Glutamine for Amelioration of Radiation and Chemotherapy Associated Mucositis during Cancer Therapy
Abstract
:1. What Is Glutamine
2. Importance of Glutamine
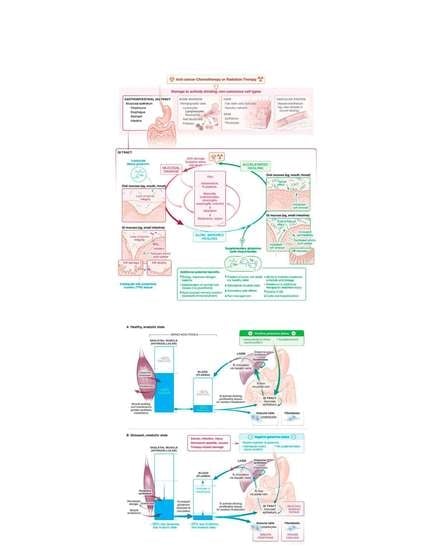
3. Oral Mucositis from Cancer Therapy: A Common Problem
4. Suggested Use of Glutamine in Mucositis Management from MASCC/ISOO 2019 Guidelines
5. Potential Mechanisms of Glutamine Activity in Mucositis Management
6. Bioavailability of Oral Glutamine Locally vs. Systemically in Mucositis Management
7. Is Glutamine Safe During Cancer Treatment
8. A Balanced Approach to Nutrition During Cancer Therapy
9. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Souba, W.W.; Smith, R.J.; Wilmore, D.W. Glutamine metabolism by the intestinal tract. J. Parenter. Enter. Nutr. 1985, 9, 608–617. [Google Scholar] [CrossRef]
- Souba, W.W.; Klimberg, V.S.; Plumley, D.A.; Salloum, R.M.; Flynn, T.C.; Bland, K.I.; Copeland, E.M., 3rd. The role of glutamine in maintaining a healthy gut and supporting the metabolic response to injury and infection. J. Surg. Res. 1990, 48, 383–391. [Google Scholar] [CrossRef]
- Klimberg, V.S.; McClellan, J.L.; Organ, C.H., Jr. Honorary Lectureship. Glutamine, cancer, and its therapy. Am. J. Surg. 1996, 172, 418–424. [Google Scholar] [CrossRef]
- Klimberg, V.S.; Souba, W.W.; Salloum, R.M.; Plumley, D.A.; Cohen, F.S.; Dolson, D.J.; Bland, K.I.; Copeland, E.M., 3rd. Glutamine-enriched diets support muscle glutamine metabolism without stimulating tumor growth. J. Surg. Res. 1990, 48, 319–323. [Google Scholar] [CrossRef]
- Smith, R.J.; Wilmore, D.W. Glutamine nutrition and requirements. J. Parenter. Enter. Nutr. 1990, 14, 94S–99S. [Google Scholar] [CrossRef] [PubMed]
- Wilmore, D.W.; Shabert, J.K. Role of glutamine in immunologic responses. Nutrition 1998, 14, 618–626. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Nyrop, K.A.; Williams, G.R.; Nishijima, T.F.; Benbow, J.M.; Muss, H.B. Skeletal Muscle Measures as Predictors of Toxicity, Hospitalization, and Survival in Patients with Metastatic Breast Cancer Receiving Taxane-Based Chemotherapy. Clin. Cancer Res. 2017, 23, 658–665. [Google Scholar] [CrossRef] [Green Version]
- Aleixo, G.F.P.; Williams, G.R.; Nyrop, K.A.; Muss, H.B.; Shachar, S.S. Muscle composition and outcomes in patients with breast cancer: Meta-analysis and systematic review. Breast Cancer Res. Treat. 2019, 177, 569–579. [Google Scholar] [CrossRef]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef]
- Fahr, M.J.; Kornbluth, J.; Blossom, S.; Schaeffer, R.; Klimberg, V.S.; Harry, M. Vars Research Award. Glutamine enhances immunoregulation of tumor growth. J. Parenter. Enter. Nutr. 1994, 18, 471–476. [Google Scholar] [CrossRef]
- Lacey, J.M.; Wilmore, D.W. Is glutamine a conditionally essential amino acid? Nutr. Rev. 1990, 48, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Stein, W.H.; Moore, S. The free amino acids of human blood plasma. J. Biol. Chem. 1954, 211, 15–26. [Google Scholar]
- Valencia, E.; Marin, A.; Hardy, G. Impact of oral L-glutamine on glutathione, glutamine, and glutamate blood levels in volunteers. Nutrition 2002, 18, 367–370. [Google Scholar] [CrossRef]
- Filho, J.C.; Bergström, J.; Stehle, P.; Fürst, P. Simultaneous measurements of free amino acid patterns of plasma, muscle and erythrocytes in healthy human subjects. Clin. Nutr. 1997, 16, 299–305. [Google Scholar] [CrossRef]
- Mcmenamy, R.H.; Lund, C.C.; Oncley, J.L. Unbound amino acid concentrations in human blood plasmas. J. Clin. Investig. 1957, 36, 1672–1679. [Google Scholar] [CrossRef]
- Al-Dasooqi, N.; Sonis, S.T.; Bowen, J.M.; Bateman, E.; Blijlevens, N.; Gibson, R.J.; Logan, R.M.; Nair, R.G.; Stringer, A.M.; Yazbeck, R. Emerging evidence on the pathobiology of mucositis. Support Care Cancer 2013, 21, 3233–3241. [Google Scholar] [CrossRef] [Green Version]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461. [Google Scholar] [CrossRef] [Green Version]
- Logan, R.M.; Al-Azri, A.R.; Bossi, P.; Stringer, A.M.; Joy, J.K.; Soga, Y.; Ranna, V.; Vaddi, A.; Raber-Durlacher, J.E.; Lalla, R.V. Systematic review of growth factors and cytokines for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2020, 28, 2485–2498. [Google Scholar] [CrossRef]
- Elad, S. The MASCC/ISOO mucositis guidelines 2019: The second set of articles and future directions. Support Care Cancer 2020, 28, 2445–2447. [Google Scholar] [CrossRef] [Green Version]
- Lalla, R.V.; Brennan, M.T.; Gordon, S.M.; Sonis, S.T.; Rosenthal, D.I.; Keefe, D.M. Oral Mucositis Due to High-Dose Chemotherapy and/or Head and Neck Radiation Therapy. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz011. [Google Scholar] [PubMed]
- Hong, B.Y.; Sobue, T.; Choquette, L.; Dupuy, A.K.; Thompson, A.; Burleson, J.A.; Salner, A.L.; Schauer, P.K.; Joshi, P.; Fox, E. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Keefe, D.M.; Schubert, M.M.; Elting, L.S.; Sonis, S.T.; Epstein, J.B.; Raber-Durlacher, J.E.; Migliorati, C.A.; McGuire, D.B.; Hutchins, R.D.; Peterson, D.E. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 2007, 109, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of oral mucositis in patients who have cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cinausero, M.; Aprile, G.; Ermacora, P.; Basile, D.; Vitale, M.G.; Fanotto, V.; Parisi, G.; Calvetti, L.; Sonis, S.T. New Frontiers in the Pathobiology and Treatment of Cancer Regimen-Related Mucosal Injury. Front. Pharmacol. 2017, 8, 354. [Google Scholar] [CrossRef] [Green Version]
- Skubitz, K.M.; Anderson, P.M. Oral glutamine to prevent chemotherapy induced stomatitis: A pilot study. J. Lab. Clin. Med. 1996, 127, 223–228. [Google Scholar] [CrossRef]
- Anderson, P.M.; Ramsay, N.K.; Shu, X.O.; Rydholm, N.; Rogosheshke, J.; Nicklow, R.; Weisdorf, D.J.; Skubitz, K.M. Effect of low-dose oral glutamine on painful stomatitis during bone marrow transplantation. Bone Marrow Transpl. 1998, 22, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Anderson, P.M.; Schroeder, G.; Skubitz, K.M. Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Cancer 1998, 83, 1433–1439. [Google Scholar] [CrossRef]
- Lalla, R.V.; Peterson, D.E. Oral mucositis. Dent. Clin. N. Am. 2005, 49, 167–184. [Google Scholar] [CrossRef]
- Aquino, V.M.; Harvey, A.R.; Garvin, J.H.; Godder, K.T.; Nieder, M.L.; Adams, R.H.; Jackson, G.B.; Sandler, E.S. A double-blind randomized placebo-controlled study of oral glutamine in the prevention of mucositis in children undergoing hematopoietic stem cell transplantation: A pediatric blood and marrow transplant consortium study. Bone Marrow Transpl. 2005, 36, 611–616. [Google Scholar] [CrossRef] [Green Version]
- Cockerham, M.B.; Weinberger, B.B.; Lerchie, S.B. Oral glutamine for the prevention of oral mucositis associated with high-dose paclitaxel and melphalan for autologous bone marrow transplantation. Ann. Pharmacother. 2000, 34, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Albertioni, F.; Rask, C.; Schroeder, H.; Peterson, C. Monitoring of methotrexate and 7-hydroxymethotrexate in saliva from children with acute lymphoblastic leukemia receiving high-dose consolidation treatment: Relation to oral mucositis. Anticancer Drugs 1997, 8, 119–124. [Google Scholar] [CrossRef] [PubMed]
- McGuire, D.B.; Altomonte, V.; Peterson, D.E.; Wingard, J.R.; Jones, R.J.; Grochow, L.B. Patterns of mucositis and pain in patients receiving preparative chemotherapy and bone marrow transplantation. Oncol. Nurs. Forum 1993, 20, 1493–1502. [Google Scholar] [PubMed]
- McGuire, D.B.; Peterson, D.B.; Muller, S.; Owen, D.C.; Slemmons, M.F.; Schubert, M.M. The 20 item oral mucositis index: Reliability and validity in bone marrow and stem cell transplant patients. Cancer Investig. 2002, 20, 893–903. [Google Scholar] [CrossRef]
- McGuire, D.B.; Yeager, K.A.; Dudley, W.N.; Peterson, D.E.; Owen, D.C.; Lin, L.S.; Wingard, J.R. Acute oral pain and mucositis in bone marrow transplant and leukemia patients: Data from a pilot study. Cancer Nurs. 1998, 21, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Schloerb, P.R.; Skikne, B.S. Oral and parenteral glutamine in bone marrow transplantation: A randomized, double-blind study. J. Parenter. Enter. Nutr. 1999, 23, 117–122. [Google Scholar] [CrossRef]
- Wingard, J.R.; Niehaus, C.S.; Peterson, D.E.; Jones, R.J.; Piantadosi, S.; Levin, L.S.; Saral, R.; Santos, G.W. Oral mucositis after bone marrow transplantation. A marker of treatment toxicity and predictor of hepatic veno-occlusive disease. Oral Surg. Oral Med. Oral Pathol. 1991, 72, 419–424. [Google Scholar] [CrossRef]
- Sonis, S.T.; Oster, G.; Fuchs, H.; Bellm, L.; Bradford, W.Z.; Edelsberg, J.; Hayden, V.; Eilers, J.; Epstein, J.B.; LeVeque, F.G. Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J. Clin. Oncol. 2001, 19, 2201–2205. [Google Scholar] [CrossRef]
- Rubenstein, E.B.; Peterson, D.E.; Schubert, M.; Keefe, D.; McGuire, D.; Epstein, J.; Elting, L.S.; Fox, P.C.; Cooksley, C.; Sonis, S.T.; et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 2004, 100 (Suppl. 9), 2026–2046. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Saunders, M.I.; Dische, S.; Bond, S.J. Radiotherapy-related early morbidity in head and neck cancer: Quantitative clinical radiobiology as deduced from the CHART trial. Radiother. Oncol. 2001, 60, 123–135. [Google Scholar] [CrossRef]
- Epstein, J.B.; Silverman, S.; Paggiarino, D.A., Jr.; Crockett, S.; Schubert, M.M.; Senzer, N.N.; Lockhart, P.B.; Gallagher, M.J.; Peterson, D.E.; Leveque, F.G. Benzydamine HCl for prophylaxis of radiation-induced oral mucositis: Results from a multicenter, randomized, double-blind, placebo-controlled clinical trial. Cancer 2001, 92, 875–885. [Google Scholar] [CrossRef]
- Huang, E.Y.; Leung, S.W.; Wang, C.J.; Chen, H.C.; Sun, L.M.; Fang, F.M.; Yeh, S.A.; Hsu, H.C.; Hsiung, C.Y. Oral glutamine to alleviate radiation-induced oral mucositis: A pilot randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 535–539. [Google Scholar] [CrossRef]
- Chen, S.C.; Lai, Y.H.; Huang, B.S.; Lin, C.Y.; Fan, K.H.; Chang, J.T. Changes and predictors of radiation-induced oral mucositis in patients with oral cavity cancer during active treatment. Eur. J. Oncol. Nurs. 2015, 19, 214–219. [Google Scholar] [CrossRef]
- Elting, L.S.; Keefe, D.M.; Sonis, S.T.; Garden, A.S.; Spijkervet, F.K.; Barasch, A.; Tishler, R.B.; Canty, T.P.; Kudrimoti, M.K.; Vera-Llonch, M. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: Demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer 2008, 113, 2704–2713. [Google Scholar] [CrossRef]
- Saunders, D.P.; Rouleau, T.; Cheng, K.; Yarom, N.; Kandwal, A.; Joy, J.; Bektas Kayhan, K.; van de Wetering, M.; Brito-Dellan, N.; Kataoka, T. Systematic review of antimicrobials, mucosal coating agents, anesthetics, and analgesics for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2020, 28, 2473–2484. [Google Scholar] [CrossRef]
- Cai, Q.; Huang, H.; Sun, X.; Xia, Z.; Li, Y.; Lin, X.; Guo, Y. Efficacy and safety of transdermal fentanyl for treatment of oral mucositis pain caused by chemotherapy. Expert. Opin. Pharmacother. 2008, 9, 3137–3144. [Google Scholar] [CrossRef]
- Huang, H.Q.; Cai, Q.Q.; Lin, X.B.; Wang, B.F.; Bu, Q.; Gao, Y.; Peng, Y.L. Transdermal fentanyl in treating severe painful mucositis caused by autologous hematopoietic stem cell transplantation. Ai Zheng 2007, 26, 390–393. [Google Scholar]
- Sobue, T.; Bertolini, M.; Thompson, A.; Peterson, D.E.; Diaz, P.I.; Dongari-Bagtzoglou, A. Chemotherapy-induced oral mucositis and associated infections in a novel organotypic model. Mol. Oral Microbiol. 2018, 33, 212–223. [Google Scholar] [CrossRef]
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.J.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017, 6, 2918–2931. [Google Scholar] [CrossRef]
- Villa, A.; Sonis, S.T. Mucositis: Pathobiology and management. Curr. Opin. Oncol. 2015, 27, 159–164. [Google Scholar] [CrossRef]
- Lalla, R.V.; Saunders, D.P.; Peterson, D.E. Chemotherapy or radiation-induced oral mucositis. Dent. Clin. N. Am. 2014, 58, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.E.P.; Cheng, K.K.F.; Chiang, K.; Kandwal, A.; Loprinzi, C.L.; Mori, T.; Potting, C.; Rouleau, T.; Toro, J.J.; Ranna, V.; et al. Systematic review of oral cryotherapy for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2019, 28, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Carrozzo, M.; Eriksen, J.G.; Bensadoun, R.J.; Boers-Doets, C.B.; Lalla, R.V.; Peterson, D.E. Oral Mucosal Injury Caused by Targeted Cancer Therapies. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz012. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.E.; Srivastava, R.; Lalla, R.V. Oral mucosal injury in oncology patients: Perspectives on maturation of a field. Oral Dis. 2015, 21, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonis, S.T. Pathobiology of mucositis. Semin. Oncol. Nurs. 2004, 20, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. Pathobiology of oral mucositis: Novel insights and opportunities. J. Support Oncol. 2007, 5 (Suppl. 4), 3–11. [Google Scholar]
- Li, H.L.; Lu, L.; Wang, X.S.; Qin, L.Y.; Wang, P.; Qiu, S.P.; Wu, H.; Huang, F.; Zhang, B.B.; Shi, H.L.; et al. Alteration of Gut Microbiota and Inflammatory Cytokine/Chemokine Profiles in 5-Fluorouracil Induced Intestinal Mucositis. Front. Cell Infect. Microbiol. 2017, 7, 455. [Google Scholar] [CrossRef]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M. Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered? Cancer Chemother. Pharmacol. 2009, 63, 239–251. [Google Scholar] [CrossRef]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Yeoh, A.S.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: Pathobiology, animal models and cytotoxic drugs. Cancer Treat. Rev. 2007, 33, 448–460. [Google Scholar] [CrossRef]
- Anderson, P.; Kaye, L. The therapeutic alliance: Adapting to the unthinkable with better information. Health Commun. 2009, 24, 775–778. [Google Scholar] [CrossRef]
- Vlassara, H.; Brownlee, M.; Manogue, K.R.; Dinarello, C.A.; Pasagian, A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: Role in normal tissue remodeling. Science 1988, 240, 1546–1548. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.; Mahoney, J.; Le Trang, N.; Pekala, P.; Cerami, A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J. Exp. Med. 1985, 161, 984–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, D.P.; Epstein, J.B.; Elad, S.; Allemano, J.; Bossi, P.; van de Wetering, M.D.; Rao, N.G.; Potting, C.; Cheng, K.K.; Freidank, A.; et al. Systematic review of antimicrobials, mucosal coating agents, anesthetics, and analgesics for the management of oral mucositis in cancer patients. Support Care Cancer 2013, 21, 3191–3207. [Google Scholar] [CrossRef] [Green Version]
- Peterson, D.E.; Ohrn, K.; Bowen, J.; Fliedner, M.; Lees, J.; Loprinzi, C.; Mori, T.; Osaguona, A.; Weikel, D.S.; Elad, S.; et al. Systematic review of oral cryotherapy for management of oral mucositis caused by cancer therapy. Support Care Cancer 2013, 21, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Yarom, N.; Hovan, A.; Bossi, P.; Ariyawardana, A.; Jensen, S.B.; Gobbo, M.; Saca-Hazboun, H.; Kandwal, A.; Majorana, A.; Ottaviani, G.; et al. Systematic review of natural and miscellaneous agents for the management of oral mucositis in cancer patients and clinical practice guidelines-part 1: Vitamins, minerals, and nutritional supplements. Support Care Cancer 2019, 27, 3997–4010. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, S.; Saha, A.; Azam, M.; Mukherjee, A.; Sur, P.K. Role of oral glutamine in alleviation and prevention of radiation-induced oral mucositis: A prospective randomized study. South Asian J. Cancer 2014, 3, 8–12. [Google Scholar]
- Tsujimoto, T.; Yamamoto, Y.; Wasa, M.; Takenaka, Y.; Nakahara, S.; Takagi, T.; Tsugane, M.; Hayashi, N.; Maeda, K.; Inohara, H.; et al. L-glutamine decreases the severity of mucositis induced by chemoradiotherapy in patients with locally advanced head and neck cancer: A double-blind, randomized, placebo-controlled trial. Oncol. Rep. 2015, 33, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Peterson, D.E.; Jones, J.B.; Petit, R.G., 2nd. Randomized, placebo-controlled trial of Saforis for prevention and treatment of oral mucositis in breast cancer patients receiving anthracycline-based chemotherapy. Cancer 2007, 109, 322–331. [Google Scholar] [CrossRef]
- Pytlik, R.; Benes, P.; Patorkova, M.; Chocenska, E.; Gregora, E.; Prochazka, B.; Kozak, T. Standardized parenteral alanyl-glutamine dipeptide supplementation is not beneficial in autologous transplant patients: A randomized, double-blind, placebo controlled study. Bone Marrow Transplant 2002, 30, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Klimberg, V.S.; Souba, W.W. The importance of intestinal glutamine metabolism in maintaining a healthy gastrointestinal tract and supporting the body’s response to injury and illness. Surg. Annu. 1990, 22, 61–76. [Google Scholar]
- Anderson, P. Predicting and facilitating survival of pediatric cancer patients: The ALC story. Pediatr. Blood Cancer 2010, 55, 1041–1042. [Google Scholar] [CrossRef]
- Anderson, P.M. Immune Therapy for Sarcomas. Adv. Exp. Med. Biol. 2017, 995, 127–140. [Google Scholar]
- De Angulo, G.; Yuen, C.; Palla, S.L.; Anderson, P.M.; Zweidler-McKay, P.A. Absolute lymphocyte count is a novel prognostic indicator in ALL and AML: Implications for risk stratification and future studies. Cancer 2008, 112, 407–415. [Google Scholar] [CrossRef]
- Moore, C.; Eslin, D.; Levy, A.; Roberson, J.; Giusti, V.; Sutphin, R. Prognostic significance of early lymphocyte recovery in pediatric osteosarcoma. Pediatr. Blood Cancer 2010, 55, 1096–1102. [Google Scholar] [CrossRef]
- Vasquez, L.; Leon, E.; Beltran, B.; Maza, I.; Oscanoa, M.; Geronimo, J. Pretreatment Neutrophil-to-Lymphocyte Ratio and Lymphocyte Recovery: Independent Prognostic Factors for Survival in Pediatric Sarcomas. J. Pediatr. Hematol. Oncol. 2017, 39, 538–546. [Google Scholar] [CrossRef]
- Rubnitz, J.E.; Campbell, P.; Zhou, Y.; Sandlund, J.T.; Jeha, S.; Ribeiro, R.C.; Inaba, H.; Bhojwani, D.; Relling, M.V.; Howard, S.C.; et al. Prognostic impact of absolute lymphocyte counts at the end of remission induction in childhood acute lymphoblastic leukemia. Cancer 2013, 119, 2061–2066. [Google Scholar] [CrossRef] [Green Version]
- DuBois, S.G.; Elterman, K.; Grier, H.E. Early lymphocyte recovery in Ewing sarcoma. J. Pediatr. Hematol. Oncol. 2007, 29, 351–352. [Google Scholar] [CrossRef]
- Souba, W.W.; Klimberg, V.S.; Copeland, E.M. Oral glutamine reduces bacterial translocation following abdominal radiation. J. Surg. Res. 1990, 48, 1–5. [Google Scholar] [CrossRef]
- Klimberg, S. Prevention of radiogenic side effects using glutamine-enriched elemental diets. Recent. Results Cancer Res. 1991, 121, 283–285. [Google Scholar]
- Klimberg, V.S.; Salloum, R.M.; Kasper, M.; Plumley, D.A.; Dolson, D.J.; Hautamaki, R.D.; Mendenhall, W.R.; Bova, F.C.; Bland, K.I.; Copeland, E.M.; et al. Oral glutamine accelerates healing of the small intestine and improves outcome after whole abdominal radiation. Arch. Surg. 1990, 125, 1040–1045. [Google Scholar] [CrossRef]
- Klimberg, V.S.; Souba, W.W.; Dolson, D.J.; Salloum, R.M.; Hautamaki, R.D.; Plumley, D.A.; Mendenhall, W.M.; Bova, F.J.; Khan, S.R.; Hackett, R.L.; et al. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer 1990, 66, 62–68. [Google Scholar] [CrossRef]
- Rubio, I.; Suva, L.J.; Todorova, V.; Bhattacharyya, S.; Kaufmann, Y.; Maners, A.; Smith, M.; Klimberg, V.S. Oral glutamine reduces radiation morbidity in breast conservation surgery. J. Parenter. Enter. Nutr. 2013, 37, 623–630. [Google Scholar] [CrossRef]
- Souba, W.W.; Klimberg, V.S.; Copeland, E.M., 3rd. Glutamine nutrition in the management of radiation enteritis. J. Parenter. Enter. Nutr. 1990, 14 (Suppl. 4), 106S–108S. [Google Scholar]
- Cao, Y.; Kennedy, R.; Klimberg, V.S. Glutamine protects against doxorubicin-induced cardiotoxicity. J. Surg. Res. 1999, 85, 178–182. [Google Scholar] [CrossRef]
- Kaufmann, Y.; Klimberg, V.S. Effect of glutamine on gut glutathione fractional release in the implanted tumor model. Nutr. Cancer 2007, 59, 199–206. [Google Scholar] [CrossRef]
- Lim, V.; Korourian, S.; Todorova, V.K.; Kaufmann, Y.; Klimberg, V.S. Glutamine prevents DMBA-induced squamous cell cancer. Oral Oncol. 2009, 45, 148–155. [Google Scholar] [CrossRef]
- Rouse, K.; Nwokedi, E.; Woodliff, J.E.; Epstein, J.; Klimberg, V.S. Glutamine enhances selectivity of chemotherapy through changes in glutathione metabolism. Ann. Surg. 1995, 221, 420–426. [Google Scholar] [CrossRef]
- Todorova, V.K.; Harms, S.A.; Kaufmann, Y.; Luo, S.; Luo, K.Q.; Babb, K.; Klimberg, V.S. Effect of dietary glutamine on tumor glutathione levels and apoptosis-related proteins in DMBA-induced breast cancer of rats. Breast Cancer Res. Treat. 2004, 88, 247–256. [Google Scholar] [CrossRef]
- Todorova, V.K.; Harms, S.A.; Luo, S.; Kaufmann, Y.; Babb, K.B.; Klimberg, V.S. Oral glutamine (AES-14) supplementation inhibits PI-3k/Akt signaling in experimental breast cancer. J. Parenter. Enter. Nutr. 2003, 27, 404–410. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Petit, R.G., 2nd; Shinal, E.; French, C. AES-14 facilitates rapid intracellular transport of high levels of L-glutamine in mucosal epithelial cells. Proc. Am. Soc. Clin. Oncol. 2000, 2002, 261b. [Google Scholar]
- Mayers, J.R.; Vander Heiden, M.G. Famine versus feast: Understanding the metabolism of tumors in vivo. Trends Biochem. Sci. 2015, 40, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.M. Supportive care after chemotherapy and radiation with a swish and swallow glutamine+ disaccharide nutritional supplement to reduce mucositis and improve enteral nutrition. In Proceedings of the 46th Congress of The International Society of Paediatric Oncology (SIOP), Toronto, ON, Canada, 22–25 October 2014. [Google Scholar]
- Todorova, V.; Vanderpool, D.; Blossom, S.; Nwokedi, E.; Hennings, L.; Mrak, R.; Klimberg, V.S. Oral glutamine protects against cyclophosphamide-induced cardiotoxicity in experimental rats through increase of cardiac glutathione. Nutrition 2009, 25, 812–817. [Google Scholar] [CrossRef]
- Klimberg, V.S.; Nwokedi, E.; Hutchins, L.F.; Pappas, A.A.; Lang, N.P.; Broadwater, J.R.; Read, R.C.; Westbrook, K.C. Glutamine facilitates chemotherapy while reducing toxicity. J. Parenter. Enter. Nutr. 1992, 16 (Suppl. 6), 83S–87S. [Google Scholar] [CrossRef]
- Klimberg, V.S.; Pappas, A.A.; Nwokedi, E.; Jensen, J.C.; Broadwater, J.R.; Lang, N.P.; Westbrook, K.C. Effect of supplemental dietary glutamine on methotrexate concentrations in tumors. Arch. Surg. 1992, 127, 1317–1320. [Google Scholar] [CrossRef]
- Rubio, I.T.; Cao, Y.; Hutchins, L.F.; Westbrook, K.C.; Klimberg, V.S. Effect of glutamine on methotrexate efficacy and toxicity. Ann. Surg. 1998, 227, 772–778. [Google Scholar] [CrossRef]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 749. [Google Scholar] [CrossRef]
- Souba, W.W.; Strebel, F.R.; Bull, J.M.; Copeland, E.M.; Teagtmeyer, H.; Cleary, K. Interorgan glutamine metabolism in the tumor-bearing rat. J. Surg. Res. 1988, 44, 720–726. [Google Scholar] [CrossRef]
- Labow, B.I.; Souba, W.W. Glutamine. World J. Surg. 2000, 24, 1503–1513. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef] [Green Version]
- Kanarek, N.; Petrova, B.; Sabatini, D.M. Dietary modifications for enhanced cancer therapy. Nature 2020, 579, 507–517. [Google Scholar] [CrossRef]
- Asselin, B.; Rizzari, C. Asparaginase pharmacokinetics and implications of therapeutic drug monitoring. Leuk. Lymphoma 2015, 56, 2273–2280. [Google Scholar] [CrossRef]
- Covini, D.; Tardito, S.; Bussolati, O.; Chiarelli, L.R.; Pasquetto, M.V.; Digilio, R.; Valentini, G.; Scotti, C. Expanding targets for a metabolic therapy of cancer: L-asparaginase. Recent Pat. Anticancer Drug Discov. 2012, 7, 4–13. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Su, Y.; Zhang, J.Y.; Antanasijevic, A.; Caffrey, M.; Schalk, A.M.; Liu, L.; Rondelli, D.; Oh, A.; Mahmud, D.L.; et al. A Novel l-Asparaginase with low l-Glutaminase Coactivity Is Highly Efficacious against Both T- and B-cell Acute Lymphoblastic Leukemias In Vivo. Cancer Res. 2018, 78, 1549–1560. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, Y.; Kornbluth, J.; Feng, Z.; Fahr, M.; Schaefer, R.F.; Klimberg, V.S. Effect of glutamine on the initiation and promotion phases of DMBA-induced mammary tumor development. J. Parenter. Enter. Nutr. 2003, 27, 411–418. [Google Scholar] [CrossRef]
- Kaufmann, Y.; Luo, S.; Johnson, A.; Babb, K.; Klimberg, V.S. Timing of oral glutamine on DMBA-induced tumorigenesis. J. Surg. Res. 2003, 111, 158–165. [Google Scholar] [CrossRef]
- Kaufmann, Y.; Spring, P.; Klimberg, V.S. Oral glutamine prevents DMBA-induced mammary carcinogenesis via upregulation of glutathione production. Nutrition 2008, 24, 462–469. [Google Scholar] [CrossRef]


| Chemotherapy Drug | Cytopenias * | Mucositis +/− GI ** |
|---|---|---|
| Busulfan (BMT) | NLRP | 2+ |
| Carboplatin | NLRP | 2+ |
| Cisplatin | NLR | 4+ |
| Cyclophosphamide iv | NLRP | 2+ |
| Cyclophosphamide (oral) | NL | 0 |
| Cytarabine | NLRP | 1+ |
| Cytarabine (high dose) | NLRP | 4+ |
| Daunomycin | NLRP | 3+ |
| Dexamethasone | L | 0 |
| Docetaxel | NLRP | 2+ |
| Doxorubicin | NLRP | 3+ |
| Doxorubicin liposomes | minimal | 2+ |
| Etoposide (BMT) | NLRP | 4+ |
| Etoposide (oral) | NLRP | 1+ |
| Etoposide iv | NLRP | 1+ |
| Everolimus | L | 2+ |
| Gemcitabine | NLRP | 1+ |
| Ifosfamide | NLRP | 0 |
| Imatinib | minimal | 0 |
| Irinotecan | NLRP | 4+ |
| Melphalan (BMT) | NLRP | 4+ |
| Methotrexate (oral) | L | 1+ |
| Methotrexate (high dose) | NLRP | 4+ |
| NAB-Paclitaxel | NLRP | 1+ |
| Pazopanib | minimal | 2+ |
| Prednisone | L | 0 |
| ^Radiation to GI tract | L | <where beam is> |
| (mucosal surfaces) | ||
| Regorafenib | minimal | 3+ |
| Sirolimus (rapamycin) | L | 1+ |
| Sorafenib | NLRP | 1+ |
| Temozolomide | NLRP | 0 |
| Temsirolimus | NLRP | 3+ |
| Topotecan | NLRP | 2+ |
| Thiotepa (BMT) | NLRP | 4+ |
| ^Total body Irradiation | NLRP | 4+ |
| Vincristine | L | 0 |
| Vinorelbine | NL | 0 |
| Information About Glutamine in Tumor Versus Treatment Effectiveness | Reference(s) |
|---|---|
| Major tumor fuel is glucose, but glutamine can also be used | [92,98,102] |
| Glutamine is a major fuel for lymphocytes and immune cells | [3,11] |
| Glutamine is needed by enterocytes to maintain intestinal health | [1] |
| Asparaginase which depletes glutamine in blood kills leukemia cells | [102,103,104] |
| Asparaginase w/o glutaminase activity is also highly effective | [105] |
| Glutamine effects glutathione levels (less in tumors, more in tissue) | [85,87,88,89] |
| Increased killing of tumors with glutamine supplementation | [85,95,106,107,108] |
| Glutamine is associated with less mucositis from chemotherapy | [26,27,28,65,68] |
| Glutamine reduces radiation side effects (helps normal tissues recover) | [42,66,80,81,82,93] |
| Glutamine’s decrease in squamous cell cancer incidence (cancer prevention) | [86] |
| Nutrients with High Glutamine | Supplement or Product (Source) |
|---|---|
| Dietary: foods high in glutamine | high protein foods (meats, fish, eggs, nuts, beans, milk) |
| High-protein milk + protein powder | FairLife milk + carnation breakfast (Nestle) |
| Water + protein powder | Beneprotein (Nestle), Whey powder, or plant protein powder |
| Protein Drinks | Boost, Ensure, Core Power, Pediasure, Peptamen |
| NG and G-tube formulas | Nutren (Nestle), Vital (Abbott), Jevity (Carewell) |
| Glutamine added to nutrient powder | Juven (Abbott) |
| Glutamine powder + maltodextrin | Glutasolve (Nestle) |
| Glutamine + sucrose+ trehalose | Healios (Enlivity) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, P.M.; Lalla, R.V. Glutamine for Amelioration of Radiation and Chemotherapy Associated Mucositis during Cancer Therapy. Nutrients 2020, 12, 1675. https://doi.org/10.3390/nu12061675
Anderson PM, Lalla RV. Glutamine for Amelioration of Radiation and Chemotherapy Associated Mucositis during Cancer Therapy. Nutrients. 2020; 12(6):1675. https://doi.org/10.3390/nu12061675
Chicago/Turabian StyleAnderson, Peter M., and Rajesh V. Lalla. 2020. "Glutamine for Amelioration of Radiation and Chemotherapy Associated Mucositis during Cancer Therapy" Nutrients 12, no. 6: 1675. https://doi.org/10.3390/nu12061675
APA StyleAnderson, P. M., & Lalla, R. V. (2020). Glutamine for Amelioration of Radiation and Chemotherapy Associated Mucositis during Cancer Therapy. Nutrients, 12(6), 1675. https://doi.org/10.3390/nu12061675