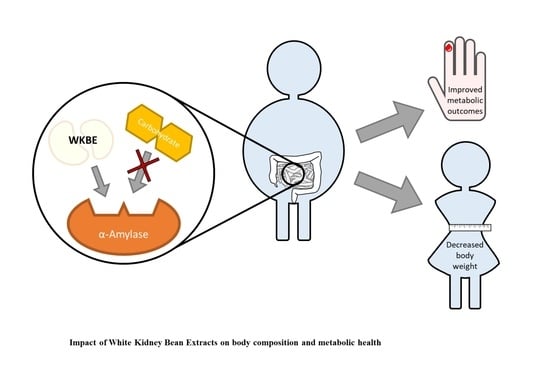
It’s No Has Bean: A Review of the Effects of White Kidney Bean Extract on Body Composition and Metabolic Health
Abstract
:1. Introduction
2. White Kidney Bean Extract
3. Evidence from Animal Studies
3.1. Effects of WKBE on Body Weight and Composition
3.2. Effects of WKBE on Cardiometabolic Markers and the Gut Microbiota
3.2.1. Blood Markers
3.2.2. Gut Microbiota Composition and Metabolism
3.2.3. Oxidative Stress Markers
4. Evidence from Human Studies
4.1. Effects of WKBE on Body Weight and Composition
4.2. Effects of WKBE on Cardiometabolic Markers
4.2.1. Blood Markers
4.2.2. Blood Pressure
4.2.3. Appetite and Hunger
4.3. Adverse Effects of WKBE
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Berry, E.M. The Obesity Pandemic-Whose Responsibility? No Blame, No Shame, Not More of the Same. Front. Nutr. 2020, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Home Page. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 March 2020).
- Andolfi, C.; Fisichella, P.M. Epidemiology of Obesity and Associated Comorbidities. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Childhood Obesity Plan: PHE’s Role in Implementation. Available online: https://www.gov.uk/government/publications/childhood-obesity-plan-phes-role-in-implementation/childhood-obesity-plan-phes-role-in-implementation (accessed on 1 March 2020).
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries Over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- Wright, S.M.; Aronne, L.J. Causes of Obesity. Abdom. Imaging 2012, 37, 730–732. [Google Scholar] [CrossRef]
- Leitner, D.R.; Frühbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and Type 2 Diabetes: Two Diseases with a Need for Combined Treatment Strategies—EASO can lead the way. Obes. Facts 2017, 5, 5–492. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Obesity and Hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef]
- Houghton, D.; Thoma, C.; Hallsworth, K.; Cassidy, S.; Hardy, T.; Burt, A.D.; Tiniakos, D.; Hollingsworth, K.G.; Taylor, R.; Day, C.P.; et al. Exercise Reduces Liver Lipids and Visceral Adiposity in Patients With Nonalcoholic Steatohepatitis in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2017, 5, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Dudekula, A.; Rachakonda, V.; Shaik, B.; Behari, J. Weight Loss in Nonalcoholic Fatty Liver Disease Patients in an Ambulatory Care Setting is Largely Unsuccessful but Correlates with Frequency of Clinic Visits. PLoS ONE 2014, 9, e111808. [Google Scholar] [CrossRef] [Green Version]
- King, N.A.; Hopkins, M.; Caudwell, P.; Stubbs, R.J.; Blundell, J.E. Individual Variability Following 12 Weeks of Supervised Exercise: Identification and Characterization of Compensation for Exercise-Induced Weight Loss. Int. J. Obes. Suppl. 2008, 32, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.E.; Markovitch, D.; Betts, J.A.; Thompson, D. Nonprescribed Physical Activity Energy Expenditure is Maintained With Structured Exercise and Implicates a Compensatory Increase in Energy Intake. Am. J. Clin. Nutr. 2010, 95, 1009–1016. [Google Scholar] [CrossRef] [Green Version]
- Sahebkar, A.; Simental-Mendía, L.E.; Reiner, Ž.; Kovanen, P.T.; Simental-Mendía, M.; Bianconi, V.; Pirro, M. Effect of Orlistat on Plasma Lipids and Body Weight: A Systematic Review and Meta-Analysis of 33 Randomized Controlled Trials. Pharmacol. Res. 2017, 122, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.; Kristensen, P.K.; Bartels, E.M.; Bliddal, H.; Astrup, A. Efficacy and Safety of the Weight-Loss Drug Rimonabant: A Meta-Analysis of Randomised Trials. Lancet 2007, 370, 1706–1713. [Google Scholar] [CrossRef]
- Kang, J.G.; Park, C.Y. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab. 2012, 36, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Phillips, B.T.; Shikora, S.A. The History of Metabolic and Bariatric Surgery: Development of Standards for Patient Safety and Efficacy. Metab. Clin. Exp. 2018, 79, 97–107. [Google Scholar] [CrossRef]
- O’Kane, M.; Parretti, H.M.; Hughes, C.A.; Sharma, M.; Woodcock, S.; Puplampu, T.; Blakemore, A.I.; Clare, K.; MacMillan, I.; Joyce, J.; et al. Guidelines for The Follow-Up of Patients Undergoing Bariatric Surgery. Clin. Obes. 2016, 6, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.; Yumuk, V.; Schlaich, M.; Nilsson, P.M.; Zahorska-Markiewicz, B.; Grassi, G.; Schmieder, R.E.; Engeli, S.; Finer, N. Joint Statement of The European Association for The Study of Obesity and the European Society of Hypertension: Obesity and Difficult to Treat Arterial Hypertension. Int. J. Hypertens. 2012, 30, 1047–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe, B.M.; Kvach, E.; Eckel, R.H. Treatment of Obesity. Circ. Res. 2016, 118, 1844–1855. [Google Scholar] [CrossRef]
- Payan, F. Structural Basis for The Inhibition of Mammalian and Insect α-amylases by Plant Protein Inhibitors. BBA-Proteins Proteom 2004, 1696, 171–180. [Google Scholar] [CrossRef]
- Bompard-Gilles, C.; Rousseau, P.; Rouge, P.; Payan, F. Substrate Mimicry in The Active Center of a Mammalian α-amylase: Structural Analysis of an Enzyme-Inhibitor Complex. Struture 1996, 4, 1441–1452. [Google Scholar] [CrossRef] [Green Version]
- Houghton, D.; Wilcox, M.D.; Brownlee, I.A.; Chater, P.I.; Seal, C.J.; Pearson, J.P. Acceptability of Alginate Enriched Bread and its Effect on Fat Digestion in Humans. Food Hydrocoll 2019, 93, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.L.; Udani, J.K. A Proprietary Alpha-Amylase Inhibitor from White Bean (Phaseolus vulgaris): A Review of Clinical Studies on Weight Loss and Glycemic Control. Nutr. J. 2011, 10, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obiro, W.C.; Zhang, T.; Jiang, B. The Nutraceutical Role of The Phaseolus Vulgaris α-amylase Inhibitor. Br. J. Nutr. 2008, 100, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onakpoya, I.; Aldaas, S.; Terry, R.; Ernst, E. The Efficacy of Phaseolus Vulgaris as a Weight-Loss Supplement: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. Br. J. Nutr 2011, 106, 196–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udani, J.; Tan, O.; Molina, J. Systematic Review and Meta-Analysis of a Proprietary Alpha-Amylase Inhibitor from White Bean (Phaseolus vulgaris L.) on Weight and Fat Loss in Humans. Foods 2018, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Qin, G.; Wang, F.; Liang, H.; Tang, S.; Shekh, K.; Wang, Y.; Li, B.; Dong, B.; Wen, P. Subchronic Study of a White Kidney Bean (Phaseolus vulgaris) Extract with α—Amylase Inhibitory Activity. Biomed Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Deglaire, A.; Moughan, P.J.; Bos, C.; Tome, D. Commercial Phaseolus Vulgaris Extract (Starch Stopper) Increases Ileal Endogenous Amino Acid and Crude Protein Losses in The Growing Rat. J. Agric. Food Chem. 2006, 54, 5197–5202. [Google Scholar] [CrossRef]
- Preuss, H.G.; Echard, B.; Bagchi, D.; Stohs, S. Inhibition by Natural Dietary Substances of Gastrointestinal Absorption of Starch and Sucrose in Rats 2. Subchronic Studies. Int. J. Med. Sci. 2007, 4, 209. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Zhu, Y.; Teng, C.; Yao, Y.; Ren, G.; Richel, A. Anti-Obesity Effects of α-amylase Inhibitor Enriched-Extract from White Common Beans (Phaseolus Vulgaris L.) Associated with The Modulation of Gut Microbiota Composition in High-Fat Diet-Induced Obese Rats. Food Funct. 2020, 11, 1624–1634. [Google Scholar] [CrossRef]
- Neil, E.S.; McGinley, J.N.; Fitzgerald, V.K.; Lauck, C.A.; Tabke, J.A.; Streeter-McDonald, M.R.; Yao, L.; Broeckling, C.D.; Weir, T.L.; Foster, M.T.; et al. White Kidney Bean (Phaseolus Vulgaris L.) Consumption Reduces Fat Accumulation in a Polygenic Mouse Model of Obesity. Nutrients 2019, 11, 2780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micheli, L.; Lucarini, E.; Trallori, E.; Avagliano, C.; de Caro, C.; Russo, R.; Calignano, A.; Ghelardini, C.; Pacini, A.; Mannelli, L.d.C. Phaseolus Vulgaris L. Extract: Alpha-amylase Inhibition Against Metabolic Syndrome in Mice. Nutrients 2019, 11, 1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tormo, M.A.; Gil-Exojo, I.; de Tejada, A.R.; Campillo, J.E. Hypoglycaemic and Anorexigenic Activities of an α-amylase Inhibitor from White Kidney Beans (Phaseolus Vulgaris) in Wistar Rats. Br. J. Nutr. 2004, 92, 785–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tormo, M.A.; Gil-Exojo, I.; De Tejada, A.R.; Campillo, J.E. White Bean Amylase Inhibitor Administered Orally Reduces Glycaemia in Type 2 Diabetic Rats. Br. J. Nutr. 2006, 96, 539–544. [Google Scholar] [PubMed]
- Song, H.; Han, W.; Yan, F.; Xu, D.; Chu, Q.; Zheng, X. Dietary Phaseolus Vulgaris Extract Alleviated Diet-Induced Obesity, Insulin Resistance and Hepatic Steatosis and Alters Gut Microbiota Composition in Mice. J. Funct. Foods 2016, 20, 236–244. [Google Scholar] [CrossRef]
- Carai, M.A.; Fantini, N.; Loi, B.; Colombo, G.; Gessa, G.L.; Riva, A.; Bombardelli, E.; Morazzoni, P. Multiple Cycles of Repeated Treatments with a Phaseolus Vulgaris Dry Extract Reduce Food Intake and Body Weight in Obese Rats. Br. J. Nutr 2011, 106, 762–768. [Google Scholar] [CrossRef] [Green Version]
- Lorrai, I.; Piga, V.; Carai, M.A.; Riva, A.; Morazzoni, P.; Gessa, G.L.; Colombo, G.; Maccioni, P. A Phaseolus Vulgaris Extract Reduces Cue-Induced Reinstatement of Chocolate Seeking in Rats. Front. Pharmacol. 2016, 7, 109. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.J.; De Oliveira, V.N.; Deconte, S.R.; Calábria, L.K.; Da Silva Moraes, A.; Espindola, F.S. Phaseolamin Treatment Prevents Oxidative Stress and Collagen Deposition in The Hearts of Streptozotocin-Induced Diabetic Rats. Diabetes Vasc. Dis. Res. 2014, 11, 110–117. [Google Scholar] [CrossRef]
- Wei, D.; Tang, K.; Wang, Q.; Estill, J.; Yao, L.; Wang, X.; Chen, Y.; Yang, K. The Use of GRADE Approach in Systematic Reviews of Animal Studies. J. Evid. Based. Med. 2016, 9, 98–104. [Google Scholar] [CrossRef]
- Preuss, H.G.; Echard, B.; Bagchi, D.; Stohs, S. Inhibition by Natural Dietary Substances of Gastrointestinal Absorption of Starch and Sucrose in Rats and Pigs: 1. Acute studies. Int. J. Med. Sci. 2007, 4, 196. [Google Scholar] [CrossRef] [Green Version]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia Muciniphila and Improved Metabolic Health During a Dietary Intervention in Obesity: Relationship with Gut Microbiome Richness and Ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial Ecology: Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative Stress and Inflammation Interactions in Human Obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef]
- Udani, J.; Singh, B.B. Blocking Carbohydrate Absorption and Weight Loss: A Clinical Trial Using a Proprietary Fractionated White Bean Extract. Altern. Ther. Health Med. 2007, 13, 32–39. [Google Scholar] [PubMed]
- Celleno, L.; Tolaini, M.V.; D’Amore, A.; Perricone, N.V.; Preuss, H.G. A Dietary Supplement Containing Standardized Phaseolus Vulgaris Extract Influences Body Composition of Overweight Men and Women. Int. J. Med. Sci. 2007, 4, 45. [Google Scholar] [CrossRef] [Green Version]
- Thom, E. A Randomized, Double-Blind, Placebo-Controlled Trial of a New Weight-Reducing Agent of Natural Origin. J. INT. MED. RES. 2000, 28, 229–233. [Google Scholar] [CrossRef] [Green Version]
- Rothacker, D. Reduction in Body Weight with a Starch Blocking Diet Aid: Starch Away Comparison with Placebo; Leiner Health Products: Carson, CA, USA, 2003. [Google Scholar]
- Grube, B.; Chong, W.F.; Chong, P.W.; Riede, L. Weight Reduction and Maintenance with IQP-PV-101: A 12-Week Randomized Controlled Study with a 24-Week Open Label Period. Obesity 2014, 22, 645–651. [Google Scholar] [CrossRef]
- Birketvedt, G.S.; Langbakk, B.; Florholmen, J. A Dietary Supplement with Bean Extract Decreases Body Weight, Body Fat, Waist Circumference and Blood Pressure in Overweight and Obese Subjects. Curr. Top. Nutraceutical. Res. 2005, 3, 137–142. [Google Scholar]
- Birketvedt, G.S.; Travis, A.; Langbakk, B.; Florholmen, J.R. Dietary Supplementation with Bean Extract Improves Lipid Profile in Overweight and Obese Subjects. Nutr. J. 2002, 18, 729–733. [Google Scholar] [CrossRef]
- Wu, X.; Xu, X.; Shen, J.; Perricone, N.V.; Preuss, H.G. Enhanced Weight Loss From a Dietary Supplement Containing Standardized Phaseolus Vulgaris Extract in Overweight Men and Women. Indian J. Appl. Res. 2010, 10, 73–79. [Google Scholar]
- Koike, T.; Koizumi, Y.; Tang, L.; Takahara, K.; Saitou, Y. The Anti-Obesity Effect and the Safety of Taking “Phaseolamin 1600 diet”. J. New Remedies Clin. 2005, 54, 1–16. [Google Scholar]
- Udani, J.; Hardy, M.; Madsen, D.C. Blocking Carbohydrate Absorption and Weight Loss: A Clinical Trial Using Phase 2 Brand Proprietary Fractionated White Bean Extract. Altern. Med. Rev. 2004, 9, 63–69. [Google Scholar] [PubMed]
- Wang, S.; Chen, L.; Yang, H.; Gu, J.; Wang, J.; Ren, F. Regular Intake of White Kidney Beans Extract (Phaseolus Vulgaris L.) Induces Weight Loss Compared to Placebo in Obese Human Subjects. Food Sci. Nutr. 2020, 8, 1315–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udani, J.K.; Singh, B.B.; Barrett, M.L.; Preuss, H.G. Lowering the Glycemic Index of White Bread Using a White Bean Extract. Nutr. J. 2009, 8, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinson, J.A.; Al Kharrat, H.; Shuta, D. Investigation of an Amylase Inhibitor on Human Glucose Absorption after Starch Consumption. Open Nutraceuticals J. 2009, 2, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and Diet-Responsive Groups of Bacteria Within The Human Colonic Microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [Green Version]
| Study | Animals | Duration | Preparation and Other Ingredients | Diet | Intervention Groups | Effects of WKBE |
|---|---|---|---|---|---|---|
| Song et al. [38] | 48 male C57BL/6J mice, | 98 days | Phase 2 (ZeLang, Nanjing, China) | High fat diet (HFD; 45% fat) and low fat diet (LFD; 10% fat) | WKBE: 50 mg/kg/d WKBE + HFD HFD: no WKBE Control: LFD | ↓ weight gain ↓ visceral fat ↓ food and calorie intake ↓ triacylglycerol ↓ total, HDL and LDL cholesterol ↓ serum adiponectin ↓ serum glucose ↓ serum insulin ↓ HOMA-IR index ↑ HOMA-IS index ↓ Firmicutes, ↑ Verrucomicrobia and Actenobacteria (phylum level) ↑ Bifidobacterium, Lactobacillus and Akkermansia (genus level) (p < 0.05 vs. HFD alone) |
| Qin et al. [29] | 80 healthy Sprague-Dawley rats | 90 days | Isolated in-house from common white kidney beans (Kunming, China) | Conventional diet | L: 1 g/kg.bw WKBE M: 2 g/kg.bw WKBE H: 4 g/kg.bw WKBE Control (no WKBE) | No effect on body weight No effect on food intake No effect on haematology markers, e.g., haemoglobin, white blood cell count, platelet count No effect on blood biochemistry, e.g., cholesterol, triglycerides, glucose (N.S. vs. control) |
| Neil et al. [34] | Experiment 1: 90 C57BL6/J mice | 84–238 days | Isolated in-house from cooked white kidney bean seed (Colorado State University, Fort Collins, CO, USA) | Not specified | WKBE: 40 g per 100 g feed Obese (HFD, no WKBE) Control (LFD, no WKBE) | ↓ weight gain ↓ subcutaneous and visceral lipid accumulation (p < 0.05 vs. HF) |
| Experiment 2: 16 male NCI C57BL6/NCr mice | 84 days | WKBE: 40 g WKBE per 100 g feed + HFD Control (HFD) | No effect on body weight or BMI No effect on feed efficiency ↓ subcutaneous, visceral, retroperitoneal, epidiymal fat mass ↑ caecal bacterial content ↑ A. muciniphila ↓ Firmicutes: Bacteroidetes No effects on total energy excreted in faeces (p < 0.05 vs. control) | |||
| Shi et al. [33] | 45 male Sprague-Dawley rats, high fat diet-induced obesity | 70 days | Isolated in-house from white common bean seeds (cultivar Longquanjiuli) (Pinzhen food Co, Haerbin, China) | HFD: 45% fat Basic diet (control) | L: 0.5% WKBE M: 1% WKBE H: 1.5% WKBE Obese (no WKBE) Control (no HFD or WKBE) | ↓ body weight at 6 (H dose) and 10 weeks (M and H dose) ↓ food intake ↓ food efficiency ratio ↓ HFD-induced intra-abdominal fat accumulation (M and H doses) ↓ HFD-induced increase in serum triglycerides (M and H doses) ↓ HFD-induced increase in LDL cholesterol ↑ total SCFAs ↑ acetic acid (H dose), propionic and isobutyric acid (M and H dose) Altered β-diversity of obese rats (H dose) ↓ Firmicutes ↑ Bacteroidetes and ↓ Firmicutes:Bacteroiedtes 30 different OTUs with H dose WKBE- including ↑ Bacteroides, Butyricicoccus, Blautia, Eubacterium and ↓ Lactobacillus and Ruminococcus (p < 0.05 vs. obese) |
| Preuss et al. [47] | 16 Sprague-Dawley rats | 63 days | Formula containing 19% dry bean extract (seed—P. Vulgaris L.), 31% hibiscus extract, 31% L-arabinose, 12% gymnema extract, 6% green tea extract leaf and 1% apple extract (AdvoCare International, Carrollton, TX, USA) | Regular rat chow, water ad libitum | WKBE: 2 g (1 g twice daily) of formula in 4 mL water (until wk.5)/sucrose solution (wk.5–9) Control: 4 mL water (until wk.5)/sucrose solution (wk.5–9) | No effect on body weight ↓ systolic BP No effect on food intake ↓ blood glucose ↓ circulating sodium and chloride ↑ circulating potassium and total protein No effect on haematology markers, e.g., haemoglobin, white blood cell count, platelet count (p < 0.05 vs. control) |
| Micheli et al. [35] | 36 male C57BL/6 mice, HFD-induced metabolic syndrome | 56 days | Extract containing α-Amylase inhibitor from common kidney bean (P. Vulgaris L.) (Beanblock®; Indena S.p.A., Milan, Italy) | HFD: 60% fat, 20% protein, 20% carbohydrate Standard diet (control): 18% fat, 24% protein, 58% carbohydrate | WKBE: 500 mg kg−1 Obese: (HFD, no WKBE) Control (normal diet) | ↓ weight gain No effect on food intake ↓ total and LDL cholesterol ↓ plasma glucose ↓ insulin tolerance ↓ plasma insulin ↓ triglycerides No effect on ghrelin ↓ oxidative stress markers protein carbonylation (in plasma but not heart tissue) ↑ cardiac antioxidant enzymes (catalase reductase and glutathione reductase, but not NADH dehydrogenase) (p < 0.05 WKBE vs. HF alone) |
| Tormo et al. [37] | Non-diabetic (ND) and type 2 diabetic (T2D) (neonatal diabetes models n0-STZ and n5-STZ) male Wistar rats. *n = not specified | 22 days | Isolated in-house from white beans (P. Vulgaris L.) (100 mg/kg body weight dissolved in 9 g NaCl/l) for 22 d to non-diabetic (ND) and type 2 diabetic (neonatal diabetes models n0-STZ and n5-STZ) male Wistar rats | Standard diet: 2.7% fat, 61.4% carbohydrate, 15.1% protein, 3.9% fibre | WKBE T2D: 100 mg/kg.bw/day dissolved in NaCl(9 g/L) T2D control: NaCl (9 g/L) daily. WKBE ND: 100 mg/kg.bw/day dissolved in NaCl(9 g/L) ND control: NaCl (9 g/L) | ↓ weight gain (WKBE ND vs. ND control) ↓ blood glucose (WKBE vs. T2D and ND controls) No effect on plasma insulin ↓ food intake (WKBE vs. T2D and ND controls) ↓ weight gain in WKBE ND vs. ND control) |
| Tormo et al. [36] | 12 adult male Wistar rats | Acute study Chronic study: 21 days (chronic) | Isolated in house from white kidney bean meal | Standard diet: 2.7% fat, 61.4% carbohydrate, 15.1% protein, 4% fibre | Acute oral administration: Starch load ± WKBE (50 mg/kg body weight) Chronic study: WKBE: 50 mg kg−1 per day Control: 9 g/L NaCl per day | Acute effects: ↓ blood glucose No effect on plasma insulin (P<0.05 vs. control; no WKBE) Chronic effects (post 21 days): ↓ weight gain ↓ blood glucose No effect on plasma insulin ↓ food intake (p < 0.05 vs. control) |
| Oliveira et al. [30] | 48 male Wistar rats, induced diabetes (streptozotocin) | 20 days | Phase 2 (Phaseolamin) (Nanjing Well Chemical Corp., Ltd., Nanjing, China) | Standard extruded chow and water ad libitum | D100: 100 mg/kg WKBE D500: 500 mg/kg WKBE D1500: 1500 mg/kg WKBE DACA: 25 mg/kg acarbose NTD (non-treated diabetic): no WKBE ND: non-diabetic, no WKBE control | ↓ body weight D100 and NTD vs. ND ↓ glycaemia (D100, D500 and D1500 vs. NTD) No effect on total cholesterol ↓ alkaline phosphatase (D1500 vs. NTD) ↓ serum urea (D500 and D1500 vs. NTD) ↑ total antioxidant status ↓ cardiac oxidative stress markers (superoxidase dismutase, catalase, malondialdehyde, D1500 vs. NTD) ↓ cardiac collagen deposition |
| Deglaire et al. [32] | 64 Sprague-Dawley male rats, protein-free diet (PFD) | 14 days | P. Vulgaris L. extract (powder removed from Starch Stopper capsules, Palmerston North, New Zealand) | Protein free diets (PFD): 0.36, 0.45 and 0.59 crude protein/100g diet | 0.4% WKBE (45 mg/day) + PFD 1.1% WKBE (120 mg/day) + PFD Control (PFD, no WKBE) | No effect on body weight No effect on food intake (vs. all groups) |
| Preuss et al. [31] | 96 Sprague-Dawley rats | Acute effects (one day) | Dry WKBE (AdvoCare International, Carrollton, TX, USA) | Regular rodent chow and water ad libitum | WKBE: 1 g WKBE (2 mL of water containing 0.5 g × 2, prior to and post CHO challenge) Control: no WKBE | ↓ blood glucose above baseline levels (p < 0.05 vs. control) |
| 2 Yorkshire pigs | Capsule containing 19% dry bean extract (seed—P. Vulgaris L.), 31% hibiscus extract, 12% gymnema extract, 6% green tea extract leaf and 1% apple extract (AdvoCare International, Carrollton, TX, USA) | Food and water ad libitum | Crossover design: WKBE: 4 capsules + 200 g sucrose and/or rice starch in water Control: 200 g sucrose and/or rice starch in water | ↓ blood glucose above baseline levels (p < 0.05 vs. control) | ||
| Carai et al. [39] | Experiment 1: 21 adult male Zucker fa/fa rats | 3 treatments, 5 days each, 20 day wash-out periods in-between | Isolated in-house from common white kidney bean | Standard rat chow: 60% carbohydrate, 4% fibre, 16% protein, 3% fat | Control: 0 mg/kg WKBE 50 mg/kg WKBE 500 mg/kg WKBE suspended in distilled water +0·5 % methylcellulose and administered orally (2 mL/kg infusion volume) | ↓ food intake ↓ body weight (p < 0.05 vs. control) |
| Experiment 2: 15 adult male Zucker fa/fa rats | Acute effects | Standard rat chow: 60% carbohydrate, 4% fibre, 16% protein, 3% fat | 0 mg/kg WKBE 50 mg/kg WKBE 500 mg/kg WKBE administered orally by 60 min before food presentation (2 mL/kg infusion volume) | ↓ glycemia (p < 0.05 vs. control) |
| Study | Design | Duration | Dose, Preparation and Other Ingredients | Participants | Effects of WKBE |
|---|---|---|---|---|---|
| Birketvedt et al. [53] and [54] | RCT | 90 days | Wellex capsules (LexMed ASA)- 900 mg WKBE per day | 62 overweight/obese (BMI >25 kg/m2) | ↓ body weight ↓ BMI ↓ body fat % ↓ WC ↓ systolic and diastolic BP ↓ total cholesterol No effect on HDL, LDL, triglycerides No effects on serum lipids or nutritional parameters (p < 0.05 vs. baseline) |
| Grube et al. [52] | RCT | 84 days | 3000 mg/day Phase 2 capsules | 117 overweight/obese (BMI 25–35 kg/m2) | ↓ body weight ↓ body fat mass ↓ WC (p < 0.001 vs. placebo) |
| Rothacker [51] | RCT | 84 days | 3000 mg/day Phase 2 capsules | 88 overweight/obese (BMI 24–32 kg/m2) | ↓ body weight (p < 0.05 vs. placebo) No effect on body fat No effect on lean body mass No effect on WC No effect on HC |
| Thom [50] | RCT | 84 days | 1200 mg/day Phase 2 capsules | 40 overweight/obese (BMI 28–39 kg/m2) | ↓ body weight ↓ BMI ↓ body fat % (p < 0.05 vs. baseline) No effect on WC No effect on HC No effect on BP |
| Wu et al. [55] | RCT | 60 days | 3000 mg/day Phase 2 capsules | 101 overweight/obese (BMI 25–40 kg/m2) | ↓ body weight ↓ waist circumference (p < 0.001 vs. placebo) No effect on HC No effect on blood biochemistry markers, e.g., cholesterol, triglycerides, blood glucose, creatinine, uric acid, apoliproteins |
| Udani et al. [57] | RCT | 56 days | 3000 mg/day Phase 2 capsules | 27 obese (BMI 30–43 kg/m2) | No effect on body weight No effect on body fat No effect on WC Trend for reduction in triglycerides (p = 0.07) No effect on blood biochemistry markers, e.g., HbA1C, total cholesterol No effect on appetite control, hunger or energy levels (vs. placebo) |
| Koike et al. [56] | Open | 56 days | 750 mg/day Phase 2 capsules | 10 (BMI 23–30 kg/m2, body fat >25% men and >30% women) | ↓ body weight ↓ BMI ↓ body fat % ↓ waist circumference ↓ hip circumference ↓ triglycerides ↓ HDL cholesterol ↓ systolic and diastolic BP (p < 0.01 vs. baseline) No effect on waist:hip ratio No effect on blood glucose No effect on total or LDL cholesterol |
| Wang et al. [58] | RCT | 35 days | 2400 mg/day WKBE capsules (Yunnan Tianbaohua Biological Resources Development) | 120 obese | ↓ body weight ↓ BMI ↓ body fat % ↓ fat mass ↓ overweight (%) ↓ subcutaneous fat thickness (triceps, subscapular, abdomen, suprailiac) ↓ waist circumference ↓ hip circumference (p < 0.01 vs. baseline) No effect on BP No effect on blood biochemistry markers, e.g., glucose, albumin, uric acid, creatinine No effects on haematological markers, e.g., haemoglobin, red blood cell count, white blood cell count |
| Celleno et al. [49] | RCT | 30 days | 445 mg/day Phase 2 capsules, with carbohydrate-rich diet | 60 overweight by 5–15 kg | ↓ body weight ↓ BMI ↓ body fat % ↓ adipose tissue thickness ↓ waist circumference ↓ hip circumference ↓ right thigh circumference (p < 0.001 vs baseline and vs placebo) |
| Udani and Singh [48] | RCT | 28 days | 2000 mg/day Phase 2 capsules (plus multi-component weight-loss program) | 25 healthy (BMI 23–31 kg/m2) | ↓ body weight ↓ waist circumference (p < 0.01 vs. baseline, but N.S. vs. placebo) No effect on fasted glucose No effect on triglycerides No effect on total cholesterol No effect on appetite control, hunger or energy levels |
| Udani et al. [59] | Open, 6-arm crossover | 1500, 2000 and 3000 mg WKBE in capsule and powder (incorporated into butter) form (consumed with white bread) | 13 healthy normoglycemic (BMI 18–25 kg/m2) | ↓ glycemic index of white bread | |
| Vinson et al. [60] | Double-blind, crossover | 1500 mg Phase 2 capsules (consumed with 4 large slices of white bread) | 11 healthy normoglycemic | ↓ peak postprandial blood glucose ↓ time of blood glucose normalisation (p < 0.05 vs. control) | |
| Double-blind, crossover | 750 mg Phase 2 capsules | 7 subjects | No effects on glucose absorption |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nolan, R.; Shannon, O.M.; Robinson, N.; Joel, A.; Houghton, D.; Malcomson, F.C. It’s No Has Bean: A Review of the Effects of White Kidney Bean Extract on Body Composition and Metabolic Health. Nutrients 2020, 12, 1398. https://doi.org/10.3390/nu12051398
Nolan R, Shannon OM, Robinson N, Joel A, Houghton D, Malcomson FC. It’s No Has Bean: A Review of the Effects of White Kidney Bean Extract on Body Composition and Metabolic Health. Nutrients. 2020; 12(5):1398. https://doi.org/10.3390/nu12051398
Chicago/Turabian StyleNolan, Ruth, Oliver M. Shannon, Natassia Robinson, Abraham Joel, David Houghton, and Fiona C. Malcomson. 2020. "It’s No Has Bean: A Review of the Effects of White Kidney Bean Extract on Body Composition and Metabolic Health" Nutrients 12, no. 5: 1398. https://doi.org/10.3390/nu12051398
APA StyleNolan, R., Shannon, O. M., Robinson, N., Joel, A., Houghton, D., & Malcomson, F. C. (2020). It’s No Has Bean: A Review of the Effects of White Kidney Bean Extract on Body Composition and Metabolic Health. Nutrients, 12(5), 1398. https://doi.org/10.3390/nu12051398