ethics Statement
2.3. Population and Sampling
2.4. Inclusion and Exclusion Criteria
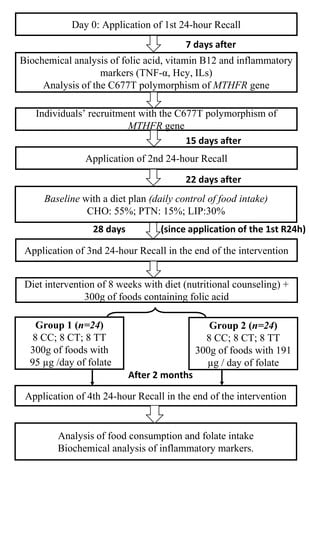
2.5. Experimental Protocol
2.6. Dietary Intervention Composition
2.7. Data Collection
2.8. Food Consumption
2.9. Collection and Isolation of Leukocyte DNA
2.10. Analysis of the C677T Polymorphism of the MTHFR Gene
- 5′-TGAAGGAGAAGGTGTCTGCGGGA-3′ (sense);
- 5′-AGGACGGTGCGGTGAGAGTG-3′ (antisense).
2.11. Statistical Analysis
3. Results
3.1. General Characteristics of the Participants by Groups
3.2. Effect of Folate Intervention on Different Genotypes of MTHFR C677T and Inflammatory Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stover, P.J. Polymorphisms in 1-carbon metabolism, epigenetics and folate-related pathologies. J. Nutr. Nutr. 2005, 4, 293–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., III; Mills, J.L.; Raiten, D.J. Biomarkers of Nutrition for Development-Folate Review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, M.R.; Lima, R.P.A.; Lisboa, J.V.C.; Chaves, T.R.; Luna, R.C.P.; do Nascimento, R.A.F.; de Oliveira, Y.; Persuhn, D.C.; Sérgio da Silva, A.; Gonçalves, M.D.C.R.; et al. Influence of the C677T Polymorphism of the MTHFR Gene on Oxidative Stress in Women With Overweight or Obesity: Response to a Dietary Folate Intervention. J. Am. Coll. Nutr. 2018, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Frosst, P.; Blom, H.J.; Milos, R.; Goyette, P.; Sheppard, C.A.; Matthews, R.G.; Boers, G.J.; den Heijer, M.; Kluijtmans, L.A.; van den Heuvel, L.P.; et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995, 10, 111–113. [Google Scholar] [CrossRef]
- Moll, S.; Varga, E.A. Homocysteine and MTHFR Mutations. Circulation 2015, 132, 6–9. [Google Scholar] [CrossRef] [Green Version]
- Tsang, B.L.; Devine, O.J.; Cordero, A.M.; Marchetta, C.M.; Mulinare, J.; Mersereau, P.; Guo, J.; Qi, Y.P.; Berry, R.J.; Rosenthal, J.; et al. Assessing the association between the methylenetetrahydrofolate reductase (MTHFR) 677C>T polymorphism and blood folate concentrations: A systematic review and meta-analysis of trials and observational studies. Am. J. Clin. Nutr. 2015, 101, 1286–1294. [Google Scholar] [CrossRef]
- Holven, K.B.; Aukrust, P.; Retterstol, K.; Hagve, T.A.; Mørkrid, L.; Ose, L.; Nenseter, M.S. Increased levels of C-reactive protein and interleukin-6 in hyperhomocysteinemic subjects. Scand. J. Clin. Lab. Investing. 2006, 66, 45–54. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, M.; Hu, X.; Zhu, J. Homocysteine stimulates nuclear factor κB activity and interleukin-6 expression in rat vascular smooth muscle cells. Cell Biol. Int. 2006, 30, 592–597. [Google Scholar] [CrossRef]
- Fuchs, D.; Avanzas, P.; Arroyo-Espliguero, R.; Jenny, M.; Consuegra-Sanchez, L.; Kaski, J.C. The role of neopterin in atherogenesis and cardiovascular risk assessment. Curr. Med. Chem. 2009, 16, 4644–4653. [Google Scholar] [CrossRef]
- Murr, C.; Winklhofer-Roob, B.M.; Schroecksnadel, K.; Maritschnegg, M.; Mangge, H.; Böhm, B.O.; Winkelmann, B.R.; März, W.; Fuchs, D. Inverse association between serum concentrations of neopterin and antioxidants in patients with and without angiographic coronary artery disease. Atherosclerosis 2009, 202, 543–549. [Google Scholar] [CrossRef]
- Wang, M.; Kaufman, R.J. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer 2014, 14, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, F.; Dai, Y.; Han, L.; Chen, S. Serum TNF-α, GTH and MDA of high-fat diet-induced obesity and obesity resistant rats. Saudi. Pharm. J. 2016, 24, 333–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, R.; Adhikari, S.; Patro, B.S.; Chattopadhyay, S.; Mukherjee, T. Free radical scavenging behavior of folic acid: Evidence for possible antioxidant activity. Free Radic. Biol. Med. 2001, 30, 1390–1399. [Google Scholar] [CrossRef]
- Mangge, H.; Becker, K.; Fuchs, D.; Gostner, J.M. Antioxidants, inflammation and cardiovascular disease. World J. Cardiol. 2014, 6, 462–477. [Google Scholar] [CrossRef]
- Shiran, A.; Remer, E.; Asmer, I.; Karkabi, B.; Zittan, E.; Cassel, A.; Barak, M.; Rozenberg, O.; Karkabi, K.; Flugelman, M.Y. Association of Vitamin B12 Deficiency with Homozygosity of the TT MTH FR C677T Genotype, Hyperhomocysteinemia, and Endothelial Cell Dysfunction. Isr. Med. Assoc. J. 2015, 17, 288–292. [Google Scholar]
- Lee, J.Y.; Shin, C.; Baik, I. Longitudinal associations between micronutrient consumption and leukocyte telomere length. J. Hum. Nutr. Diet. 2017, 30, 236–243. [Google Scholar] [CrossRef]
- Hiraoka, M.; Kagawa, Y. Genetic polymorphisms and folate status. Congenit. Anom. 2017, 57, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Bandarra, D.; Biddlestone, J.; Mudie, S.; Müller, H.A.J.; Rocha, S. HIF-1α restricts NF-κB-dependent gene expression to control innate immunity signals. Dis. Models Mech. 2015, 8, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Caprau, D.; Romitti, P.; Christensen, K.; Murray, J.C. Genotype frequencies and linkage disequilibrium in the CEPH human diversity panel for variants in folate pathway genes MTHFR, MTHFD, MTRR, RFC1, and GCP2. Birth Defects Res. A Clin. Mol. Teratol. 2003, 67, 545–549. [Google Scholar] [CrossRef]
- Wu, C.H.; Huang, T.C.; Lin, B.F. Folate deficiency affects dendritic cell function and subsequent T helper cell differentiation. J. Nutr. Biochem. 2017, 41, 65–72. [Google Scholar] [CrossRef]
- Otten, J.J.; Hellwig, J.P.; Linda, D. Dietary Reference Intakes: The Essential Guide to Nutrient; National Academies Press: Washington, DC, USA, 2006; pp. 245–253. [Google Scholar]
- Liew, S.C. Folic acid and diseases—Supplement it or not? Rev. Assoc. Med. Bras. 2016, 62, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Santos Nunes, M.K.; Silva, A.S.; Wanderley de Queiroga Evangelista, I.; Modesto Filho, J.; Alves Pegado Gomes, C.N.; Ferreira do Nascimento, R.A.; Pordeus Luna, R.C.; de Carvalho Costa, M.J.; Paulo de Oliveira, N.F.; Camati Persuhn, D. Analysis of the DNA methylation profiles of miR-9-3, miR-34a, and miR-137 promoters in patients with diabetic retinopathy and nephropathy. J. Diabetes Complicat. 2018, 32, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.J.C. Resultado Final PPSUS 2013. II Ciclo de Diagnóstico e Intervenção da Situação Alimentar, Nutricional e das Doenças não Transmissíveis mais Prevalentes da População do Município de João Pessoa/PB [Cycle II of Diagnosis and Intervention of the Food, Nutritional and Non-Communicable Diseases Status of the Population of the Municipality of João Pessoa (II DISANDNT/JP)]. João Pessoa: Foundation for Support and Research Support/PPSUS/PB/Ministry of Health/National Research Council/CNPq/João Pessoa Prefecture. Public Notice 001/2013, Number EFP_00008187. Available online: http://fapesq.rpp.br/editais-resultados/resultadofinalppsus2013.pdf/view (accessed on 9 July 2019).
- Bolfarine, H.; Bussab, W. de O. Sampling Elements, 1st ed.; Edgard Blucher: São Paulo, Brazil, 2005. [Google Scholar]
- de Medeiros Cavalcante, I.G.; Silva, A.S.; Costa, M.J.; Persuhn, D.C.; Issa, C.T.; de Luna Freire, T.L.; da Conceição Rodrigues Gonçalves, M. Effect of vitamin D3 supplementation and influence of BsmI polymorphism of the VDR gene of the inflammatory profile and oxidative stress in elderly women with vitamin D insufficiency: Vitamin D3 megadose reduces inflammatory markers. Exp. Gerontol. 2015, 66, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Massa, N.M.L.; Silva, A.S.; de Oliveira, C.V.C.; Costa, M.J.C.; Persuhn, D.C.; Barbosa, C.V.S.; Gonçalves, M.D. Supplementation with Watermelon Extract Reduces Total Cholesterol and LDL Cholesterol in Adults with Dyslipidemia under the Influence of the MTHFR C677T Polymorphism. J. Am. Coll. Nutr. 2016, 35, 514–520. [Google Scholar] [CrossRef]
- Issa, C.T.M.I.; Silva, A.S.; Toscano, L.T.; Medeiros, M.S.; Persuhn, D.C.; da Silva Diniz, A.; de Carvalho Costa, M.J.; Rodrigues Gonçalves Mda, C. Relationship between cardiometabolic profile, vitamin D status and BsmI polymorphism of the VDR gene in non-institutionalized elderly subjects: Cardiometabolic profile, vitamin D status and BsmI polymorphism of the VDR gene in non-institutionalized elderly subjects. Exp. Gerontol. 2016, 81, 56–64. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum: Hillsdale, MI, USA, 1988. [Google Scholar]
- Switzeny, O.J.; Müllner, E.; Wagner, K.H.; Brath, H.; Aumüller, E.; Haslberger, A.G. Vitamin and antioxidant rich diet increases MLH1 promoter DNA methylation in DMT2 subjects. Clin. Epigenet. 2012, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and T of HBC in A (Adult TPI. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421.
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American heart association nutrition committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [Green Version]
- Dietary Guidelines Advisory Committee. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans. Part D: The Science Base. Section 2: Nutrient Adequacy; USDA: Washington, DC, USA, 2010.
- Costa, M.J.C. Nutrição Clínica: Uso do Sistema de Equivalentes na Prática Dietoterápica, 2nd ed.; Editora da UFPB: João Pessoa, Brazil, 2013. [Google Scholar]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry; Report of A WHO Expert Committee; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- Kuo, K.; Still, R.; Mcdowell, I.; Biochem, M. Standardization (External and Internal) of HPLC Assay for Plasma Homocysteine. Clin. Chem. 1997, 43, 1653–1669. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, C.M.; Huff, D.L.; Gunter, E.W. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin. Chem. 1999, 45, 290–292. [Google Scholar] [CrossRef] [Green Version]
- Elefteriades, J.A. Mini-CABG: A step forward or backward? The point of view. J. Cardiothorac. Vasc. Anesth. 1997, 11, 661–668. [Google Scholar] [CrossRef]
- Kroegel, C.; Antony, V.B. Immunobiology of pleural inflammation: Potential implications for pathogenesis, diagnosis and therapy. Eur. Respir. J. 1997, 10, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Xirouchaki, N.; Tzanakis, N.; Bouros, D.; Kyriakou, D.; Karkavitsas, N.; Alexandrakis, M.; Siafakas, N. Diagnostic value of interleukin-1alpha, interleukin-6, and tumor necrosis factor in pleural effusions. Chest 2002, 121, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Benitez, J.M.; Barros, R.T.; Cevallos, C.V.G.; Rizzo, L.V.; Kokron, C.; Woronik, V.; Kalil, M.B.J. Comparação da secreção de citocinas (IL-4, IL-5, IL-6, IL-10) entre pacientes com nefropatia da IgA e deficiência de IgA. Rev. Bras. Alerg Imunopatol. 2004, 27, 82–93. [Google Scholar]
- Junqueira, C.L.C.; Pereira De Sant’Anna, P.R.; Junqueira Monteiro, A.S.; Franco de Oliverira, J.M.; Martins Romêo Filho, L.S. Associação de Marcadores Inflamatórios e Níveis Tensionais em Indivíduos Hipertensos com Diabetes Mellitus Tipo 2 Inflammatory Markers and Blood Pressure Levels in Hypertensive Patients with Type 2 Diabetes. Rev. da SOCERJ 2005, 18, 392–396. [Google Scholar]
- de Carvalho Pereira, D.; Lima, R.P.A.; de Lima, R.T.; Gonçalves, M.D.C.R.; de Morais, L.C.S.L.; Franceschini, S.D.C.C.; Filizola, R.G.; de Moraes, R.M.; Asciutti, L.S.R.; de Carvalho Costa, M.J. Association between obesity and calcium:phosphorus ratio in the habitual diets of adults in a city of Northeastern Brazil: An epidemiological study. Nutr. J. 2013, 12, 90. [Google Scholar] [CrossRef] [Green Version]
- Asciutti, L.S.R.; Rivera, M.A.A.; Costa, M.J.C.; Lima, F.E.L.; Imperiano, E.; Arruda, M.S.; Bandeira, M.G.; Arruda, N.S. Manual de Porções Média em Tamanho Real Baseado No Programa Dietsys Para Estudo de Base Populacional; de Nutrição, D., Ed.; Universidade Federal da Paraíba: João Pessoa, Brazil, 2005. [Google Scholar]
- USDA. U. S. Department of Agriculture Agricultural Research Service: Fontes de Folato. 2015. Available online: https://ndb.nal.usda.gov/ndb/search/list (accessed on 17 November 2017).
- EPIC—European Prospective Investigation into Cancer and Nutrition. Multiple Source Method—MSM. Available online: https://msm.dife.de/tps/msm/ (accessed on 17 December 2017).
- MSM. The Multiple Source Method. Departament of Epidemiology of the German Institute of Human Nutrition Potsdam. Available online: https://msm.dife.de/tps/msm/ (accessed on 17 November 2017).
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef] [Green Version]
- Arruda, V.R.; von Zuben, P.M.; Chiaparini, L.C.; Annichino-Bizzacchi, J.M.; Costa, F.F. The mutation Ala677>Val in the methylene tetrahydrofolate reductase gene: A risk factor for arterial disease and venous thrombosis. Thromb. Haemost. 1997, 77, 818–821. [Google Scholar] [CrossRef]
- Venn, B.J.; Mann, J.L.; Williams, S.M.; Riddell, L.J.; Chisholm, A.; Harper, M.J.; Aitken, W.; Rossaak, J.L. Assessment of three levels of folic acid on serum folate and plasma homocysteine: A randomised placebo-controlled double-blind dietary intervention trial. Eur. J. Clin. Nutr. 2002, 56, 748–754. [Google Scholar] [CrossRef]
- Ashfield-Watt, P.A.L.; Pullin, C.H.; Whiting, J.M.; Clark, Z.E.; Moat, S.J.; Newcombe, R.G.; Burr, M.L.; Lewis, M.J.; Powers, H.J.; McDowell, I.F.W. Methylenetetrahydrofolate reductase 677CT genotype modulates homocysteine responses to a folate-rich diet or a low dose folic acid supplement: A randomized controlled trial. Am. J. Clin. Nutr. 2002, 76, 180–186. [Google Scholar] [CrossRef]
- Ashfield-Watt, P.A.L.; Whiting, J.M.; Clark, Z.E.; Moat, S.J.; Newcombe, R.G.; Burr, M.L.; McDowell, I.F.W. A comparison of the effect of advice to eat either “5-a-day” fruit and vegetables or folic acid-fortified foods on plasma folate and homocysteine. Eur. J. Clin. Nutr. 2003, 57, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, P.; de Groot, L.C. Dietary determinants of plasma homocysteine concentrations. Semin. Vasc. Med. 2005, 5, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Bogers, R.P.; Dagnelie, P.C.; Bast, A.; van Leeuwen, M.; van Klaveren, J.D.; van den Brandt, P.A. Effect of increased vegetable and fruit consumption on plasma folate and homocysteine concentrations. Nutrition 2007, 23, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawashima, A.; Madarame, T.; Koike, H.; Komatsu, Y.; Wise, J.A. Four week supplementation with mixed fruit and vegetable juice concentrates increased protective serum antioxidants and folate and decreased plasma homocysteine in Japanese subjects. Asia Pac. J. Clin. Nutr. 2007, 16, 411–421. [Google Scholar] [PubMed]
- Zappacosta, B.; Mastroiacovo, P.; Persichilli, S.; Pounis, G.; Ruggeri, S.; Minucci, A.; Carnovale, E.; Andria, G.; Ricci, R.; Scala, I.; et al. Homocysteine Lowering by Folate-Rich Diet or Pharmacological Supplementations in Subjects with Moderate hyperhomocysteinemia. Nutrients 2013, 5, 1531–1543. [Google Scholar] [CrossRef]
- Kagawa, Y.; Hiraoka, M.; Kageyama, M.; Kontai, Y.; Yurimoto, M.; Nishijima, C.; Sakamoto, K. Medical cost savings in Sakado City and worldwide achieved by preventing disease by folic acid fortification. Congenit. Anom. 2017, 57, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Brouwer, I.A.; van Dusseldorp, M.; Thomas, C.M.; Duran, M.; Hautvast, J.G.A.J.; Eskes, T.K.A.B.; Steegers-Theunissen, R.P. Lowdose folic acid supplementation decreases plasma homocysteine concentrations: A randomized trial. Am. J. Clin. Nutr. 1999, 69, 99–104. [Google Scholar] [CrossRef]
- Arias, L.D.; Parra, B.E.; Muñoz, A.M.; Cárdenas, D.L.; Duque, T.G.; Manjarrés, L.M. Study Exploring the Effects of Daily Supplementation with 400 μg of Folic Acid on the Nutritional Status of Folate in Women of Reproductive Age. Birth Defects Res. 2017, 109, 564–573. [Google Scholar] [CrossRef]
- Anderson, C.A.; Beresford, S.A.; McLerran, D.; Lampe, J.W.; Deeb, S.; Feng, Z.; Motulsky, A.G. Response of serum and red blood cell folate concentrations to folic acid supplementation depends on methylenetetrahydrofolate reductase C677T genotype: Results from a crossover trial. Mol. Nutr. Food Res. 2013, 57, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Hiraoka, M.; Kato, K.; Saito, Y.; Yasuda, K.; Kagawa, Y. Gene-nutrient and gene-gene interactions of controlled folate intake by Japanese women. Biochem. Biophys. Res. Commun. 2004, 316, 1210–1216. [Google Scholar] [CrossRef]
- Cabo, R.; Hernes, S.; Slettan, A.; Haugen, M.; Ye, S.; Blomhoff, R.; Mansoor, M.A. Effect of genetic polymorphisms involved in folate metabolism on the concentration of serum folate and plasma total homocysteine (p-tHcy) in healthy subjects after short-term folic acid supplementation: A randomized, double blind, crossover study. Genes Nutr. 2015, 10, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colson, N.J.; Naug, H.L.; Nikbakht, E.; Zhang, P.; McCormack, J. The impact of MTHFR 677 C/T genotypes on folate status markers: A meta-analysis of folic acid intervention studies. Eur. J. Nutr. 2017, 56, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Guinotte, C.L.; Burns, M.G.; Axume, J.A.; Hata, H.; Urrutia, T.F.; Alamilla, A.; McCabe, D.; Singgih, A.; Cogger, E.A.; Caudill, M.A. Methylenetetrahydrofolate reductase 677C-->T variant modulates folate status response to controlled folate intakes in young women. J. Nutr. 2003, 133, 1272–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saldanha, J.F.; de O Leal, V.; Stenvinkel, P.; Carraro-Eduardo, J.C.; Mafra, D. Resveratrol: Why Is It a Promising Therapy for Chronic Kidney Disease Patients? Oxid. Med. Cell. Longev. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Connaughton, R.M.; McMorrow, A.M.; McGillicuddy, F.C.; Lithander, F.E.; Roche, H.M. Impact of anti-inflammatory nutrients on obesity-associated metabolic-inflammation from childhood through to adulthood. Proc. Nutr. Soc. 2016, 75, 115–124. [Google Scholar] [CrossRef]
- Wang, G.; Dai, J.; Mao, J.; Zeng, X.; Yang, X.; Wang, X. Folic acid reverses hyper-responsiveness of LPS-induced chemokine secretion from monocytes in patients with hyperhomocysteinemia. Atherosclerosis 2005, 179, 395–402. [Google Scholar] [CrossRef]
- Singhal, G.; Jaehne, E.J.; Corrigan, F.; Toben, C.; Baune, B.T. Inflammasomes in neuroinflammation and changes in brain function: A focused review. Front. Neurosci. 2014, 8, 315. [Google Scholar] [CrossRef] [Green Version]
- Bonfigli, A.R.; Spazzafumo, L.; Prattichizzo, F.; Bonafè, M.; Mensà, E.; Micolucci, L.; Giuliani, A.; Fabbietti, P.; Testa, R.; Boemi, M. Leukocyte telomere length and mortality risk in patients with type 2 diabetes. Oncotarget 2016, 7, 50835–50844. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Keku, T.O.; Martin, C.; Galanko, J.; Woosley, J.T.; Schroeder, J.C.; Satia, J.A.; Halabi, S.; Sandler, R.S. Circulating Levels of Inflammatory Cytokines and Risk of Colorectal Adenomas. Cancer Res. 2008, 68, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Soria, G.; Ofri-Shahak, M.; Haas, I.; Yaal-Hahoshen, N.; Leider-Trejo, L.; Leibovich-Rivkin, T.; Weitzenfeld, P.; Meshel, T.; Shabtai, E.; Gutman, M.; et al. Inflammatory mediators in breast cancer: Coordinated expression of TNFα & IL-1β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transition. BMC Cancer 2011, 11, 130. [Google Scholar]
- Hehlgans, T.; Pfeffer, K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: Players, rules and the games. Immunology 2005, 115, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, C.L.; Kennedy, E.B.; Roche, H.M. Metabolic Inflammation-Differential Modulation by Dietary Constituents. Nutrients 2016, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Farinha, J.B.; Steckling, F.M.; Stefanello, S.T.; Cardoso, M.S.; Nunes, L.S.; Barcelos, R.P.; Duarte, T.; Kretzmann, N.A.; Mota, C.B.; Bresciani, G. Response of oxidative stress and inflammatory biomarkers to a 12-week aerobic exercise training in women with metabolic syndrome. Sports Med. Open 2015, 1, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.J.; Mcallister, M.J.; Slusher, A.L.; Webb, H.E.; Mock, J.T.; Acevedo, E.O. Obesity-related oxidative stress: The impact of physical activity and diet manipulation. Sports Med. Open 2015, 1, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, X.; Li, J.; Cui, Y.; Liu, Z.; Zhao, Z.; Ge, J.; Guan, D.; Hu, J.; Wang, Y.; Zhang, F.; et al. MTHFR C677T and MTR A2756G polymorphisms and the homocysteine lowering efficacy of different doses of folic acid in hypertensive Chinese adults. Nutr. J. 2012, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Cuskelly, G.J.; McNulty, H.; Scott, J.M. Effect of increasing dietary folate on red-cell folate: Implications for prevention of neural tube defects. Lancet 1996, 347, 657–659. [Google Scholar] [CrossRef]
- Bonaa, K.H.; Njølstad, I.; Ueland, P.M.; Schirmer, H.; Tyerdal, A.; Steigen, T.; Wang, H.; Nordrehaug, J.E.; Arnesen, E.; Rasmussen, K. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N. Engl. J. Med. 2006, 354, 1578–1588. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elmawla, M.A.; Rizk, S.M.; Youssry, I.; Shaheen, A.A. Impact of genetic polymorphism of methylenetetrahydrofolate reductase C677T on development of hyperhomocysteinemia and related oxidative changes in Egyptian b-thalassemia major patients. PLoS ONE 2016, 11, e0155070. [Google Scholar] [CrossRef]
- McDonald, D.; Glusman, G.; Price, N.D. Personalized nutrition through big data. Nat. Biotechnol. 2016, 34, 152–154. [Google Scholar] [CrossRef]
- Noecker, C.; Borenstein, E. Getting Personal about Nutrition. Trends Mol. Med. 2016, 22, 83–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacka, F.N.; Maes, M.; Pasco, J.A.; Williams, L.J.; Berk, M. Nutrient intakes and the common mental disorders in women. J. Affect. Disord. 2012, 141, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Vollset, S.E.; Clarke, R.; Lewington, S.; Ebbing, M.; Halsey, J.; Lonn, E.; Armitage, J.; Manson, J.E.; Hankey, G.J.; Spence, J.D.; et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: Meta-analyses of data on 50,000 individuals. Lancet 2013, 381, 1029–1036. [Google Scholar] [CrossRef] [Green Version]
- Clarke, R.; Bennett, D.; Parish, S.; Lewington, S.; Skeaff, M.; Eussen, S.J.; Lewerin, C.; Stott, D.J.; Armitage, J.; Hankey, G.J.; et al. Effects of homocysteine lowering with B vitamins on cognitive aging: Metaanalysis of 11 trials with cognitive data on 22,000 individuals. Am. J. Clin. Nutr. 2014, 100, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, D.E.; El-Sohemy, A. A randomized trial of genetic information for personalized nutrition. Genes Nutr. 2012, 7, 559–566. [Google Scholar] [CrossRef] [Green Version]

| Group | Gene | est_d | n | Significance | Power |
|---|---|---|---|---|---|
| 2 | CC | 1.48 | 8 | 5% | 94.5% |
| 2 | CT | 1.96 | 8 | 5% | 99.7% |
| 2 | TT | 1.89 | 8 | 5% | 99.5% |
| 1 | CC | 0.43 | 8 | 5% | 18.5% |
| 1 | CT | 0.69 | 8 | 5% | 78.7% |
| 1 | TT | 1.40 | 8 | 5% | 92.1% |
| Group | Gene | n | SD_Before FA | Mean_Before FA | SD_After FA | Mean_After FA |
|---|---|---|---|---|---|---|
| 2 | CC | 8 | 5.30 | 12.95 | 7.27 | 22.22 |
| 2 | CT | 8 | 1.62 | 13.49 | 3.10 | 18.11 |
| 2 | TT | 8 | 5.12 | 16.53 | 4.36 | 25.48 |
| 1 | CC | 8 | 4.09 | 13.18 | 3.79 | 14.89 |
| 1 | CT | 8 | 3.98 | 13.32 | 4.96 | 16.40 |
| 1 | TT | 8 | 4.60 | 15.01 | 6.30 | 22.63 |
| Biomarkers | Group 1 (95 µg/Day) | Group 2 (191 µg/Day) | p-Value |
|---|---|---|---|
| Age (years) | 44.33 ± 9.26 | 44.88 ± 12.47 | 0.8802 |
| BMI (kg/m2) | 30.47 ± 4.82 | 29.88 ± 3.01 | 0.6236 |
| WC (cm) | 91.78 ± 12.02 | 91.63 ± 7.96 | 0.9600 |
| Folate (µg) | 150.90 ± 42.73 | 144.40 ± 43.47 | 0.6034 |
| Folic Acid (μg/mL) | 13.83 ± 4.13 | 14.32 ± 4.46 | 0.6222 |
| Vitamin B12 (pg/mL) | 304.66 ± 133.05 | 253.14 ± 103.58 | 0.1417 |
| Homocysteine (μmol/L) | 11.65 ± 5.99 | 9.64 ± 2.44 | 0.1404 |
| TNF-α (pg/mL) | 6.14 ± 7.26 | 6.51 ± 7.90 | 0.8661 |
| IL-1β (pg/mL) | 3.88 ± 3.90 | 5.11 ± 4.92 | 0.3429 |
| IL-6 (pg/mL) | 4.42 ± 3.85 | 4.49 ± 3.47 | 0.9894 |
| Group 1 95 µg/Day | |||||||||
| Pre | Post | p-value | Pre | Post | p-value | Pre | Post | p-value | |
| CC | CC | CT | CT | TT | TT | ||||
| Folate (μg) | 149.0 ± 14.43 | 292.0 ± 65.24 | 0.0004 * | 170.0 ± 50.43 | 254.0 ± 85.27 | 0.0910 | 154.0 ± 53.84 | 316.0 ± 63.06 | 0.0003 * |
| FA (μg/mL) | 13.18 ± 4.08 | 14.88 ± 3.79 | 0.1318 | 13.32 ± 3.97 | 16.4 ± 4.95 | 0.0011 * | 15.00 ± 4.60 | 22.62 ± 6.30 | 0.0249 * |
| Vit. B12 (μg/mL) | 268.6± 92.63 | 271.0 ± 92.16 | 0.3185 | 345.5 ± 179.14 | 355.2 ± 202.59 | 0.7991 | 299.8 ± 118.65 | 273.6 ± 83.60 | 0.3162 |
| Hcy (μmol/L) | 13.61 ± 13.10 | 7.83 ± 2.51 | 0.2408 | 10.98 ± 6.11 | 9.86 ± 4.43 | 0.6812 | 19.33 ± 12.75 | 14.91 ± 10.40 | 0.4603 |
| TNF-α (pg/mL) | 5.80 ± 8.33 | 4.45 ± 3.54 | 0.6489 | 9.52 ± 8.63 | 2.84 ± 2.09 | 0.0517 | 3.10 ± 2.34 | 4.30 ± 6.99 | 0.5959 |
| IL-6 (pg/mL) | 4.26 ± 5.10 | 4.48 ± 2.29 | 0.9184 | 5.16 ± 1.60 | 2.82 ± 2.96 | 0.0685 | 3.85 ± 4.39 | 3.29 ± 3.07 | 0.8310 |
| IL-1β (pg/mL) | 3.50 ± 4.44 | 3.85 ± 1.78 | 0.8280 | 5.43 ± 4.50 | 1.55 ± 1.79 | 0.0224* | 2.71 ± 2.34 | 3.69 ± 4.58 | 0.6713 |
| Group 2 191 µg/Day | |||||||||
| Pre | Post | p-value | Pre | Post | p-value | Pre | Post | p-value | |
| CC | CC | CT | CT | TT | TT | ||||
| Folate (μg) | 132.0 ± 32.73 | 352.0 ± 73.07 | 0.0000 * | 128.0 ± 41.17 | 410.0 ± 372.35 | 0.0000 * | 184.0 ± 34.54 | 376.0 ± 68.32 | 0.0000 * |
| FA (μg/mL) | 12.94 ± 5.29 | 22.21 ± 7.26 | 0.0033 * | 13.48 ± 1.61 | 18.11 ± 3.10 | 0.0016 * | 16.52 ± 5.11 | 25.48 ± 4.35 | 0.0046 * |
| Vi. B12 (μg/mL) | 251.8 ± 113.13 | 237.5 ± 52.69 | 0.7174 | 261.2 ± 139.79 | 258.0 ± 113.03 | 0.8429 | 246.3 ± 52.71 | 228.6 ± 78.15 | 0.6492 |
| Hcy (μmol/L) | 8.97 ± 1.52 | 7.60 ± 1.79 | 0.1207 | 9.91 ± 3.70 | 8.01 ± 2.27 | 0.2374 | 10.05 ± 1.66 | 7.11 ± 0.77 | 0.0005 * |
| TNF-α (pg/mL) | 7.73 ± 12.34 | 5.46 ± 9.38 | 0.5132 | 5.02 ± 4.59 | 1.66 ± 2.31 | 0.1614 | 6.78 ± 5.24 | 1.66 ± 0.53 | 0.0004 * |
| IL-6 (pg/mL) | 3.93 ± 4.70 | 3.63 ± 3.41 | 0.8980 | 3.86 ± 3.76 | 1.84 ± 2.03 | 0.2224 | 5.67 ± 0.93 | 0.66 ± 0.74 | 0.0000 * |
| IL-1β (pg/mL) | 3.17 ± 5.74 | 17.62 ± 1.63 | 0.2610 | 6.85 ± 5.82 | 3.01 ± 3.43 | 0.1565 | 5.32 ± 2.20 | 1.73 ± 2.47 | 0.0007 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisboa, J.V.d.C.; Ribeiro, M.R.; Luna, R.C.P.; Lima, R.P.A.; do Nascimento, R.A.F.; Monteiro, M.G.C.A.; Lima, K.Q.d.F.; Fechine, C.P.N.d.S.; de Oliveira, N.F.P.; Persuhn, D.C.; et al. Food Intervention with Folate Reduces TNF-α and Interleukin Levels in Overweight and Obese Women with the MTHFR C677T Polymorphism: A Randomized Trial. Nutrients 2020, 12, 361. https://doi.org/10.3390/nu12020361
Lisboa JVdC, Ribeiro MR, Luna RCP, Lima RPA, do Nascimento RAF, Monteiro MGCA, Lima KQdF, Fechine CPNdS, de Oliveira NFP, Persuhn DC, et al. Food Intervention with Folate Reduces TNF-α and Interleukin Levels in Overweight and Obese Women with the MTHFR C677T Polymorphism: A Randomized Trial. Nutrients. 2020; 12(2):361. https://doi.org/10.3390/nu12020361
Chicago/Turabian StyleLisboa, Jéssica Vanessa de Carvalho, Marina Ramalho Ribeiro, Rafaella Cristhine Pordeus Luna, Raquel Patrícia Ataíde Lima, Rayner Anderson Ferreira do Nascimento, Mussara Gomes Cavalcante Alves Monteiro, Keylha Querino de Farias Lima, Carla Patrícia Novaes dos Santos Fechine, Naila Francis Paulo de Oliveira, Darlene Camati Persuhn, and et al. 2020. "Food Intervention with Folate Reduces TNF-α and Interleukin Levels in Overweight and Obese Women with the MTHFR C677T Polymorphism: A Randomized Trial" Nutrients 12, no. 2: 361. https://doi.org/10.3390/nu12020361
APA StyleLisboa, J. V. d. C., Ribeiro, M. R., Luna, R. C. P., Lima, R. P. A., do Nascimento, R. A. F., Monteiro, M. G. C. A., Lima, K. Q. d. F., Fechine, C. P. N. d. S., de Oliveira, N. F. P., Persuhn, D. C., Veras, R. C., Gonçalves, M. d. C. R., Ferreira, F. E. L. d. L., Lima, R. T., da Silva, A. S., Diniz, A. d. S., de Almeida, A. T. C., de Moraes, R. M., Verly Junior, E., & Costa, M. J. d. C. (2020). Food Intervention with Folate Reduces TNF-α and Interleukin Levels in Overweight and Obese Women with the MTHFR C677T Polymorphism: A Randomized Trial. Nutrients, 12(2), 361. https://doi.org/10.3390/nu12020361