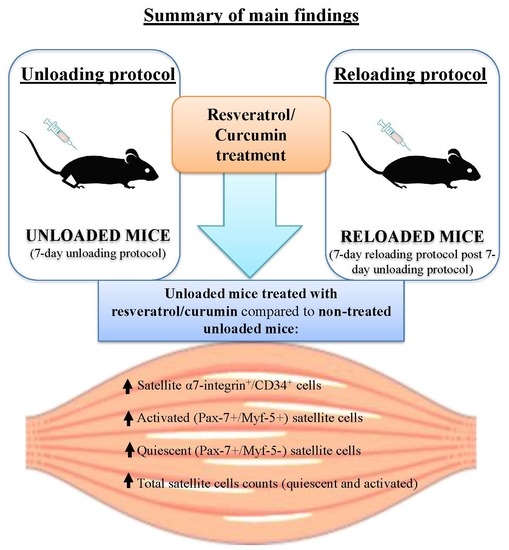
Satellite Cells and Markers of Muscle Regeneration during Unloading and Reloading: Effects of Treatment with Resveratrol and Curcumin
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Animal Experiments
2.2. Ethics
2.3. In-Vivo Measurements in the Mice
2.4. Sacrifice and Sample Collection
2.5. Tissue Embedding
2.6. Biological Analyses
2.7. Statistical Analysis
3. Results
3.1. Physiological Characteristics of the Study Animals
3.2. Structural Phenotypic Characteristics
3.3. Sirtuin-1 Content and Activity
3.4. Satellite Cell Counts
3.5. Myogenic Markers of Muscle Regeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Editorial Support
References
- Shrikrishna, D.; Patel, M.; Tanner, R.; Seymour, J.M.; Connolly, B.; Puthucheary, Z.; Walsh, S.L.; Bloch, S.; Sidhu, P.; Hart, N.; et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur. Respir. J. 2012, 40, 1115–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, S.F.; Rohm, M.; Herzig, S.; Diaz, M.B. Cancer Cachexia: More Than Skeletal Muscle Wasting. Trends Cancer 2018, 4, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E. Impact of Physical Activity and Exercise on Chronic Obstructive Pulmonary Disease Phenotypes: The Relevance of Muscle Adaptation. Arch. Bronconeumol. 2019, 55, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.; Pascual, S.; Castro-Acosta, A.; Hernández-Carcereny, C.; Castelo, R.; Márquez-Martín, E.; Montón, C.; Palou, A.; Faner, R.; Furlong, L.I.; et al. The Biomepoc Project: Personalized Biomarkers and Clinical Profiles in Chronic Obstructive Pulmonary Disease. Archivos de Bronconeumología (Engl. Ed.) 2019, 55, 93–99. [Google Scholar] [CrossRef]
- Gea, J.; Martínez-Llorens, J. Muscle Dysfunction in Chronic Obstructive Pulmonary Disease: Latest Developments. Archivos de Bronconeumología (Engl. Ed.) 2019, 55, 237–238. [Google Scholar] [CrossRef]
- Chacon-Cabrera, A.; Gea, J.; Barreiro, E. Short- and Long-Term Hindlimb Immobilization and Reloading: Profile of Epigenetic Events in Gastrocnemius. J. Cell. Physiol. 2016, 232, 1415–1427. [Google Scholar] [CrossRef] [Green Version]
- Chacon-Cabrera, A.; Lund-Palau, H.; Gea, J.; Barreiro, E. Time-Course of Muscle Mass Loss, Damage, and Proteolysis in Gastrocnemius following Unloading and Reloading: Implications in Chronic Diseases. PLoS ONE 2016, 11, e0164951. [Google Scholar] [CrossRef]
- Guitart, M.; Lloreta, J.; García, L.M.; Barreiro, E. Muscle regeneration potential and satellite cell activation profile during recovery following hindlimb immobilization in mice. J. Cell. Physiol. 2018, 233, 4360–4372. [Google Scholar] [CrossRef] [Green Version]
- Marquis, K.; Debigaré, R.; Lacasse, Y.; Leblanc, P.; Jobin, J.; Carrier, G.; Maltais, F. Midthigh Muscle Cross-Sectional Area Is a Better Predictor of Mortality than Body Mass Index in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2002, 166, 809–813. [Google Scholar] [CrossRef]
- Maltais, F.; Decramer, M.; Casaburi, R.; Barreiro, E.; Burelle, Y.; Debigare, R.; Dekhuijzen, P.N.R.; Franssen, F.; Gayan-Ramirez, G.; Gea, J.; et al. An official American Thoracic Society/European Respiratory Society statement: Update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014, 189, e15–e62. [Google Scholar] [CrossRef] [Green Version]
- Wall, B.T.; Dirks, M.L.; Snijders, T.; Senden, J.M.G.; Dolmans, J.; Van Loon, L. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol. 2013, 210, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.A.; Labugger, R.; Collier, C.; Brison, R.J.; Iscoe, S.; Van Eyk, J.E. Fast and Slow Skeletal Troponin I in Serum from Patients with Various Skeletal Muscle Disorders: A Pilot Study. Clin. Chem. 2005, 51, 966–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, S.; Kuroda, K.; Le Grand, F.; Rudnicki, M.A. Asymmetric Self-Renewal and Commitment of Satellite Stem Cells in Muscle. Cell 2007, 129, 999–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozdziak, P.; Pulvermacher, P.M.; Schultz, E. Muscle regeneration during hindlimb unloading results in a reduction in muscle size after reloading. J. Appl. Physiol. 2001, 91, 183–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueira, L.; Trisko, B.M.; Lima-Rosa, F.L.; Jackson, J.; Lund-Palau, H.; Yamaguchi, M.; Breen, E.C. Cigarette smoke directly impairs skeletal muscle function through capillary regression and altered myofibre calcium kinetics in mice fatigue resistance and myofibre calcium handling, and these changes ultimately affect contractile efficiency of locomotor muscles independent of a change in lung function. J. Physiol. 2018, 596, 14. [Google Scholar]
- Suetta, C.; Frandsen, U.; Mackey, A.L.; Jensen, L.; Hvid, L.G.; Bayer, M.L.; Petersson, S.J.; Schrøder, H.D.; Andersen, J.L.; Aagaard, P.; et al. Ageing is associated with diminished muscle re-growth and myogenic precursor cell expansion early after immobility-induced atrophy in human skeletal muscle. J. Physiol. 2013, 591, 3789–3804. [Google Scholar] [CrossRef]
- Arentson-Lantz, E.J.; English, K.L.; Paddon-Jones, D.; Fry, C.S. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J. Appl. Physiol. 2016, 120, 965–975. [Google Scholar] [CrossRef] [Green Version]
- Jackson, J.R.; Ryan, M.J.; Hao, Y.; E Alway, S. Mediation of endogenous antioxidant enzymes and apoptotic signaling by resveratrol following muscle disuse in the gastrocnemius muscles of young and old rats. Am. J. Physiol. Integr. Comp. Physiol. 2010, 299, 1572–1581. [Google Scholar] [CrossRef]
- Donnelly, L.; Newton, R.; Kennedy, G.E.; Fenwick, P.S.; Leung, R.H.F.; Ito, K.; Russell, R.E.; Barnes, P.J. Anti-inflammatory effects of resveratrol in lung epithelial cells: Molecular mechanisms. Am. J. Physiol. Cell. Mol. Physiol. 2004, 287, L774–L783. [Google Scholar] [CrossRef] [Green Version]
- Jarolim, S.; Millen, J.; Heeren, G.; Laun, P.; Goldfarb, D.S.; Breitenbach, M. A novel assay for replicative lifespan in Saccharomyces cerevisiae. FEMS Yeast Res. 2004, 5, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Cheng, X.; Cui, Y.; Xia, Q.; Yan, X.; Zhang, M.; Lan, G.; Liu, J.; Shan, T.; Huang, Y. Resveratrol regulates skeletal muscle fibers switching through the AdipoR1-AMPK-PGC-1α pathway. Food Funct. 2019, 10, 3334–3343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Chen, S.; Li, Z.; Zhao, X.; Li, W.; Sun, Y.; Zhang, Z.; Ling, W.; Feng, X. Effects and mechanisms of resveratrol on the amelioration of oxidative stress and hepatic steatosis in KKAy mice. Nutr. Metab. 2014, 11, 35. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; He, Z.; Mao, C.; Shui, X.; Cai, L. Therapeutic Effects of Resveratrol Liposome on Muscle Injury in Rats. Med. Sci. Monit. 2019, 25, 2377–2385. [Google Scholar] [CrossRef]
- Hsu, Y.-J.; Ho, C.-S.; Lee, M.-C.; Ho, C.-S.; Huang, C.-C.; Kan, N.-W. Protective Effects of Resveratrol Supplementation on Contusion Induced Muscle Injury. Int. J. Med. Sci. 2020, 17, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Bennett, B.T.; Mohamed, J.S.; E Alway, S. Effects of Resveratrol on the Recovery of Muscle Mass Following Disuse in the Plantaris Muscle of Aged Rats. PLoS ONE 2013, 8, e83518. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Xiao, J.; Sheng, X.; Zhang, X.; Guo, M. Curcumin protects against myocardial infarction-induced cardiac fibrosis via SIRT1 activation in vivo and in vitro. Drug Des. Dev. Ther. 2016, 10, 1267–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabowska, W.; Suszek, M.; Wnuk, M.; Lewinska, A.; Wasiak, E.; Sikora, E.; Bielak-Zmijewska, A. Curcumin elevates sirtuin level but does not postpone in vitro senescence of human cells building the vasculature. Oncotarget 2016, 7, 19201–19213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poylin, V.; Fareed, M.U.; O’Neal, P.; Alamdari, N.; Reilly, N.; Menconi, M.; Hasselgren, P.-O. The NF-kappaB inhibitor curcumin blocks sepsis-induced muscle proteolysis. Mediat. Inflamm. 2008, 2008, 317851. [Google Scholar] [CrossRef] [Green Version]
- Jin, B.; Li, Y.-P. Curcumin prevents lipopolysaccharide-induced atrogin-1/MAFbx upregulation and muscle mass loss. J. Cell. Biochem. 2007, 100, 960–969. [Google Scholar] [CrossRef] [Green Version]
- Mañas-García, L.; Bargalló, N.; Gea, J.; Barreiro, E. Muscle Phenotype, Proteolysis, and Atrophy Signaling During Reloading in Mice: Effects of Curcumin on the Gastrocnemius. Nutrients 2020, 12, 388. [Google Scholar] [CrossRef] [Green Version]
- Thaloor, D.; Miller, K.J.; Gephart, J.; Mitchell, P.O.; Pavlath, G.K. Systemic administration of the NF-κB inhibitor curcumin stimulates muscle regeneration after traumatic injury. Am. J. Physiol. Cell Physiol. 1999, 277, C320–C329. [Google Scholar] [CrossRef]
- Lang, S.M.; Kazi, A.A.; Hong-Brown, L.; Lang, C.H. Delayed Recovery of Skeletal Muscle Mass following Hindlimb Immobilization in mTOR Heterozygous Mice. PLoS ONE 2012, 7, e38910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol Ameliorates Aging-Related Metabolic Phenotypes by Inhibiting cAMP Phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-C.; Yang, M.-H.; Tung, H.-C.; Chang, C.-Y.; Tsai, Y.-L.; Huang, J.-P.; Yen, T.-H.; Hung, L.-M. Resveratrol exhibits differential protective effects on fast- and slow-twitch muscles in streptozotocin-induced diabetic rats J. Diabetes 2014, 6, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Vazeille, E.; Slimani, L.; Claustre, A.; Magne, H.; Labas, R.; Bechet, D.; Taillandier, D.; Dardevet, M.; Astruc, T.; Attaix, D.; et al. Curcumin treatment prevents increased proteasome and apoptosome activities in rat skeletal muscle during reloading and improves subsequent recovery. J. Nutr. Biochem. 2012, 23, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Gutierres, V.O.; Campos, M.L.; Arcaro, C.A.; Assis, R.P.; Baldan-Cimatti, H.M.; Peccinini, R.G.; Paula-Gomes, S.; Kettelhut, I.C.; Baviera, A.M.; Brunetti, I.L. Curcumin Pharmacokinetic and Pharmacodynamic Evidences in Streptozotocin-Diabetic Rats Support the Antidiabetic Activity to Be via Metabolite(s). Evid. Based Complement. Altern. Med. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baltaci, S.B.; Mogulkoc, R.; Baltaci, A.K. Resveratrol and exercise (review). Biomed. Rep. 2016, 5, 525–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.-W.; Su, Y.-K.; Bamodu, O.A.; Hueng, D.-Y.; Lee, W.-H.; Huang, C.-C.; Deng, L.; Hsiao, M.; Chien, M.; Yeh, C.-T.; et al. The Disruption of the β-Catenin/TCF-1/STAT3 Signaling Axis by 4-Acetylantroquinonol B Inhibits the Tumorigenesis and Cancer Stem-Cell-Like Properties of Glioblastoma Cells, In Vitro and In Vivo. Cancers 2018, 10, 491. [Google Scholar] [CrossRef] [Green Version]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of Substances to Laboratory Animals: Routes of Administration and Factors to Consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Barreiro, E.; Puig-Vilanova, E.; Marin-Corral, J.; Chacon-Cabrera, A.; Salazar-Degracia, A.; Mateu, X.; Maestu, L.P.; Garcia-Arumi, E.; Andreu, A.L.; Molina, L. Therapeutic Approaches in Mitochondrial Dysfunction, Proteolysis, and Structural Alterations of Diaphragm and Gastrocnemius in Rats with Chronic Heart Failure. J. Cell. Physiol. 2015, 231, 1495–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthel, L.K.; Raymond, P.A. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J. Histochem. Cytochem. 1990, 38, 1383–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkie, G.S.; Schirmer, E.C. Purification of Nuclei and Preparation of Nuclear Envelopes from Skeletal Muscle. Adv. Struct. Saf. Stud. 2008, 463, 23–41. [Google Scholar] [CrossRef] [Green Version]
- Vilà, L.; Elias, I.; Roca, C.; Ribera, A.; Ferre, T.; Casellas, A.; Lage, R.; Franckhauser, S.; Bosch, F. AAV8-mediated Sirt1 gene transfer to the liver prevents high carbohydrate diet-induced nonalcoholic fatty liver disease. Mol. Ther. Methods Clin. Dev. 2014, 1, 14039. [Google Scholar] [CrossRef] [PubMed]
- Pasut, A.; Oleynik, P.; Rudnicki, M.A. Isolation of Muscle Stem Cells by Fluorescence Activated Cell Sorting Cytometry. Adv. Struct. Saf. Stud. 2011, 798, 53–64. [Google Scholar] [CrossRef]
- Kuang, J.; Yan, X.; Genders, A.; Granata, C.; Bishop, D.J. An overview of technical considerations when using quantitative real-time PCR analysis of gene expression in human exercise research. PLoS ONE 2018, 13, e0196438. [Google Scholar] [CrossRef] [PubMed]
- Touchberry, C.D.; Wacker, M.J.; Richmond, S.R.; Whitman, S.A.; Godard, M.P. Age-Related Changes in Relative Expression of Real-Time PCR Housekeeping Genes in Human Skeletal Muscle. J. Biomol. Tech. 2006, 17, 157–162. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Tanno, M.; Horio, Y. The effects of resveratrol and SIRT1 activation on dystrophic cardiomyopathy. Ann. N. Y. Acad. Sci. 2015, 1348, 46–54. [Google Scholar] [CrossRef]
- He, J.; Xie, H.; Wu, S. Dietary supplementation of curcumin alleviates NF-κB-dependent skeletal muscle wasting in rat. Endocr. Metab. Immune Disord. Drug Targets 2016, 16, 1. [Google Scholar] [CrossRef]
- Ryall, J.; Dell’Orso, S.; Derfoul, A.; Juan, A.; Zare, H.; Feng, X.; Clermont, D.; Koulnis, M.; Gutierrez-Cruz, G.; Fulco, M.; et al. The NAD (+)-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell 2015, 16, 171–183. [Google Scholar] [CrossRef] [Green Version]
- Alway, S.E.; McCrory, J.L.; Kearcher, K.; Vickers, A.; Frear, B.; Gilleland, D.L.; Bonner, D.E.; Thomas, J.M.; Donley, D.A.; Lively, M.W.; et al. Resveratrol Enhances Exercise-Induced Cellular and Functional Adaptations of Skeletal Muscle in Older Men and Women. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2017, 72, 1595–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackey, A.L.; Kjaer, M.; Charifi, N.; Henriksson, J.; Bojsen-Møller, J.; Holm, L.; Kadi, F. Assessment of satellite cell number and activity status in human skeletal muscle biopsies. Muscle Nerve 2009, 40, 455–465. [Google Scholar] [CrossRef]
- Pardo, P.S.; Boriek, A.M. The physiological roles of Sirt1 in skeletal muscle. Aging 2011, 3, 430–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.; Conboy, M.J.; Conboy, I.M. Sirt1-Independent Rescue of Muscle Regeneration by Resveratrol in Type I Diabetes. J. Diabetes Metab. 2013, 4. [Google Scholar] [CrossRef] [Green Version]








| Non-Immobilized (N = 10) | 7dI (N = 10) | 7dI + Res (N = 10) | 7dI + Cur (N = 10) | 7dR (N = 10) | 7dR + Res (N = 10) | 7dR + Cur (N = 10) | |||
| Food intake (g/24 h) | 3.15 (0.10) | 3.10 (0.03) | 3.17 (0.13) | 3.02 (0.56) | 3.08 (0.47) | 3.11 (0.17) | 3.48 (0.65) | ||
| Total body weight gain (%) | +7.28 (2.02) | −1.60 (1.82), *** | +0.36 (1.49) | −0.73 (1.20) | +3.23 (1.45), §§ | +6.40 (1.92) | +5.94 (1.30) | ||
| Gastrocnemius weight (g) | 0.115 (0.003) | 0.085 (0.05), * | 0.091 (0.007) | 0.089 (0.003) | 0.097 (0.004), § | 0.099 (0.004) | 0.097 (0.006) | ||
| Limb strength gain (%) | +11.44 (5.10) | −5.45 (1.12), * | −0.61 (1.45) | −2.88 (1.81) | +7.00 (2.39), §§ | +7.71 (2.08) | +7.60 (1.99) | ||
| Resveratrol | Curcumin | ||||||||
| Immobilization effect p-value | Treatment effect p-value | Interaction effect p-value | Immobilization effect p-value | Treatment effect p-value | Interaction effect p-value | ||||
| Food intake (g/24 h) | 0.919 | 0.454 | 0.750 | 0.640 | 0.426 | 0.308 | |||
| Total body weight gain (%) | 0.0001 | 0.052 | 0.621 | 0.0001 | 0.126 | 0.423 | |||
| Gastrocnemius weight (g) | 0.0001 | 0.078 | 0.274 | 0.0001 | 0.270 | 0.270 | |||
| Limb strength gain (%) | 0.048 | 0.547 | 0.637 | 0.0171 | 0.763 | 0.890 | |||
| Non-Immobilized (N = 10) | 7dI (N = 10) | 7dI + Res (N = 10) | 7dI + Cur (N = 10) | 7dR (N = 10) | 7dR + Res (N = 10) | 7dR + Cur (N = 10) | |||
| Muscle fiber type, % | |||||||||
| Type I fibers | 15.37 (2.99) | 16.93 (2.88) | 14.68 (2.56) | 18.27 (6.00) | 16.25 (7.23) | 15.21 (8.28) | 14.95 (4.84) | ||
| Type II fibers | 81.96 (5.74) | 82.94 (3.09) | 85.31 (2.56) | 81.72 (6.00) | 83.74 (7.23) | 84.78 (8.28) | 85.04 (4.84) | ||
| Muscle fiber size (CSA) | |||||||||
| Cross-sectional area, type I fibers | 1246.64 (129.93) | 886.56 (115.34), ** | 982.45 (114.50) | 843.59 (133.10) | 923.18 (108.64) | 1010.70 (111.03) | 1035.55 (117.76) | ||
| Cross-sectional area, type II fibers | 1256.52 (105.47) | 914.31 (134.64), * | 1002.68 (114.12) | 948.48 (115.00) | 1079.80 (119.22), § | 1256.11 (127.88) (p = 0.075) | 1117.72 (120.27) | ||
| Muscle hybrid fiber, % | 0.66 (0.10) | 3.55 (1.80), ** | 3.14 (1.05) | 2.60 (0.67) | 3.34 (1.72) | 2.11 (1.10) | 2.04 (0.99) | ||
| Cross-sectional area, hybrid fibers | 1014.60 (86.57) | 908.03 (150.27) | 916.61 (187.89) | 787.31 (88.64) | 751.68 (167.49) | 857.90 (128.07) | 971.03 (167.32), # | ||
| Resveratrol | Curcumin | ||||||||
| Immobilization effect p-value | Treatment effect p-value | Interaction effect p-value | Immobilization effect p-value | Treatment effect p-value | Interaction effect p-value | ||||
| Type I fibers, % | 0.890 | 0.275 | 0.850 | 0.195 | 0.726 | 0.908 | |||
| Type II fibers, % | 0.890 | 0.275 | 0.850 | 0.325 | 0.926 | 0.423 | |||
| Cross-sectional area, type I fibers | 0.750 | 0.156 | 0.832 | 0.210 | 0.770 | 0.470 | |||
| Cross-sectional area, type II fibers | 0.010 | 0.147 | 0.713 | 0.0140 | 0.563 | 0.911 | |||
| Muscle hybrid fiber, % | 0.810 | 0.352 | 0.990 | 0.455 | 0.587 | 0.674 | |||
| Cross-sectional area, hybrid fibers | 0.515 | 0.694 | 0.576 | 0.283 | 0.621 | 0.0187 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mañas-García, L.; Guitart, M.; Duran, X.; Barreiro, E. Satellite Cells and Markers of Muscle Regeneration during Unloading and Reloading: Effects of Treatment with Resveratrol and Curcumin. Nutrients 2020, 12, 1870. https://doi.org/10.3390/nu12061870
Mañas-García L, Guitart M, Duran X, Barreiro E. Satellite Cells and Markers of Muscle Regeneration during Unloading and Reloading: Effects of Treatment with Resveratrol and Curcumin. Nutrients. 2020; 12(6):1870. https://doi.org/10.3390/nu12061870
Chicago/Turabian StyleMañas-García, Laura, Maria Guitart, Xavier Duran, and Esther Barreiro. 2020. "Satellite Cells and Markers of Muscle Regeneration during Unloading and Reloading: Effects of Treatment with Resveratrol and Curcumin" Nutrients 12, no. 6: 1870. https://doi.org/10.3390/nu12061870
APA StyleMañas-García, L., Guitart, M., Duran, X., & Barreiro, E. (2020). Satellite Cells and Markers of Muscle Regeneration during Unloading and Reloading: Effects of Treatment with Resveratrol and Curcumin. Nutrients, 12(6), 1870. https://doi.org/10.3390/nu12061870