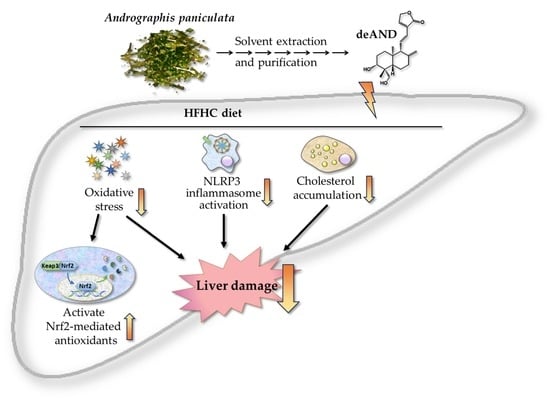
A Diterpenoid, 14-Deoxy-11, 12-Didehydroandrographolide, in Andrographis paniculata Reduces Steatohepatitis and Liver Injury in Mice Fed a High-Fat and High-Cholesterol Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Studies
2.3. Determinations of Fat Contents in the Liver and Feces
2.4. Determination of Glutathione, Glutathione-Related Enzyme Activities, and Lipid Peroxidation in Liver
2.5. Western Blot Analysis
2.6. Quntitative Real-Time Polymerase Chain Reaction (Q-PCR) Analysis
2.7. Determination of Glutamate in the Plasma and Liver
2.8. Statistical Analysis
3. Results
3.1. Plasma Biochemical Parameters
3.2. Histological Examination
3.3. GSH and GSH-Related Enzyme Activities, Lipid Peroxidation, and Tumor Necrosis Factor-Alpha (TNF)-α Content
3.4. Apoptosis Index in Liver
3.5. Fecal Cholesterol and Total Bile Acid Contents
3.6. NLRP3 Inflammasome Activation
3.7. Antioxidant Enzyme Activity
3.8. Glutamate Levels in Plasma and the Liver
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iqbal, U.; Perumpail, B.J.; Akhtar, D.; Kim, D.; Ahmed, A. The Epidemiology, Risk Profiling and Diagnostic Challenges of Nonalcoholic Fatty Liver. Medicines (Basel) 2019, 6, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Fernández-Galilea, M.; Martínez-Fernández, L.; González-Muniesa, P.; Pérez-Chávez, A.; Martínez, J.A.; Moreno-Aliaga, M.J. Oxidative Stress and Non-Alcoholic Fatty Liver Disease: Effects of Omega-3 Fatty Acid Supplementation. Nutrients 2019, 11, 872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioannou, G.N.; Haigh, W.G.; Thorning, D.; Savard, C. Hepatic cholesterol crystals and crown-like structures distinguish NASH from simple steatosis. J. Lipid Res. 2013, 54, 1326–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, M.V.; Michelotti, G.A.; Xie, G.; Almeida-Pereira, T.; Boursier, J.; Bohnic, B.; Guy, C.D.; Diehl, A.M. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS ONE 2015, 10, e0127991. [Google Scholar] [CrossRef] [Green Version]
- Ahn, M.; Kim, J.; Choi, Y.; Ekanayake, P.; Chun, J.Y.; Yang, D.; Kim, G.O.; Shin, T. Fermented black radish (Raphanus sativus L. var. niger) attenuates methionine and choline deficient diet-induced nonalcoholic fatty liver disease in mice. Food Sci. Nutr. 2019, 9, 3327–3337. [Google Scholar] [CrossRef] [Green Version]
- Tu, L.N.; Showalter, M.R.; Cajka, T.; Fan, S.; Pillai, V.V.; Fiehn, O.; Selvaraj, V. Metabolomic characteristics of cholesterol-induced non-obese nonalcoholic fatty liver disease in mice. Sci. Rep. 2017, 7, 6120. [Google Scholar] [CrossRef]
- Ioannou, G.N. The Role of Cholesterol in the Pathogenesis of NASH. Trends Endocrinol. Metab. 2016, 27, 84–95. [Google Scholar] [CrossRef]
- Yao, H.T.; Lee, P.F.; Lii, C.K.; Liu, Y.T.; Chen, S.H. Freshwater clam extract reduces liver injury by lowering cholesterol accumulation, improving dysregulated cholesterol synthesis and alleviating inflammation in high-fat, high-cholesterol and cholic acid diet-induced steatohepatitis in mice. Food Funct. 2018, 19, 4876–4887. [Google Scholar] [CrossRef]
- Matsuzawa, N.; Takamura, T.; Kurita, S.; Misu, H.; Ota, T.; Ando, H.; Yokoyama, M.; Honda, M.; Zen, Y.; Nakanuma, Y.; et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology 2007, 46, 1392–1403. [Google Scholar] [CrossRef]
- Hirsch, N.; Konstantinov, A.; Anavi, S.; Aronis, A.; Hagay, Z.; Madar, Z.; Tirosh, O. Prolonged feeding with green tea polyphenols exacerbates cholesterol-induced fatty liver disease in mice. Mol. Nutr. Food Res. 2016, 60, 2542–2553. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D. Phytonutrients as therapeutic agents. J. Complement. Integr. Med. 2014, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Crisóstomo, I.; Fernández-Martínez, E.; Cariño-Cortés, R.; Betanzos-Cabrera, G.; Bobadilla-Lugo, R.A. Phytosterols and Triterpenoids for Prevention and Treatment of Metabolic-related Liver Diseases and Hepatocellular Carcinoma. Curr. Pharm. Biotechnol. 2019, 20, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.W.; Lin, B.F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 13, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koteswara, R.Y.; Vimalamma, G.; Rao, C.V.; Tzeng, Y.M. Flavonoids and andrographolides from Andrographis paniculata. Phytochemistry 2004, 65, 2317–2321. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; He, H.; Xia, G.Y.; Zhou, K.L.; Qiu, F. A new flavonoid from the aerial parts of Andrographis paniculata. Nat. Prod. Res. 2014, 28, 138–143. [Google Scholar] [CrossRef]
- Chua, L.S. Review on liver inflammation and antiinflammatory activity of Andrographis paniculata for hepatoprotection. Phytother. Res. 2014, 28, 1589–1598. [Google Scholar] [CrossRef]
- Wang, L.; Cao, F.; Zhu, L.L.; Liu, P.; Shang, Y.R.; Liu, W.H.; Dong, X.; Bao, H.D.; Gong, P.; Wang, Z.Y. Andrographolide impairs alpha-naphthyl isothiocyanate-induced cholestatic liver injury in vivo. J. Nat. Med. 2019, 73, 388–396. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, S.R.; Chai, L.; Zhao, J.; Wang, Y.; Wang, Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit. Rev. Food Sci. Nutr. 2019, 59, S17–S29. [Google Scholar] [CrossRef]
- Pholphana, N.; Rangkadilok, N.; Thongnest, S.; Ruchirawat, S.; Ruchirawat, M.; Satayavivad, J. Determination and variation of three active diterpenoids in Andrographis paniculata (Burm.f.) Nees. Phytochem. Anal. 2004, 15, 365–371. [Google Scholar] [CrossRef]
- Guan, S.P.; Kong, L.R.; Cheng, C.; Lim, J.C.; Wong, W.F. Protective role of 14-deoxy-11,12-didehydroandrographolide, a noncytotoxic analogue of andrographolide, in allergic airway inflammation. J. Nat. Prod. 2011, 74, 1484–1490. [Google Scholar] [CrossRef]
- Yen, C.C.; Liu, Y.T.; Lin, Y.J.; Yang, Y.C.; Chen, C.C.; Yao, H.T.; Chen, H.W.; Lii, C.K. Bioavailability of the diterpenoid 14-deoxy-11,12-didehydroandrographolide in rats and up-regulation of hepatic drug-metabolizing enzyme and drug transporter expression. Phytomedicine 2019, 61, 152841. [Google Scholar] [CrossRef] [PubMed]
- Awang, K.; Abdullah, N.H.; Hadi, A.H.; Su-Fong, Y. Cardiovascular activity of labdane diterpenes from Andrographis paniculata in isolated rat hearts. J. Biomed. Biotechnol. 2012, 2012, 876458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, W.; Li, Y.; Chen, S.; Wang, M.; Zhang, A.; Zhou, H.; Chen, H.; Jin, M. 14-Deoxy-11, 12-dehydroandrographolide exerts anti-influenza A virus activity and inhibits replication of H5N1 virus by restraining nuclear export of viral ribonucleoprotein complexes. Antiviral. Res. 2015, 118, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Morita, H.; Tezuka, Y. Preferentially cytotoxic constituents of Andrographis paniculata and their preferential cytotoxicity against human pancreatic cancer cell lines. Nat. Prod. Commun. 2015, 10, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.K.; Muhammad, T.S.; Tan, M.L. 14-Deoxy-11,12-didehydroandrographolide induces DDIT3-dependent endoplasmic reticulum stress-mediated autophagy in T-47D breast carcinoma cells. Toxicol. Appl. Pharmacol. 2016, 300, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Yoopan, N.; Thisoda, P.; Rangkadilok, N.; Sahasitiwat, S.; Pholphana, N.; Ruchirawat, S.; Satayavivad, J. Cardiovascular effects of 14-deoxy-11,12-didehydroandrographolide and Andrographis paniculata extracts. Planta Med. 2007, 73, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Carleton, H.M. Carleton’s Histological Techniques, 5th ed.; Oxford University Press: London, UK, 1980. [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Guan, X.; Hoffman, B.; Dwivedi, C.; Matthees, D.P. A simultaneous liquid chromatography/mass spectrometric assay of glutathione, cysteine, homocysteine and their disulfides in biological samples. J. Pharm. Biomed. Anal. 2003, 31, 251–261. [Google Scholar] [CrossRef]
- Uehiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissue by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Yao, H.T.; Hsu, Y.R.; Lii, C.K.; Lin, A.H.; Chang, K.H.; Yang, H.T. Effect of commercially available green and black tea beverages on drug-metabolizing enzymes and oxidative stress in Wistar rats. Food Chem. Toxicol. 2014, 70, 120–127. [Google Scholar] [CrossRef]
- Yao, H.T.; Lii, C.K.; Chou, R.H.; Lin, J.H.; Yang, H.T.; Chiang, M.T. Effect of chitosan on hepatic drug metabolizing enzymes and oxidative stress in rats fed low and high-fat diets. J. Agric. Food Chem. 2010, 58, 5187–5193. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Chan, I.L.; Yang, T.H.; Liu, S.H.; Chiang, M.T. Supplementation of chitosan alleviates high-fat diet-enhanced lipogenesis in rats via adenosine monophosphate (AMP)-activated protein kinase activation and inhibition of lipogenesis-associated genes. J. Agric. Food Chem. 2015, 25, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Shakib, N.; Khadem Ansari, M.H.; Karimi, P.; Rasmi, Y. Neuroprotective mechanism of low-dose sodium nitrite in oxygen-glucose deprivation model of cerebral ischemic stroke in PC12 cells. EXCLI J. 2019, 18, 229–242. [Google Scholar] [PubMed]
- Min, H.K.; Kapoor, A.; Fuchs, M.; Mirshahi, F.; Zhou, H.; Maher, J.; Kellum, J.; Warnick, R.; Contos, M.J.; Sanyal, A.J. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012, 15, 665–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinué, Á.; Herrero-Cervera, A.; González-Navarro, H. Understanding the Impact of Dietary Cholesterol on Chronic Metabolic Diseases through Studies in Rodent Models. Nutrients 2018, 10, 939. [Google Scholar] [CrossRef] [Green Version]
- Gan, L.T.; Van Rooyen, D.M.; Koina, M.E.; McCuskey, R.S.; Teoh, N.C.; Farrell, G.C. Hepatocyte free cholesterol lipotoxicity results from JNK1-mediated mitochondrial injury and is HMGB1 and TLR4-dependent. J. Hepatol. 2014, 61, 1376–1384. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, D.; Wree, A.; Povero, D.; Solís, N.; Hernandez, A.; Pizarro, M.; Moshage, H.; Torres, J.; Feldstein, A.E.; Cabello-Verrugio, C.; et al. Andrographolide Ameliorates Inflammation and Fibrogenesis and Attenuates Inflammasome Activation in Experimental Non-Alcoholic Steatohepatitis. Sci. Rep. 2017, 14, 3491. [Google Scholar] [CrossRef]
- Yang, J.J.; Tao, H.; Huang, C.; Li, J. Nuclear erythroid 2-related factor 2: A novel potential therapeutic target for liver fibrosis. Food Chem. Toxicol. 2013, 59, 421–427. [Google Scholar] [CrossRef]
- Li, J.; Woolbright, B.L.; Zhao, W.; Wang, Y.; Matye, D.; Hagenbuch, B.; Jaeschke, H.; Li, T. Sortilin 1 Loss-of-Function Protects Against Cholestatic Liver Injury by Attenuating Hepatic Bile Acid Accumulation in Bile Duct Ligated Mice. Toxicol. Sci. 2018, 161, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, S.; Furfaro, A.L.; Domenicotti, C.; Odetti, P.; Cottalasso, D.; Marinari, U.M.; Pronzato, M.A.; Traverso, N. Supplementation with N-acetylcysteine and taurine failed to restore glutathione content in liver of streptozotocin-induced diabetics rats but protected from oxidative stress. Biochim. Biophys. Acta 2005, 1741, 48–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaassen, C.D.; Reisman, S.A. Nrf2 the rescue: Effects of the antioxidative/electrophilic response on the liver. Toxicol. Appl. Pharmacol. 2010, 244, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safaei, A.; Arefi Oskouie, A.; Mohebbi, S.R.; Rezaei-Tavirani, M.; Mahboubi, M.; Peyvandi, M.; Okhovatian, F.; Zamanian-Azodi, M. Metabolomic analysis of human cirrhosis, hepatocellular carcinoma, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis diseases. Gastroenterol. Hepatol. Bed Bench 2016, 9, 158–173. [Google Scholar] [PubMed]
- Garattini, S. Glutamic acid, twenty years later. J. Nutr. 2000, 130, 901S–909S. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, C.G. A review of glutamate receptors I: Current understanding of their biology. J. Toxicol. Pathol. 2008, 21, 25–51. [Google Scholar] [CrossRef]








| Control | HFHC | HFHC + 0.05% deAND | HFHC + 0.1% deAND | |
|---|---|---|---|---|
| Casein | 20 | 20 | 20 | 20 |
| Soybean oil | 2.5 | 2.5 | 2.5 | 2.5 |
| Lard | 2 | 25 | 25 | 25 |
| Sucrose | 7 | 7 | 7 | 7 |
| Corn starch | 58.3 | 34.55 | 34.5 | 34.45 |
| Cellulose | 5 | 5 | 5 | 5 |
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 |
| Cholesterol | 0.5 | 0.5 | 0.5 | |
| Cholic acid | 0.25 | 0.25 | 0.25 | |
| AIN93 vitamin mixture a | 1 | 1 | 1 | 1 |
| AIN93 mineral mixture | 4 | 4 | 4 | 4 |
| deAND | 0.05 | 0.1 | ||
| Total calories (Kcal/100 g diet) | 381.7 | 494.9 | 494.7 | 494.5 |
| Gene | Forward Primer | Reverse Primer | Fragment Size |
|---|---|---|---|
| Nlrp3 | 5′-GAAGAAGAGAGGAGAGGAGGTCG-3′ | 5′-TTCACCAGTCTGGAAGAACAGGCAAC-3′ | 89 |
| Caspase 1 | 5′-TTACTGCTATGGACAAGGCACG-3′ | 5′-GCTGATGGAGCTGATTGAAGCT-3′ | 142 |
| Il-1β | 5′-TTTGAAGAAGAGCCCTCCTC-3′ | 5′-AGGTGCTGATGTACCAGTTG-3′ | 436 |
| Nrf2 | 5′- GATGACCATGAGTCGCTTGC-3′ | 5′- CCTGATGAGGGGCAGTGAAG-3′ | 73 |
| Gapdh | 5′-GCCTGGAGAAACCTGCCAAGTATG-3′ | 5′-GGGAGTTGCTGTTGAAGTCGCA-3′ | 213 |
| Control | HFHC | HFHC + 0.05% deAND | HFHC + 0.1% deAND | |
|---|---|---|---|---|
| Total cholesterol (mg/dL) | 125.5 ± 9.8 | 454.3 ± 38.2 * | 360.0 ± 52.0 # | 345.3 ± 86.1 # |
| Triglyceride (mg/dL) | 154.3 ± 24.3 | 97.7 ± 21.7 * | 113.5 ± 34.3 | 89.8 ± 14.0 |
| ALT (U/L) | 25.8 ± 12.6 | 232.8 ± 134.7 * | 44.0 ± 10.5 # | 49.4 ± 18.9 # |
| AST (U/L) | 90.3 ± 39.3 | 424.8 ± 173.6 * | 131.0 ± 70.3 # | 122.0 ± 43.2 # |
| Hs-CRP (mg/dL) | 0.28 ± 0.02 | 0.62 ± 0.14 * | 0.52 ± 0.03 | 0.56 ± 0.11 |
| IL-1β (pg/mL) | 2.95 ± 0.96 | 2.82 ± 1.07 | 1.99 ± 0.38 | 2.34 ± 0.69 |
| Control | HFHC | HFHC + 0.05% deAND | HFHC + 0.1% deAND | |
|---|---|---|---|---|
| GSH (nmol/mg protein) | 2.81 ± 0.99 | 0.26 ± 0.03 * | 0.24 ± 0.05 | 0.32 ± 0.04 # |
| TBARS (nmol MDA/mg protein) | 0.79 ± 0.14 | 0.73 ± 0.14 | 0.76 ± 0.22 | 0.81 ± 0.14 |
| GSH peroxidase (nmol/min/mg protein) | 330.7 ± 62.0 | 283.3 ± 24.2 | 268.3 ± 72.3 | 243.2 ± 43.1 |
| GSH reductase (nmol/min/mg protein) | 49.6 ± 3.5 | 43.6 ± 2.9 * | 48.1 ± 5.8 | 50.6 ± 5.3 # |
| GSH-S-transferase (nmol/min/mg protein) | 317.7 ± 64.6 | 370.0 ± 39.9 | 297.2 ± 57.9 | 373.9 ± 86.7 |
| TNF-α (pg/mg protein) | 38.1 ± 7.5 | 67.7 ± 15.5 * | 54.4 ± 6.0 | 43.6 ± 12.0 # |
| IL-1β (pg/mg protein) | 0.04 ± 0.01 | 0.09 ± 0.06 * | 0.06 ± 0.01 | 0.05 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-T.; Chen, H.-W.; Lii, C.-K.; Jhuang, J.-H.; Huang, C.-S.; Li, M.-L.; Yao, H.-T. A Diterpenoid, 14-Deoxy-11, 12-Didehydroandrographolide, in Andrographis paniculata Reduces Steatohepatitis and Liver Injury in Mice Fed a High-Fat and High-Cholesterol Diet. Nutrients 2020, 12, 523. https://doi.org/10.3390/nu12020523
Liu Y-T, Chen H-W, Lii C-K, Jhuang J-H, Huang C-S, Li M-L, Yao H-T. A Diterpenoid, 14-Deoxy-11, 12-Didehydroandrographolide, in Andrographis paniculata Reduces Steatohepatitis and Liver Injury in Mice Fed a High-Fat and High-Cholesterol Diet. Nutrients. 2020; 12(2):523. https://doi.org/10.3390/nu12020523
Chicago/Turabian StyleLiu, Yun-Ta, Haw-Wen Chen, Chong-Kuei Lii, Jia-Hua Jhuang, Chin-Shiu Huang, Mei-Ling Li, and Hsien-Tsung Yao. 2020. "A Diterpenoid, 14-Deoxy-11, 12-Didehydroandrographolide, in Andrographis paniculata Reduces Steatohepatitis and Liver Injury in Mice Fed a High-Fat and High-Cholesterol Diet" Nutrients 12, no. 2: 523. https://doi.org/10.3390/nu12020523
APA StyleLiu, Y. -T., Chen, H. -W., Lii, C. -K., Jhuang, J. -H., Huang, C. -S., Li, M. -L., & Yao, H. -T. (2020). A Diterpenoid, 14-Deoxy-11, 12-Didehydroandrographolide, in Andrographis paniculata Reduces Steatohepatitis and Liver Injury in Mice Fed a High-Fat and High-Cholesterol Diet. Nutrients, 12(2), 523. https://doi.org/10.3390/nu12020523