Protective Effects of Medicinal Plant Decoctions on Macrophages in the Context of Atherosclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Preparation of the Decoctions
2.3. LDL Isolation
2.4. LDL Oxidation
2.5. Dil-ox-LDL Production
2.6. Cell Culture
2.7. Neutral Red Assay
2.8. Fluorescence and Confocal Microscopy
2.9. Flow Cytometry
2.10. Evaluation of NF-κB Activation
2.11. Statistics
3. Results and Discussion
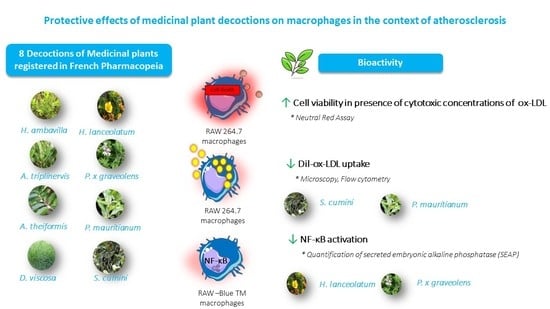
3.1. Medicinal Plant Decoctions Reduce ox-LDL Cytotoxic Conditions
3.2. Medicinal Plant Decoctions Reduce the Dil-ox-LDL Uptake by RAW 264.7 Macrophages
3.3. Medicinal Plant Decoctions Reduce Lipopolysaccharide-Induced NFkB Activation in RAW-Blue™ Macrophages
4. Conclusions
- -
- Murine macrophages were used and stimulated by human LDLs. This experimental design is commonly used in the literature. In addition, this cellular model was chosen as we plan to carry out in vivo studies to assess the bioactivity of decoctions in a mouse model of atherosclerosis. Furthermore, isolation of LDLs in mice is difficult to achieve due to limited blood volume [55].
- -
- The precise compounds and mechanisms responsible for the inhibition of the uptake of ox-LDL by P mauritianum and S cumini decoctions would deserve further investigation but cannot be explained by the presence of a single compound. Each decoction constitutes a complex set of molecules that can exert their effects synergistically. In addition, mechanistic aspects could to be investigated (for example, the study of the impact of decoctions on the expression of scavengers’ receptors).
- -
- In a physiological context, polyphenols can be transformed into secondary metabolites by intestinal of hepatic enzymes (including both host and microbiota enzymes).
- -
- In order to be able to evaluate the curative or preventive potential of the different decoctions tested, our in vitro study would benefit from being extended by studies on an in vivo model.
- -
- In summary, we demonstrated the effects of eight medicinal plant decoctions on ox-LDL cytotoxicity; we then showed that certain medicinal plant decoctions were able to significantly limit ox-LDL uptake and subsequent foam cell formation. Finally, we report the potential of decoctions to inhibit NF-κB activation, suggesting that herbal teas may prevent the activation of inflammatory signaling pathways.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of Potentially Modifiable Risk Factors Associated with Myocardial Infarction in 52 Countries (the INTERHEART Study): Case-Control Study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Stary, H.C.; Chandler, A.B.; Glagov, S.; Guyton, J.R.; Insull, W.; Rosenfeld, M.E.; Schaffer, S.A.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A Definition of Initial, Fatty Streak, and Intermediate Lesions of Atherosclerosis. A Report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler. Thromb. J. Vasc. Biol. 1994, 14, 840–856. [Google Scholar] [CrossRef] [Green Version]
- Chistiakov, D.A.; Melnichenko, A.A.; Orekhov, A.N.; Bobryshev, Y.V. How Do Macrophages Sense Modified Low-Density Lipoproteins? Int. J. Cardiol. 2017, 230, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.T.; Parthasarathy, S.; Fong, L.G.; Steinberg, D. Oxidatively Modified Low Density Lipoproteins: A Potential Role in Recruitment and Retention of Monocyte/Macrophages during Atherogenesis. Proc. Natl. Acad. Sci. USA 1987, 84, 2995–2998. [Google Scholar] [CrossRef] [Green Version]
- Gerrity, R.G. The Role of the Monocyte in Atherogenesis: II. Migration of Foam Cells from Atherosclerotic Lesions. Am. J. Pathol. 1981, 103, 191–200. [Google Scholar] [PubMed]
- Maguire, E.M.; Pearce, S.W.A.; Xiao, Q. Foam Cell Formation: A New Target for Fighting Atherosclerosis and Cardiovascular Disease. Vascul. Pharmacol. 2019, 112, 54–71. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.; Trigatti, B.L. Macrophage Apoptosis and Necrotic Core Development in Atherosclerosis: A Rapidly Advancing Field with Clinical Relevance to Imaging and Therapy. Can. J. Cardiol. 2017, 33, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Mechanisms of Foam Cell Formation in Atherosclerosis. J. Mol. Med. 2017, 95, 1153–1165. [Google Scholar] [CrossRef]
- Tabas, I.; Bornfeldt, K.E. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ. Res. 2016, 118, 653–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicero, A.F.G.; Kuwabara, M.; Johnson, R.; Bove, M.; Fogacci, F.; Rosticci, M.; Giovannini, M.; D’Addato, S.; Borghi, C. Brisighella Heart Study group LDL-Oxidation, Serum Uric Acid, Kidney Function and Pulse-Wave Velocity: Data from the Brisighella Heart Study Cohort. Int. J. Cardiol. 2018, 261, 204–208. [Google Scholar] [CrossRef]
- Ricci, C.; Ruscica, M.; Camera, M.; Rossetti, L.; Macchi, C.; Colciago, A.; Zanotti, I.; Lupo, M.G.; Adorni, M.P.; Cicero, A.F.G.; et al. PCSK9 Induces a Pro-Inflammatory Response in Macrophages. Sci. Rep. 2018, 8, 2267. [Google Scholar] [CrossRef] [Green Version]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef]
- Bobryshev, Y.V.; Ivanova, E.A.; Chistiakov, D.A.; Nikiforov, N.G.; Orekhov, A.N. Macrophages and Their Role in Atherosclerosis: Pathophysiology and Transcriptome Analysis. BioMed Res. Int. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Kapate, N.; Shields, C.W.; Mitragotri, S. Drug Delivery to Macrophages: A Review of Targeting Drugs and Drug Carriers to Macrophages for Inflammatory Diseases. Adv. Drug Deliv. Rev. 2019, 165–166. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.L.; Singh, V.; Barthwal, M.K. Macrophages: An Elusive yet Emerging Therapeutic Target of Atherosclerosis. Med. Res. Rev. 2008, 28, 483–544. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 Richest Dietary Sources of Polyphenols: An Application of the Phenol-Explorer Database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.-K.; Lee, H.-J.; Shih, Y.-W.; Chyau, C.-C.; Wang, C.-J. Mulberry Anthocyanin Extracts Inhibit LDL Oxidation and Macrophage-Derived Foam Cell Formation Induced by Oxidative LDL. J. Food Sci. 2008, 73, H113–H121. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rodríguez, M.L.; García-Chávez, E.; Berhow, M.; de Mejia, E.G. Anti-Inflammatory and Anti-Oxidant Effect of Calea Urticifolia Lyophilized Aqueous Extract on Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. J. Ethnopharmacol. 2016, 188, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Lee, T.-S.; Chiang, A.-N. Quercetin Enhances ABCA1 Expression and Cholesterol Efflux through a P38-Dependent Pathway in Macrophages. J. Lipid Res. 2012, 53, 1840–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Liu, M.-H.; Hu, H.-J.; Feng, H.; Fan, X.-J.; Zou, W.; Pan, Y.; Hu, X.; Wang, Z. Curcumin Enhanced Cholesterol Efflux by Upregulating ABCA1 Expression through AMPK-SIRT1-LXRα Signaling in THP-1 Macrophage-Derived Foam Cells. DNA Cell Biol. 2015, 34, 561–572. [Google Scholar] [CrossRef]
- Lavergne, R. Plantes Médicinales Indigènes: Tisanerie et Tisaneurs de la Réunion; Université Montpellier II—Sciences et Techniques du Languedoc: Montpellier, France, 1989. [Google Scholar]
- Checkouri, E.; Reignier, F.; Robert-Da Silva, C.; Meilhac, O. Evaluation of Polyphenol Content and Antioxidant Capacity of Aqueous Extracts from Eight Medicinal Plants from Reunion Island: Protection against Oxidative Stress in Red Blood Cells and Preadipocytes. Antioxidants 2020, 9, 959. [Google Scholar] [CrossRef] [PubMed]
- Ghaddar, B.; Veeren, B.; Rondeau, P.; Bringart, M.; d’Hellencourt, C.L.; Meilhac, O.; Bascands, J.-L.; Diotel, N. Impaired Brain Homeostasis and Neurogenesis in Diet-Induced Overweight Zebrafish: A Preventive Role from A. Borbonica Extract. Sci. Rep. 2020, 10, 14496. [Google Scholar] [CrossRef]
- Marimoutou, M.; Le Sage, F.; Smadja, J.; d’Hellencourt, C.L.; Gonthier, M.-P.; Robert-Da Silva, C. Antioxidant Polyphenol-Rich Extracts from the Medicinal Plants Antirhea Borbonica, Doratoxylon Apetalum and Gouania Mauritiana Protect 3T3-L1 Preadipocytes against H2O2, TNFα and LPS Inflammatory Mediators by Regulating the Expression of Superoxide Dismutase and NF-ΚB Genes. J. Inflamm. 2015, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Le Sage, F.; Meilhac, O.; Gonthier, M.-P. Anti-Inflammatory and Antioxidant Effects of Polyphenols Extracted from Antirhea Borbonica Medicinal Plant on Adipocytes Exposed to Porphyromonas Gingivalis and Escherichia Coli Lipopolysaccharides. Pharmacol. Res. 2017, 119, 303–312. [Google Scholar] [CrossRef]
- Taïlé, J.; Arcambal, A.; Clerc, P.; Gauvin-Bialecki, A.; Gonthier, M.-P. Medicinal Plant Polyphenols Attenuate Oxidative Stress and Improve Inflammatory and Vasoactive Markers in Cerebral Endothelial Cells during Hyperglycemic Condition. Antioxidants 2020, 9, 573. [Google Scholar] [CrossRef]
- Luo, Y.; Sun, G.; Dong, X.; Wang, M.; Qin, M.; Yu, Y.; Sun, X. Isorhamnetin Attenuates Atherosclerosis by Inhibiting Macrophage Apoptosis via PI3K/AKT Activation and HO-1 Induction. PLoS ONE 2015, 10, e0120259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Feng, J.; Zhang, J.; Kang, X.; Qian, D. Quercetin Modulates AMPK/SIRT1/NF-ΚB Signaling to Inhibit Inflammatory/Oxidative Stress Responses in Diabetic High Fat Diet-Induced Atherosclerosis in the Rat Carotid Artery. Exp. Ther. Med. 2020, 20, 280. [Google Scholar] [CrossRef]
- Romero, M.; Jiménez, R.; Sánchez, M.; López-Sepúlveda, R.; Zarzuelo, M.J.; O’Valle, F.; Zarzuelo, A.; Pérez-Vizcaíno, F.; Duarte, J. Quercetin Inhibits Vascular Superoxide Production Induced by Endothelin-1: Role of NADPH Oxidase, Uncoupled ENOS and PKC. Atherosclerosis 2009, 202, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.-L.; Hung, C.-H.; Chan, S.-H.; Hsieh, P.-L.; Ou, H.-C.; Cheng, Y.-H.; Chu, P.-M. Chlorogenic Acid Protects Against OxLDL-Induced Oxidative Damage and Mitochondrial Dysfunction by Modulating SIRT1 in Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, e1700928. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Liang, B.; Zhang, Y.; Wang, Y.; Tang, J.; Shi, G. Kaempferol Alleviates Ox-LDL-Induced Apoptosis by up-Regulation of Autophagy via Inhibiting PI3K/Akt/MTOR Pathway in Human Endothelial Cells. Cardiovasc. Pathol. 2017, 31, 57–62. [Google Scholar] [CrossRef]
- Meilhac, O.; Escargueil-Blanc, I.; Thiers, J.-C.; Salvayre, R.; Nègre-Salvayre, A. Bcl-2 Alters the Balance between Apoptosis and Necrosis, but Does Not Prevent Cell Death Induced by Oxidized Low Density Lipoproteins. FASEB J. 1999, 13, 485–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Xu, S.; Huang, Y.; Xie, Y.; Lan, T.; Le, K.; Chen, J.; Chen, S.; Gao, S.; Xu, X.; Shen, X.; et al. Evaluation of Foam Cell Formation in Cultured Macrophages: An Improved Method with Oil Red O Staining and DiI-OxLDL Uptake. Cytotechnology 2010, 62, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Stary Herbert, C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W.; Rosenfeld, M.E.; Schwartz Colin, J.; Wagner William, D.; Wissler Robert, W. A Definition of Advanced Types of Atherosclerotic Lesions and a Histological Classification of Atherosclerosis. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef]
- Jonasson, L.; Holm, J.; Skalli, O.; Bondjers, G.; Hansson, G.K. Regional Accumulations of T Cells, Macrophages, and Smooth Muscle Cells in the Human Atherosclerotic Plaque. Arteriosclerosis 1986, 6, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, S.; Yang, B.; Fan, Y.; Deng, X. Effect of Oxidized Low-Density Lipoprotein Concentration Polarization on Human Smooth Muscle Cells’ Proliferation, Cycle, Apoptosis and Oxidized Low-Density Lipoprotein Uptake. J. R. Soc. Interface 2011, 9, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.-Z.; Teng, X.; Zhang, Z.-B.; Zheng, C.-J.; Chen, S.-H. Mangiferin Inhibits Apoptosis and Oxidative Stress via BMP2/Smad-1 Signaling in Dexamethasone-Induced MC3T3-E1 Cells. Int. J. Mol. Med. 2018, 41, 2517–2526. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Han, F.; Gao, G.; Liu, M. Mangiferin Exert Cardioprotective and Anti-Apoptotic Effects in Heart Failure Induced Rats. Life Sci. 2020, 249, 117476. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yao, H.; Wang, S.; Li, H.; Hou, X. Mangiferin Inhibits Apoptosis and Autophagy Induced by Staphylococcus Aureus in RAW264.7 Cells. J. Inflamm. Res. 2020, 13, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Basu, S.K.; Falck, J.R.; Ho, Y.K.; Goldstein, J.L. The Scavenger Cell Pathway for Lipoprotein Degradation: Specificity of the Binding Site That Mediates the Uptake of Negatively-Charged LDL by Macrophages. J. Supramol. Struct. 1980, 13, 67–81. [Google Scholar] [CrossRef]
- Hoppe, G.; O’Neil, J.; Hoff, H.F. Inactivation of Lysosomal Proteases by Oxidized Low Density Lipoprotein Is Partially Responsible for Its Poor Degradation by Mouse Peritoneal Macrophages. J. Clin. Investig. 1994, 94, 1506–1512. [Google Scholar] [CrossRef] [Green Version]
- Chistiakov, D.A.; Bobryshev, Y.V.; Orekhov, A.N. Changes in Transcriptome of Macrophages in Atherosclerosis. J. Cell. Mol. Med. 2015, 19, 1163–1173. [Google Scholar] [CrossRef]
- Cookson, F.B. The Origin of Foam Cells in Atherosclerosis. Br. J. Exp. Pathol. 1971, 52, 62–69. [Google Scholar]
- Cao, H.; Jia, Q.; Yan, L.; Chen, C.; Xing, S.; Shen, D. Quercetin Suppresses the Progression of Atherosclerosis by Regulating MST1-Mediated Autophagy in Ox-LDL-Induced RAW264.7 Macrophage Foam Cells. Int. J. Mol. Sci. 2019, 20, 6093. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of Inflammation in the Pathogenesis of Atherosclerosis and Therapeutic Interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnevale, R.; Nocella, C.; Petrozza, V.; Cammisotto, V.; Pacini, L.; Sorrentino, V.; Martinelli, O.; Irace, L.; Sciarretta, S.; Frati, G.; et al. Localization of Lipopolysaccharide from Escherichia Coli into Human Atherosclerotic Plaque. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Giovannini, C.; Masella, R. Role of Polyphenols in Cell Death Control. Nutr. Neurosci. 2012, 15, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Louis, X.L.; Thandapilly, S.J.; Kalt, W.; Vinqvist-Tymchuk, M.; Aloud, B.M.; Raj, P.; Yu, L.; Le, H.; Netticadan, T. Blueberry Polyphenols Prevent Cardiomyocyte Death by Preventing Calpain Activation and Oxidative Stress. Food Funct. 2014, 5, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.M.; Lee, C.H.; Lee, H.; Moon, B.; Lee, C.Y. Relative Antioxidant and Cytoprotective Activities of Common Herbs. Food Chem. 2008, 106, 929–936. [Google Scholar] [CrossRef]
- Cheng, D.; Zhang, X.; Tang, J.; Kong, Y.; Wang, X.; Wang, S. Chlorogenic Acid Protects against Aluminum Toxicity via MAPK/Akt Signaling Pathway in Murine RAW264.7 Macrophages. J. Inorg. Biochem. 2019, 190, 113–120. [Google Scholar] [CrossRef]
- Varshney, R.; Gupta, S.; Roy, P. Cytoprotective Effect of Kaempferol against Palmitic Acid-Induced Pancreatic β-Cell Death through Modulation of Autophagy via AMPK/MTOR Signaling Pathway. Mol. Cell. Endocrinol. 2017, 448, 1–20. [Google Scholar] [CrossRef]
- Tolosa, L.; Rodeiro, I.; Donato, M.T.; Herrera, J.A.; Delgado, R.; Castell, J.V.; Gómez-Lechón, M.J. Multiparametric Evaluation of the Cytoprotective Effect of the Mangifera Indica L. Stem Bark Extract and Mangiferin in HepG2 Cells. J. Pharm. Pharmacol. 2013, 65, 1073–1082. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Checkouri, E.; Ramin-Mangata, S.; Diotel, N.; Viranaicken, W.; Marodon, C.; Reignier, F.; Robert-Da Silva, C.; Meilhac, O. Protective Effects of Medicinal Plant Decoctions on Macrophages in the Context of Atherosclerosis. Nutrients 2021, 13, 280. https://doi.org/10.3390/nu13010280
Checkouri E, Ramin-Mangata S, Diotel N, Viranaicken W, Marodon C, Reignier F, Robert-Da Silva C, Meilhac O. Protective Effects of Medicinal Plant Decoctions on Macrophages in the Context of Atherosclerosis. Nutrients. 2021; 13(1):280. https://doi.org/10.3390/nu13010280
Chicago/Turabian StyleCheckouri, Eloïse, Stéphane Ramin-Mangata, Nicolas Diotel, Wildriss Viranaicken, Claude Marodon, Franck Reignier, Christine Robert-Da Silva, and Olivier Meilhac. 2021. "Protective Effects of Medicinal Plant Decoctions on Macrophages in the Context of Atherosclerosis" Nutrients 13, no. 1: 280. https://doi.org/10.3390/nu13010280
APA StyleCheckouri, E., Ramin-Mangata, S., Diotel, N., Viranaicken, W., Marodon, C., Reignier, F., Robert-Da Silva, C., & Meilhac, O. (2021). Protective Effects of Medicinal Plant Decoctions on Macrophages in the Context of Atherosclerosis. Nutrients, 13(1), 280. https://doi.org/10.3390/nu13010280