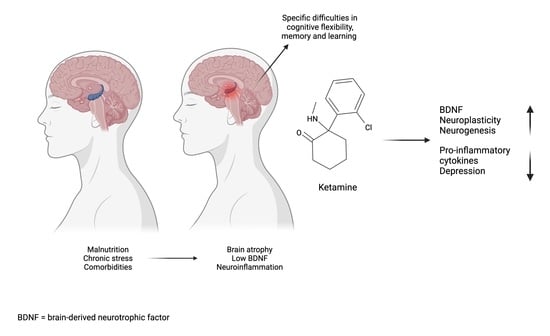
Ketamine as a Treatment for Anorexia Nervosa: A Narrative Review
Abstract
:1. Introduction: An Overview of Ketamine
2. Materials and Methods
3. The Neurobiological and Psychological Effects of Ketamine
4. Anorexia Nervosa
5. The Use of Ketamine in Commonly Comorbid Psychiatric Disorders
5.1. Depression
5.2. Obsessive-Compulsive Disorder
5.3. Autism Spectrum Disorder (ASD)
5.4. Anxiety Disorders
5.4.1. Generalised Anxiety Disorder and Social Anxiety Disorder
5.4.2. Post-Traumatic Stress Disorder
5.5. Substance and Alcohol Use Disorders
6. Ketamine and the Neurobiology of Anorexia Nervosa
6.1. Neurotransmitters
6.1.1. Serotonin
6.1.2. Dopamine
6.2. Neuroplasticity and Neuromorphology
6.3. Neuropsychology
7. Ketamine as a Treatment for Anorexia Nervosa
7.1. Current Research
7.2. Side Effects and Safety Concerns
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurdi, M.S.; Theerth, K.A.; Deva, R.S. Ketamine: Current applications in anesthesia, pain, and critical care. Anesth. Essays Res. 2014, 8, 283. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Model List of Essential Medicines: 21st List; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Mion, G.; Villevieille, T. Ketamine pharmacology: An update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci. Ther. 2013, 19, 370–380. [Google Scholar] [CrossRef]
- Domino, E.F. Taming the ketamine tiger. Anesthesiology 2010, 113, 678–684. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, R.S.; Rosenblat, J.D.; Nemeroff, C.B.; Sanacora, G.; Murrough, J.W.; Berk, M.; Brietzke, E.; Dodd, S.; Gorwood, P.; Ho, R. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: An international expert opinion on the available evidence and implementation. Am. J. Psychiatry 2021, 178, 383–399. [Google Scholar] [CrossRef]
- Chong, C.; Schug, S.; Page-Sharp, M.; Ilett, K. Bioavailability of ketamine after oral or sublingual administration. Pain Med. 2006, 7, 469. [Google Scholar] [CrossRef] [Green Version]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Georgiou, P.; Fischell, J.; Elmer, G.I.; Alkondon, M.; Yuan, P.; Pribut, H.J.; Singh, N.S. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016, 533, 481–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domino, E.F. Neurobiology of phencyclidine (Sernyl), a drug with an unusual spectrum of pharmacological activity. Int. Rev. Neurobio. 1964, 6, 303–347. [Google Scholar]
- Fontana, A. Terapia atidpresiva con ketamine [Antidepressive therapy with ketamine]. Acta Psiquiat. Psicol. Amer. Lat. Psychiatr. Psychol. Proc. Lat. Am. 1974, 20, 32. [Google Scholar]
- Wilkinson, S.T.; Toprak, M.; Turner, M.S.; Levine, S.P.; Katz, R.B.; Sanacora, G. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am. J. Psychiatry 2017, 174, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.H.; Landeros-Weisenberger, A.; Coughlin, C.; Mulqueen, J.; Johnson, J.A.; Gabriel, D.; Reed, M.O.; Jakubovski, E.; Bloch, M.H. Ketamine for social anxiety disorder: A randomized, placebo-controlled crossover trial. Neuropsychopharmacology 2018, 43, 325–333. [Google Scholar] [CrossRef]
- Ezquerra-Romano, I.I.; Lawn, W.; Krupitsky, E.; Morgan, C.J.A. Ketamine for the treatment of addiction: Evidence and potential mechanisms. Neuropharmacology 2018, 142, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Mateus, C.F.; Malcolm, R.J.; Brady, K.T.; Back, S.E. Efficacy of ketamine in the treatment of substance use disorders: A systematic review. Front. Psychiatry 2018, 9, 277. [Google Scholar] [CrossRef] [Green Version]
- Worrell, S.D.; Gould, T.J. Therapeutic Potential of Ketamine for Alcohol Use Disorder. Neurosci. Biobehav. Rev. 2021, 126, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Feder, A.; Parides, M.K.; Murrough, J.W.; Perez, A.M.; Morgan, J.E.; Saxena, S.; Kirkwood, K.; Aan Het Rot, M.; Lapidus, K.A.; Wan, L.B.; et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: A randomized clinical trial. JAMA Psychiatry 2014, 71, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Leary, T.; Litwin, G.H.; Metzner, R. Reactions to psilocybin administered in a supportive environment. J. Nerv. Ment. Dis. 1963, 137, 561–573. [Google Scholar]
- Metzner, R.; Leary, T. On programming psychedelic experiences. Psychedelic. Rev. 1967, 9, 5–19. [Google Scholar]
- Hartogsohn, I. Constructing drug effects: A history of set and setting. Drug Sci. Policy Law 2017, 3, 2050324516683325. [Google Scholar] [CrossRef]
- Johnson, M.W.; Richards, W.A.; Griffiths, R.R. Human hallucinogen research: Guidelines for safety. J. Psychopharmacol. 2008, 22, 603–620. [Google Scholar]
- Carhart-Harris, R.L.; Roseman, L.; Haijen, E.; Erritzoe, D.; Watts, R.; Branchi, I.; Kaelen, M. Psychedelics and the essential importance of context. J. Psychopharmacol. 2018, 32, 725–731. [Google Scholar] [CrossRef]
- Andrade, C. Ketamine for depression, 4: In what dose, at what rate, by what route, for how long, and at what frequency? J. Clin. Psychiatry 2017, 78, e852–e857. [Google Scholar] [CrossRef]
- Loo, C.; Gálvez, V.; O’keefe, E.; Mitchell, P.; Hadzi-Pavlovic, D.; Leyden, J.; Harper, S.; Somogyi, A.; Lai, R.; Weickert, C. Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr. Scand. 2016, 134, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Dakwar, E.; Anerella, C.; Hart, C.; Levin, F.; Mathew, S.; Nunes, E. Therapeutic infusions of ketamine: Do the psychoactive effects matter? Drug Alcohol. Depend. 2014, 136, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Zarate, C.A.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 2006, 63, 856–864. [Google Scholar] [CrossRef]
- Krystal, J.H.; Sanacora, G.; Duman, R.S. Rapid-acting glutamatergic antidepressants: The path to ketamine and beyond. Biol. Psychiatry 2013, 73, 1133–1141. [Google Scholar] [CrossRef] [Green Version]
- Duman, R.S.; Li, N.; Liu, R.J.; Duric, V.; Aghajanian, G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 2012, 62, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Zhang, J.-C.; Han, M.; Yao, W.; Yang, C.; Ren, Q.; Ma, M.; Chen, Q.-X.; Hashimoto, K. Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology 2016, 233, 3647–3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.-C.; Yao, W.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Han, M.; Hashimoto, K. Comparison of ketamine, 7, 8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology 2015, 232, 4325–4335. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Rovnaghi, C.R.; Anand, K.J. Ketamine affects the neurogenesis of rat fetal neural stem progenitor cells via the PI3K/Akt-p27 signaling pathway. Birth. Defects Res. B Dev. Reprod. Toxicol. 2014, 101, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Yamada, J.; Jinno, S. Potential link between antidepressant-like effects of ketamine and promotion of adult neurogenesis in the ventral hippocampus of mice. Neuropharmacology 2019, 158, 107710. [Google Scholar] [CrossRef]
- Ma, Z.; Zang, T.; Birnbaum, S.G.; Wang, Z.; Johnson, J.E.; Zhang, C.L.; Parada, L.F. TrkB dependent adult hippocampal progenitor differentiation mediates sustained ketamine antidepressant response. Nat. Commun. 2017, 8, 1668. [Google Scholar]
- Deyama, S.; Duman, R.S. Neurotrophic mechanisms underlying the rapid and sustained antidepressant actions of ketamine. Pharmacol. Biochem. Behav. 2020, 188, 172837. [Google Scholar]
- Wang, N.; Yu, H.Y.; Shen, X.F.; Gao, Z.Q.; Yang, C.; Yang, J.J.; Zhang, G.F. The rapid antidepressant effect of ketamine in rats is associated with down-regulation of pro-inflammatory cytokines in the hippocampus. Ups. J. Med. Sci. 2015, 120, 241–248. [Google Scholar] [CrossRef]
- Clarke, M.; Razmjou, S.; Prowse, N.; Dwyer, Z.; Litteljohn, D.; Pentz, R.; Anisman, H.; Hayley, S. Ketamine modulates hippocampal neurogenesis and pro-inflammatory cytokines but not stressor induced neurochemical changes. Neuropharmacology 2017, 112, 210–220. [Google Scholar] [CrossRef]
- Kiraly, D.D.; Horn, S.R.; Van Dam, N.T.; Costi, S.; Schwartz, J.; Kim-Schulze, S.; Patel, M.; Hodes, G.E.; Russo, S.J.; Merad, M.; et al. Altered peripheral immune profiles in treatment-resistant depression: Response to ketamine and prediction of treatment outcome. Transl. Psychiatry 2017, 7, e1065. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Y.; Zhou, Y.; Zheng, W.; Liu, W.; Wang, C.; Lan, X.; Deng, X.; Xu, Y.; Zhang, B.; Ning, Y. Alterations of multiple peripheral inflammatory cytokine levels after repeated ketamine infusions in major depressive disorder. Transl Psychiatry 2020, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Li, C.T.; Lin, W.C.; Hong, C.J.; Tu, P.C.; Bai, Y.M.; Cheng, C.M.; Su, T.P. Rapid inflammation modulation and antidepressant efficacy of a low-dose ketamine infusion in treatment-resistant depression: A randomized, double-blind control study. Psychiatry Res. 2018, 269, 207–211. [Google Scholar] [CrossRef]
- Park, M.; Newman, L.E.; Gold, P.W.; Luckenbaugh, D.A.; Yuan, P.; Machado-Vieira, R.; Zarate, C.A., Jr. Change in cytokine levels is not associated with rapid antidepressant response to ketamine in treatment-resistant depression. J. Psychiatr. Res. 2017, 84, 113–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birmingham, C.L.; Su, J.; Hlynsky, J.A.; Goldner, E.M.; Gao, M. The mortality rate from anorexia nervosa. Int. J. Eat. Disord. 2005, 38, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Franko, D.L.; Keshaviah, A.; Eddy, K.T.; Krishna, M.; Davis, M.C.; Keel, P.K.; Herzog, D.B. A longitudinal investigation of mortality in anorexia nervosa and bulimia nervosa. Am. J. Psychiatry 2013, 170, 917–925. [Google Scholar] [CrossRef]
- Eddy, K.T.; Tabri, N.; Thomas, J.J.; Murray, H.B.; Keshaviah, A.; Hastings, E.; Edkins, K.; Krishna, M.; Herzog, D.B.; Keel, P.K.; et al. Recovery from anorexia nervosa and bulimia nervosa at 22-year follow-up. J. Clin. Psychiatry 2017, 78, 184–189. [Google Scholar] [CrossRef]
- Støving, R.K.; Andries, A.; Brixen, K.; Bilenberg, N.; Hørder, K. Gender differences in outcome of eating disorders: A retrospective cohort study. Psychiatry Res. 2011, 186, 362–366. [Google Scholar] [CrossRef]
- Treasure, J.; Willmott, D.; Ambwani, S.; Cardi, V.; Clark Bryan, D.; Rowlands, K.; Schmidt, U. Cognitive interpersonal model for anorexia nervosa revisited: The perpetuating factors that contribute to the development of the severe and enduring illness. J. Clin. Med. 2020, 9, 630. [Google Scholar] [CrossRef] [Green Version]
- Treasure, J.; Oyeleye, O.; Bonin, E.M.; Zipfel, S.; Fernandez-Aranda, F. Optimising care pathways for adult anorexia nervosa. What is the evidence to guide the provision of high-quality, cost-effective services? Eur. Eat. Disord. Rev. 2021, 29, 306–315. [Google Scholar] [CrossRef]
- Hay, P.J.; Touyz, S.; Sud, R. Treatment for severe and enduring anorexia nervosa: A review. Aust. N. Z. J. Psychiatry 2012, 46, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Wonderlich, S.A.; Bulik, C.M.; Schmidt, U.; Steiger, H.; Hoek, H.W. Severe and enduring anorexia nervosa: Update and observations about the current clinical reality. Int. J. Eat. Disord. 2020, 53, 1303–1312. [Google Scholar] [CrossRef]
- Jagielska, G.; Kacperska, I. Outcome, comorbidity and prognosis in anorexia nervosa. Psychiatr. Pol. 2017, 51, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Ulfvebrand, S.; Birgegård, A.; Norring, C.; Högdahl, L.; von Hausswolff-Juhlin, Y. Psychiatric comorbidity in women and men with eating disorders results from a large clinical database. Psych. Res. 2015, 230, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.H.; Kaplan, A.S.; Garfinkel, P.E.; Rockert, W.; Toner, B.; Abbey, S.E. Depression in anorexia nervosa and bulimia nervosa: Discriminating depressive symptoms and episodes. J. Psychosom. Res. 1994, 38, 773–782. [Google Scholar] [CrossRef]
- Jordan, J.; Joyce, P.R.; Carter, F.A.; Horn, J.; McIntosh, V.V.; Luty, S.E.; McKenzie, J.M.; Frampton, C.M.; Mulder, R.T.; Bulik, C.M. Specific and nonspecific comorbidity in anorexia nervosa. Int. J. Eat. Disord. 2008, 41, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Hepp, U.; Spindler, A.; Schnyder, U.; Kraemer, B.; Milos, G. Post-traumatic stress disorder in women with eating disorders. Eat. Weight Disord. 2007, 12, e24–e27. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Rodríguez, M.L.; Ann Von Holle, T.; Thornton, L.M.; Klump, K.L.; Brandt, H.; Crawford, S.; Fichter, M.M.; Halmi, K.A.; Huber, T.; Johnson, C.; et al. Post traumatic stress disorder in anorexia nervosa. Psychosom. Med. 2011, 73, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaye, W.H.; Bulik, C.M.; Thornton, L.; Barbarich, N.; Masters, K.; Group, P.F.C. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am. J. Psychiatry 2004, 161, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Otani, R.; Iguchi, T.; Ishii, R.; Uchida, S.; Okada, A.; Kitayama, S.; Koyanagi, K.; Suzuki, Y.; Suzuki, Y.; et al. Prevalence of autism spectrum disorder and autistic traits in children with anorexia nervosa and avoidant/restrictive food intake disorder. Biopsychosoc. Med. 2021, 15, 9. [Google Scholar] [CrossRef]
- Bulik, C.M.; Sullivan, P.F.; Joyce, P.R. Temperament, character and suicide attempts in anorexia nervosa, bulimia nervosa and major depression. Acta Psychiatr. Scand 1999, 100, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Boehm, I.; Flohr, L.; Steding, J.; Holzapfel, L.; Seitz, J.; Roessner, V.; Ehrlich, S. The Trajectory of Anhedonic and Depressive Symptoms in Anorexia Nervosa: A Longitudinal and Cross-Sectional Approach. Eur. Eat. Disord. Rev. 2018, 26, 69–74. [Google Scholar] [CrossRef]
- Pompili, M.; Mancinelli, I.; Girardi, P.; Ruberto, A.; Tatarelli, R. Suicide in anorexia nervosa: A meta-analysis. Int. J. Eat. Disord. 2004, 36, 99–103. [Google Scholar] [CrossRef]
- Franko, D.L.; Tabri, N.; Keshaviah, A.; Murray, H.B.; Herzog, D.B.; Thomas, J.J.; Coniglio, K.; Keel, P.K.; Eddy, K.T. Predictors of long-term recovery in anorexia nervosa and bulimia nervosa: Data from a 22-year longitudinal study. J. Psychiatr. Res. 2018, 96, 183–188. [Google Scholar] [CrossRef]
- Engel, S.G.; Wonderlich, S.A.; Crosby, R.D.; Mitchell, J.E.; Crow, S.; Peterson, C.B.; Le Grange, D.; Simonich, H.K.; Cao, L.; Lavender, J.M.; et al. The role of affect in the maintenance of anorexia nervosa: Evidence from a naturalistic assessment of momentary behaviors and emotion. J. Abnorm. Psychol. 2013, 122, 709–719. [Google Scholar] [CrossRef] [Green Version]
- Raney, T.J.; Thornton, L.M.; Berrettini, W.; Brandt, H.; Crawford, S.; Fichter, M.M.; Halmi, K.A.; Johnson, C.; Kaplan, A.S.; LaVia, M.; et al. Influence of overanxious disorder of childhood on the expression of anorexia nervosa. Int. J. Eat. Disord. 2008, 41, 326–332. [Google Scholar] [CrossRef]
- Bulik, C.M.; Sullivan, P.F.; Fear, J.I.; Joyce, P.R. Eating disorders and antecedent anxiety disorders: A controlled study. Acta Psychiatr. Scand. 1997, 96, 101–107. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. Manual for the State-Trait Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Wagner, A.; Aizenstein, H.; Venkatraman, V.K.; Fudge, J.; May, J.C.; Mazurkewicz, L.; Frank, G.K.; Bailer, U.F.; Fischer, L.; Nguyen, V. Altered reward processing in women recovered from anorexia nervosa. Am. J. Psychiatry 2007, 164, 1842–1849. [Google Scholar] [CrossRef]
- Kaye, W.; Bailer, U.; Frank, G.; Henry, S.; Price, J.; Meltzer, C.; Becker, C.; Ziolko, S.; Mathis, C.; Wagner, A. Serotonin transporter binding after recovery from eating disorders. Psychopharmacology 2008, 197, 521–522. [Google Scholar] [CrossRef]
- Kong, S.; Bernstein, K. Childhood trauma as a predictor of eating psychopathology and its mediating variables in patients with eating disorders. J. Clin. Nurs. 2009, 18, 1897–1907. [Google Scholar] [CrossRef]
- Wade, T.D.; Bulik, C.M.; Neale, M.; Kendler, K.S. Anorexia nervosa and major depression: Shared genetic and environmental risk factors. Am. J. Psychiatry 2000, 157, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.J.; Yilmaz, Z.; Thornton, L.M.; Hübel, C.; Coleman, J.R.; Gaspar, H.A.; Bryois, J.; Hinney, A.; Leppä, V.M.; Mattheisen, M. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 2019, 51, 1207–1214. [Google Scholar] [CrossRef] [Green Version]
- Cederlöf, M.; Thornton, L.M.; Baker, J.; Lichtenstein, P.; Larsson, H.; Rück, C.; Bulik, C.M.; Mataix-Cols, D. Etiological overlap between obsessive-compulsive disorder and anorexia nervosa: A longitudinal cohort, multigenerational family and twin study. World Psychiatry 2015, 14, 333–338. [Google Scholar] [CrossRef] [Green Version]
- de Vos, J.; Houtzager, L.; Katsaragaki, G.; van de Berg, E.; Cuijpers, P.; Dekker, J. Meta analysis on the efficacy of pharmacotherapy versus placebo on anorexia nervosa. J. Eat. Disord. 2014, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Esketamine for treatment resistant depression is not recommended by NICE. BMJ 2020, 368, m329. [Google Scholar] [CrossRef]
- Short, B.; Fong, J.; Galvez, V.; Shelker, W.; Loo, C.K. Side-effects associated with ketamine use in depression: A systematic review. Lancet Psychiatry 2018, 5, 65–78. [Google Scholar] [CrossRef]
- Fanta, S.; Kinnunen, M.; Backman, J.T.; Kalso, E. Population pharmacokinetics of S-ketamine and norketamine in healthy volunteers after intravenous and oral dosing. Eur. J. Clin. Pharmacol. 2015, 71, 441–447. [Google Scholar] [CrossRef]
- Gill, H.; Gill, B.; Rodrigues, N.B.; Lipsitz, O.; Rosenblat, J.D.; El-Halabi, S.; Nasri, F.; Mansur, R.B.; Lee, Y.; McIntyre, R.S. The effects of ketamine on cognition in treatment-resistant depression: A systematic review and priority avenues for future research. Neurosci. Biobehav. Rev. 2020, 120, 78–85. [Google Scholar] [CrossRef]
- Kim, S.; Rush, B.S.; Rice, T.R. A systematic review of therapeutic ketamine use in children and adolescents with treatment-resistant mood disorders. Eur. Child Adolesc. Psychiatry 2020, 30, 1485–1501. [Google Scholar] [CrossRef] [PubMed]
- Sos, P.; Klirova, M.; Novak, T.; Kohutova, B.; Horacek, J.; Palenicek, T. Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuroendocrinol. Lett. 2013, 34, 101–107. [Google Scholar]
- Luckenbaugh, D.A.; Niciu, M.J.; Ionescu, D.F.; Nolan, N.M.; Richards, E.M.; Brutsche, N.E.; Guevara, S.; Zarate, C.A. Do the dissociative side effects of ketamine mediate its antidepressant effects? J. Affect. Disord. 2014, 159, 56–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belser, A.B.; Agin-Liebes, G.; Swift, T.C.; Terrana, S.; Devenot, N.; Friedman, H.L.; Guss, J.; Bossis, A.; Ross, S. Patient experiences of psilocybin-assisted psychotherapy: An interpretative phenomenological analysis. J. Humanist. Psychol. 2017, 57, 354–388. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, R.R.; Johnson, M.W.; Richards, W.A.; Richards, B.D.; McCann, U.; Jesse, R. Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects. Psychopharmacology 2011, 218, 649–665. [Google Scholar] [CrossRef] [Green Version]
- Morgan, C.J.; Curran, H.V.; Independent Scientific Committee on Drugs (ISCD). Ketamine use: A review. Addiction 2012, 107, 27–38. [Google Scholar] [CrossRef]
- Shiroma, P.R.; Thuras, P.; Wels, J.; Albott, C.S.; Erbes, C.; Tye, S.; Lim, K.O. Neurocognitive performance of repeated versus single intravenous subanesthetic ketamine in treatment resistant depression. J. Affect. Disord. 2020, 277, 470–477. [Google Scholar] [CrossRef]
- Martinotti, G.; Chiappini, S.; Pettorruso, M.; Mosca, A.; Miuli, A.; Di Carlo, F.; D’Andrea, G.; Collevecchio, R.; Di Muzio, I.; Sensi, S.L. Therapeutic Potentials of Ketamine and Esketamine in Obsessive–Compulsive Disorder (OCD), Substance Use Disorders (SUD) and Eating Disorders (ED): A Review of the Current Literature. Brain Sci. 2021, 11, 856. [Google Scholar] [CrossRef]
- Veraart, J.K.; Kamphuis, J.; Schlegel, M.; Schoevers, R.A. Oral S-ketamine effective after deep brain stimulation in severe treatment-resistant depression and extensive comorbidities. BMJ Case Rep. 2021, 14, e238135. [Google Scholar] [CrossRef]
- Wink, L.K.; Reisinger, D.L.; Horn, P.; Shaffer, R.C.; O’Brien, K.; Schmitt, L.; Dominick, K.R.; Pedapati, E.V.; Erickson, C.A. Brief Report: Intranasal Ketamine in Adolescents and Young Adults with Autism Spectrum Disorder—Initial Results of a Randomized, Controlled, Crossover, Pilot Study. J. Autism Dev. Disord. 2021, 51, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Kastner, T.; Walsh, K.; Shulman, L.; Alam, F.; Flood, S. Ketamine and the core symptoms of autism. Int. J. Disabil. Hum. Dev. 2016, 15, 121–123. [Google Scholar] [CrossRef]
- Wink, L.K.; Anne, M.; Shaffer, R.C.; Pedapati, E.; Friedmann, K.; Schaefer, T.; Erickson, C.A. Intranasal ketamine treatment in an adult with autism spectrum disorder. J. Clin. Psychiatry 2014, 75, 835–836. [Google Scholar] [CrossRef]
- Glue, P.; Neehoff, S.; Sabadel, A.; Broughton, L.; Le Nedelec, M.; Shadli, S.; McNaughton, N.; Medlicott, N.J. Effects of ketamine in patients with treatment-refractory generalized anxiety and social anxiety disorders: Exploratory double-blind psychoactive-controlled replication study. J. Psychopharmacol. 2020, 34, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Glue, P.; Medlicott, N.J.; Neehoff, S.; Surman, P.; Lam, F.; Hung, N.; Hung, C.T. Safety and efficacy of extended release ketamine tablets in patients with treatment-resistant depression and anxiety: Open label pilot study. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320922474. [Google Scholar] [CrossRef]
- Girgenti, M.J.; Ghosal, S.; LoPresto, D.; Taylor, J.R.; Duman, R.S. Ketamine accelerates fear extinction via mTORC1 signaling. Neurobiol. Dis. 2017, 100, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Duek, O.; Kelmendi, B.; Pietrzak, R.H.; Harpaz-Rotem, I. Augmenting the treatment of PTSD with ketamine—A review. Curr. Treat Options Psychiatry 2019, 6, 143–153. [Google Scholar] [CrossRef]
- Feder, A.; Rutter, S.B.; Schiller, D.; Charney, D.S. The emergence of ketamine as a novel treatment for posttraumatic stress disorder. Adv. Pharmacol. 2020, 89, 261–286. [Google Scholar]
- Asim, M.; Bing, W.; Bo, H.; Xiaoguang, W. Ketamine For Post-Traumatic Stress Disorders And It’s Possible Therapeutic Mechanism. Neurochem. Int. 2021, 146, 105044. [Google Scholar] [CrossRef]
- Pradhan, B.; Mitrev, L.; Moaddell, R.; Wainer, I.W. d-Serine is a potential biomarker for clinical response in treatment of post-traumatic stress disorder using (R,S)-ketamine infusion and TIMBER psychotherapy: A pilot study. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 831–839. [Google Scholar] [CrossRef]
- Das, R.K.; Gale, G.; Walsh, K.; Hennessy, V.E.; Iskandar, G.; Mordecai, L.A.; Brandner, B.; Kindt, M.; Curran, H.V.; Kamboj, S.K. Ketamine can reduce harmful drinking by pharmacologically rewriting drinking memories. Nat. Commun. 2019, 10, 5187. [Google Scholar] [CrossRef] [PubMed]
- Artigas, F. Serotonin receptors involved in antidepressant effects. Pharmacol. Ther. 2013, 137, 119–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauthier, C.; Hassler, C.; Mattar, L.; Launay, J.M.; Callebert, J.; Steiger, H.; Melchior, J.C.; Falissard, B.; Berthoz, S.; Mourier-Soleillant, V.; et al. Symptoms of depression and anxiety in anorexia nervosa: Links with plasma tryptophan and serotonin metabolism. Psychoneuroendocrinology 2014, 39, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Bailer, U.F.; Kaye, W.H. Serotonin: Imaging findings in eating disorders. Curr. Top. Behav. Neurosci. 2011, 6, 59–79. [Google Scholar]
- Bailer, U.F.; Price, J.C.; Meltzer, C.C.; Mathis, C.A.; Frank, G.K.; Weissfeld, L.; McConaha, C.W.; Henry, S.E.; Brooks-Achenbach, S.; Barbarich, N.C.; et al. Altered 5-HT 2A receptor binding after recovery from bulimia-type anorexia nervosa: Re-lationships to harm avoidance and drive for thinness. Neuropsychopharmacology 2004, 29, 1143–1155. [Google Scholar] [CrossRef]
- Chen, J.; Kang, Q.; Jiang, W.; Fan, J.; Zhang, M.; Yu, S.; Zhang, C. The 5-HTTLPR confers susceptibility to anorexia nervosa in Han Chinese: Evidence from a case-control and family-based study. PLoS ONE 2015, 10, e0119378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calati, R.; De Ronchi, D.; Bellini, M.; Serretti, A. The 5-HTTLPR polymorphism and eating disorders: A meta-analysis. Int. J. Eat. Disord. 2011, 44, 191–199. [Google Scholar] [CrossRef]
- Nacmias, B.; Ricca, V.; Tedde, A.; Mezzani, B.; Rotella, C.M.; Sorbi, S. 5-HT2A receptor gene polymorphisms in anorexia nervosa and bulimia nervosa. Neurosci. Lett. 1999, 277, 134–136. [Google Scholar] [CrossRef]
- Gorwood, P.; Ades, J.; Bellodi, L.F.; Cellini, E.; Collier, D.A.; Di Bella, D.; Di Bernardo, M.; Estivill, X.; Fernandez-Aranda, F.; Gratacos, M.; et al. The 5-HT 2A−1438G/A polymorphism in anorexia nervosa: A combined analysis of 316 trios from six European centres. Mol. Psychiatry 2002, 7, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Kaye, W.H.; Frank, G.K.; Bailer, U.F.; Henry, S.E.; Meltzer, C.C.; Price, J.C.; Mathis, C.A.; Wagner, A. Serotonin alterations in anorexia and bulimia nervosa: New insights from imaging studies. Physiol. Behav. 2005, 85, 73–81. [Google Scholar] [CrossRef]
- Pham, T.; Mendez-David, I.; Defaix, C.; Guiard, B.; Tritschler, L.; David, D.; Gardier, A. Ketamine treatment involves medial prefrontal cortex serotonin to induce a rapid antidepressant-like activity in BALB/cJ mice. Neuropharmacology 2017, 112, 198–209. [Google Scholar] [CrossRef]
- Yamanaka, H.; Yokoyama, C.; Mizuma, H.; Kurai, S.; Finnema, S.J.; Halldin, C.; Doi, H.; Onoe, H. A possible mechanism of the nucleus accumbens and ventral pallidum 5-HT1B receptors underlying the antidepressant action of ketamine: A PET study with macaques. Transl. Psychiatry 2014, 4, e342. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, K.G.; Müller, H.K.; Elfving, B.; Dale, E.; Wegener, G.; Sanchez, C. Potential involvement of serotonergic signaling in ketamine’s antidepressant actions: A critical review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 71, 27–38. [Google Scholar] [CrossRef]
- Berridge, K.C.; Robinson, T.E. Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol. 2016, 71, 670. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Sandstrom, S.M.; Denenberg, V.H.; Palmiter, R.D. Distinguishing whether dopamine regulates liking, wanting, and/or learning about rewards. Behav. Neurosci. 2005, 119, 5. [Google Scholar] [CrossRef]
- Berridge, K.C.; Robinson, T.E. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain. Res. Rev. 1998, 28, 309–369. [Google Scholar] [CrossRef]
- Kontis, D.; Theochari, E. Dopamine in anorexia nervosa: A systematic review. Behav. Pharmacol. 2012, 23, 496–515. [Google Scholar] [CrossRef]
- O’Hara, C.B.; Campbell, I.C.; Schmidt, U. A reward-centred model of anorexia nervosa: A focussed narrative review of the neurological and psychophysiological literature. Neurosci. Biobehav. Rev. 2015, 52, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Bergh, C.; Södersten, P. Anorexia nervosa, self-starvation and the reward of stress. Nat. Med. 1996, 2, 21–22. [Google Scholar] [CrossRef]
- Zink, C.F.; Weinberger, D.R. Cracking the moody brain: The rewards of self starvation. Nat. Med. 2010, 16, 1382–1383. [Google Scholar] [CrossRef]
- Sterpenich, V.; Vidal, S.; Hofmeister, J.; Michalopoulos, G.; Bancila, V.; Warrot, D.; Dayer, A.; Desseilles, M.; Aubry, J.M.; Kosel, M.; et al. Increased reactivity of the mesolimbic reward system after ketamine injection in patients with treatment-resistant major depressive disorder. Anesthesiology 2019, 130, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Kotoula, V.; Stringaris, A.; Mackes, N.; Mazibuko, N.; Hawkins, P.C.; Furey, M.; Curran, H.V.; Mehta, M.A. Ketamine Modulates the Neural Correlates of Reward Processing in Unmedicated Patients in Remission from Depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, in press. [Google Scholar] [CrossRef]
- Mkrtchian, A.; Evans, J.W.; Kraus, C.; Yuan, P.; Kadriu, B.; Nugent, A.C.; Roiser, J.P.; Zarate, C.A. Ketamine modulates fronto-striatal circuitry in depressed and healthy individuals. Mol. Psychiatry 2021, 26, 3292–3301. [Google Scholar] [CrossRef]
- Belujon, P.; Grace, A.A. Restoring mood balance in depression: Ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol. Psychiatry 2014, 76, 927–936. [Google Scholar] [CrossRef] [Green Version]
- Gu, Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience 2002, 111, 815–835. [Google Scholar] [CrossRef]
- Marzo, A.; Bai, J.; Otani, S. Neuroplasticity regulation by noradrenaline in mammalian brain. Curr. Neuropharmacol. 2009, 7, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Dalton, B.; Bartholdy, S.; Robinson, L.; Solmi, M.; Ibrahim, M.A.; Breen, G.; Schmidt, U.; Himmerich, H. A meta-analysis of cytokine concentrations in eating disorders. J. Psychiatr. Res. 2018, 103, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Solmi, M.; Veronese, N.; Favaro, A.; Santonastaso, P.; Manzato, E.; Sergi, G.; Correll, C.U. Inflammatory cytokines and anorexia nervosa: A meta-analysis of cross-sectional and longitudinal studies. Psychoneuroendocrinology 2015, 51, 237–252. [Google Scholar] [CrossRef]
- Dalton, B.; Leppanen, J.; Campbell, I.C.; Chung, R.; Breen, G.; Schmidt, U.; Himmerich, H. A longitudinal analysis of cytokines in anorexia nervosa. Brain Behav. Immun. 2020, 85, 88–95. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Chrousos, G.P. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann. N. Y. Acad Sci. 2002, 966, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.E.; Cohen, S.; Ritchey, A.K. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychol. 2002, 21, 531. [Google Scholar] [CrossRef]
- Hänsel, A.; Hong, S.; Cámara, R.J.; Von Kaenel, R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci. Biobehav. Rev. 2010, 35, 115–121. [Google Scholar] [CrossRef]
- Kubera, M.; Obuchowicz, E.; Goehler, L.; Brzeszcz, J.; Maes, M. In animal models, psychosocial stress-induced (neuro) inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 744–759. [Google Scholar] [CrossRef]
- Himmerich, H.; Fischer, J.; Bauer, K.; Kirkby, K.C.; Sack, U.; Krügel, U. Stress-induced cytokine changes in rats. Eur. Cytokine Netw. 2013, 24, 97–103. [Google Scholar] [CrossRef]
- Krügel, U.; Fischer, J.; Bauer, K.; Sack, U.; Himmerich, H. The impact of social isolation on immunological parameters in rats. Arch. Toxicol. 2014, 88, 853–855. [Google Scholar] [CrossRef]
- Miller, M.W.; Lin, A.P.; Wolf, E.J.; Miller, D.R. Oxidative stress, inflammation, and neuroprogression in chronic PTSD. Harv. Rev. Psychiatry 2018, 26, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Benedek, D.; Fullerton, C.; Forsten, R.; Naifeh, J.; Li, X.; Hu, X.; Li, H.; Jia, M.; Xing, G. PTSD risk is associated with BDNF Val66Met and BDNF overexpression. Mol. Psychiatry 2014, 19, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Suliman, S.; Hemmings, S.M.; Seedat, S. Brain-Derived Neurotrophic Factor (BDNF) protein levels in anxiety disorders: Systematic review and meta-regression analysis. Front. Integr. Neurosci. 2013, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in fear-and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 2017, 42, 254–270. [Google Scholar] [CrossRef] [Green Version]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keeler, J.L.; Robinson, L.; Keeler-Schäffeler, R.; Dalton, B.; Treasure, J.; Himmerich, H. Growth factors in Anorexia Nervosa: A systematic review and meta-analysis of cross-sectional and longitudinal data. World J. Biol. Psychiatry. in press.
- Seitz, J.; Bühren, K.; von Polier, G.G.; Heussen, N.; Herpertz-Dahlmann, B.; Konrad, K. Morphological changes in the brain of acutely ill and weight-recovered patients with anorexia nervosa. Z. Kinder Jugendpsychiatr. Psychother. 2014, 42, 7–18. [Google Scholar] [CrossRef]
- Seitz, J.; Konrad, K.; Herpertz-Dahlmann, B. Extend, pathomechanism and clinical consequences of brain volume changes in anorexia nervosa. Curr. Neuropharmacol. 2018, 16, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Keeler, J.; Patsalos, O.; Thuret, S.; Ehrlich, S.; Tchanturia, K.; Himmerich, H.; Treasure, J. Hippocampal volume, function, and related molecular activity in anorexia nervosa: A scoping review. Expert Rev. Clin. Pharmacol. 2020, 13, 1367–1387. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, G.; Guzmán-Quevedo, O.; Aragão, R.D.S.; Bolaños-Jiménez, F. Early malnutrition results in long-lasting impairments in pattern-separation for overlapping novel object and novel location memories and reduced hippocampal neurogenesis. Sci. Rep. 2016, 6, 21275. [Google Scholar]
- Andrade, J.; Madeira, M.; Paula-Barbosa, M. Effects of long-term malnutrition and rehabilitation on the hippocampal formation of the adult rat. A morphometric study. J. Anat. 1995, 187, 379. [Google Scholar] [PubMed]
- Neves, G.; Cooke, S.F.; Bliss, T.V. Synaptic plasticity, memory and the hippocampus: A neural network approach to causality. Nat. Rev. Neurosci. 2008, 9, 65–75. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.-M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef]
- Roberto, C.A.; Mayer, L.E.; Brickman, A.M.; Barnes, A.; Muraskin, J.; Yeung, L.K.; Steffener, J.; Sy, M.; Hirsch, J.; Stern, Y. Brain tissue volume changes following weight gain in adults with anorexia nervosa. Int. J. Eat. Disord. 2011, 44, 406–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seitz, J.; Walter, M.; Mainz, V.; Herpertz-Dahlmann, B.; Konrad, K.; von Polier, G. Brain volume reduction predicts weight development in adolescent patients with anorexia nervosa. J. Psychiatr Res. 2015, 68, 228–237. [Google Scholar] [CrossRef]
- Barbarich-Marsteller, N.C.; Fornal, C.A.; Takase, L.F.; Bocarsly, M.E.; Arner, C.; Walsh, B.T.; Hoebel, B.G.; Jacobs, B.L. Activity-based anorexia is associated with reduced hippocampal cell proliferation in adolescent female rats. Behav. Brain Res. 2013, 236, 251–257. [Google Scholar] [CrossRef]
- Yang, C.; Yang, J.; Luo, A.; Hashimoto, K. Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites. Transl. Psychiatry 2019, 9, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdallah, C.G.; Jackowski, A.; Salas, R.; Gupta, S.; Sato, J.R.; Mao, X.; Coplan, J.D.; Shungu, D.C.; Mathew, S.J. The nucleus accumbens and ketamine treatment in major depressive disorder. Neuropsychopharmacology 2017, 42, 1739–1746. [Google Scholar] [CrossRef]
- Aleksandrova, L.R.; Phillips, A.G.; Wang, Y.T. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J. Psychiatry Neurosci. 2017, 42, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bomba, M.; Marfone, M.; Brivio, E.; Oggiano, S.; Broggi, F.; Neri, F.; Nacinovich, R. Autobiographical memory in adolescent girls with anorexia nervosa. Eur. Eat. Disord. Rev. 2014, 22, 479–486. [Google Scholar] [CrossRef]
- Huber, J.; Salatsch, C.; Ingenerf, K.; Schmid, C.; Maatouk, I.; Weisbrod, M.; Herzog, W.; Friederich, H.C.; Nikendei, C. Characteristics of disorder-related autobiographical memory in acute anorexia nervosa patients. Eur. Eat. Disord. Rev. 2015, 23, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Nikendei, C.; Funiok, C.; Pfüller, U.; Zastrow, A.; Aschenbrenner, S.; Weisbrod, M.; Herzog, W.; Friederich, H.-C. Memory performance in acute and weight-restored anorexia nervosa patients. Psychol. Med. 2011, 41, 829–838. [Google Scholar] [CrossRef]
- Kemps, E.; Tiggemann, M.; Wade, T.; Ben-Tovim, D.; Breyer, R. Selective working memory deficits in anorexia nervosa. Eur. Eat. Disord. Rev. 2006, 14, 97–103. [Google Scholar] [CrossRef]
- Keeler, J.; Lambert, E.; Olivola, M.; Owen, J.; Xia, J.; Thuret, S.; Himmerich, H.; Cardi, V.; Treasure, J. Lower pattern recognition memory scores in anorexia nervosa. J. Eat. Disord. 2021, 9, 49. [Google Scholar] [CrossRef]
- Godley, J.; Tchanturia, K.; MacLeod, A.; Schmidt, U. Future-directed thinking in eating disorders. Br. J. Clin. Psychol. 2001, 40, 281–295. [Google Scholar] [CrossRef]
- Manuel, A.; Wade, T.D. Emotion regulation in broadly defined anorexia nervosa: Association with negative affective memory bias. Behav. Res. Ther. 2013, 51, 417–424. [Google Scholar] [CrossRef]
- Schacter, D.L.; Addis, D.R. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 773–786. [Google Scholar] [CrossRef]
- Miles, S.; Gnatt, I.; Phillipou, A.; Nedeljkovic, M. Cognitive flexibility in acute anorexia nervosa and after recovery: A systematic review. Clin. Psychol. Rev. 2020, 81, 101905. [Google Scholar] [CrossRef]
- Morgan, C.J.; Curran, H.V. Acute and chronic effects of ketamine upon human memory: A review. Psychopharmacology 2006, 188, 408–424. [Google Scholar] [CrossRef]
- Veen, C.; Jacobs, G.; Philippens, I.; Vermetten, E. Subanesthetic dose ketamine in posttraumatic stress disorder: A role for reconsolidation during trauma-focused psychotherapy? In Behavioral Neurobiology of PTSD; Springer: Cham, Switzerland, 2018; pp. 137–162. [Google Scholar]
- Lambert, E.; Treasure, J.; Purves, K.L.; McGregor, T.; Bergou, N.; Kan, C.; Breen, G.; Eley, T.C.; Cardi, V. Fear conditioning in women with anorexia nervosa and healthy controls: A preliminary study. J. Abnorm. Psychol. 2021, 130, 490. [Google Scholar] [CrossRef] [PubMed]
- Cardi, V.; Matteo, R.D.; Corfield, F.; Treasure, J. Social reward and rejection sensitivity in eating disorders: An investigation of attentional bias and early experiences. World J. Biol. Psychiatry 2013, 14, 622–633. [Google Scholar] [CrossRef]
- Hasler, G. Toward specific ways to combine ketamine and psychotherapy in treating depression. CNS Spectr. 2020, 25, 445–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocker, K.; Hasler, G.; Hartmann, M. The altered-state-of-consciousness (ASC) aspect of a feeling of lightness is reported to be associated with antidepressant benefits by depressed individuals receiving ketamine infusions: A systematic analysis of internet video testimonials. Psychother. Psychosom. 2019, 88, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Mollaahmetoglu, M.; Keeler, J.; Ashbullby, K.J.; Argyri, E.K.; Grabski, M.; Morgan, C.J. “This is something that changed my life”: A qualitative study of patients’ experiences in a clinical trial of ketamine treatment for alcohol use disorders. Front. Psychiatry 2021, 12, 1356. [Google Scholar] [CrossRef]
- Ehrlich, S.; Geisler, D.; Ritschel, F.; King, J.A.; Seidel, M.; Boehm, I.; Breier, M.; Clas, S.; Weiss, J.; Marxen, M.; et al. Elevated cognitive control over reward processing in recovered female patients with anorexia nervosa. J. Psychiatry Neurosci. 2015, 40, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friederich, H.C.; Herzog, W. Cognitive-behavioral flexibility in anorexia nervosa. Behav. Neurobio. Eat. Disord. 2010, 6, 111–123. [Google Scholar]
- Rich, E. Anorexic dis (connection): Managing anorexia as an illness and an identity. Sociol. Health Illn. 2006, 28, 284–305. [Google Scholar] [CrossRef]
- Bowden, H. A phenomenological study of anorexia nervosa. Philos. Psychiatry Psychol. 2012, 19, 227–241. [Google Scholar]
- Skårderud, F. Eating one’s words, part I:‘concretised metaphors’ and reflective function in anorexia nervosa—An interview study. Eur. Eat. Disord. Rev. Prof. J. Eat. Disord. Assoc. 2007, 15, 163–174. [Google Scholar] [CrossRef]
- Olatunji, B.O.; Levinson, C.; Calebs, B. A network analysis of eating disorder symptoms and characteristics in an inpatient sample. Psychiatry Res. 2018, 262, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Tantillo, M.; Sanftner, J.; Hauenstein, E. Restoring connection in the face of disconnection: An integrative approach to understanding and treating anorexia nervosa. Adv. Eat. Disord. 2013, 1, 21–38. [Google Scholar] [CrossRef]
- Arkell, J.; Robinson, P. A pilot case series using qualitative and quantitative methods: Biological, psychological and social outcome in severe and enduring eating disorder (anorexia nervosa). Int. J. Eat. Disord. 2008, 41, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Dechant, E.; Boyle, B.; Ross, R.A. Ketamine in a Patient with Comorbid Anorexia and MDD. J. Womens Health Dev. 2020, 3, 373–375. [Google Scholar] [CrossRef]
- Mills, I.H.; Park, G.R.; Manara, A.R.; Merriman, R.J. Treatment of compulsive behaviour in eating disorders with intermittent ketamine infusions. QJM 1998, 91, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, T.; Trunko, M.E.; Feifel, D.; Lopez, E.; Peterson, D.; Frank, G.K.; Kaye, W. A longitudinal case series of IM ketamine for patients with severe and enduring eating disorders and comorbid treatment-resistant depression. Clin. Case Rep. 2021, 9, e03869. [Google Scholar] [CrossRef] [PubMed]
- Scolnick, B.; Zupec-Kania, B.; Calabrese, L.; Aoki, C.; Hildebrandt, T. Remission from Chronic Anorexia Nervosa With Ketogenic Diet and Ketamine: Case Report. Front. Psychiatry 2020, 11, 763. [Google Scholar] [CrossRef]
- Miller, K.K.; Grinspoon, S.K.; Ciampa, J.; Hier, J.; Herzog, D.; Klibanski, A. Medical findings in outpatients with anorexia nervosa. Arch. Intern. Med. 2005, 165, 561–566. [Google Scholar] [CrossRef] [Green Version]
- Noppers, I.M.; Niesters, M.; Aarts, L.P.; Bauer, M.C.; Drewes, A.M.; Dahan, A.; Sarton, E.Y. Drug-induced liver injury following a repeated course of ketamine treatment for chronic pain in CRPS type 1 patients: A report of 3 cases. Pain 2011, 152, 2173–2178. [Google Scholar] [CrossRef]
- Sear, J.W. Ketamine hepato-toxicity in chronic pain management: Another example of unexpected toxicity or a predicted result from previous clinical and pre-clinical data? Pain 2011, 152, 1946–1947. [Google Scholar] [CrossRef]
- Li, C.-C.; Wu, S.-T.; Cha, T.-L.; Sun, G.-H.; Yu, D.-S.; Meng, E. A survey for ketamine abuse and its relation to the lower urinary tract symptoms in Taiwan. Sci. Rep. 2019, 9, 7240. [Google Scholar] [CrossRef] [PubMed]
- Winstock, A.R.; Mitcheson, L.; Gillatt, D.A.; Cottrell, A.M. The prevalence and natural history of urinary symptoms among recreational ketamine users. BJU Int. 2012, 110, 1762–1766. [Google Scholar] [CrossRef] [PubMed]
- Shahani, R.; Streutker, C.; Dickson, B.; Stewart, R.J. Ketamine-associated ulcerative cystitis: A new clinical entity. Urology 2007, 69, 810–812. [Google Scholar] [CrossRef]
- Stheneur, C.; Bergeron, S.; Lapeyraque, A.-L. Renal complications in anorexia nervosa. Eat. Weight. Disord. -Stud. Anorex. Bulim. Obes. 2014, 19, 455–460. [Google Scholar] [CrossRef]
- Cockhill, L.A.; Remick, R.A. Blood pressure effects of monoamine oxidase inhibitors—The highs and lows. Can. J. Psychiatry 1987, 32, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D.; Krystal, J.H.; Putnam, F.W.; Southwick, S.M.; Marmar, C.; Charney, D.S.; Mazure, C.M. Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS). J. Trauma. Stress 1998, 11, 125–136. [Google Scholar] [CrossRef]
- Lahti, A.C.; Koffel, B.; LaPorte, D.; Tamminga, C.A. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 1995, 13, 9–19. [Google Scholar] [CrossRef]
- Dessain, A.; Bentley, J.; Treasure, J.; Schmidt, U.; Himmerich, H. Patients’ and Carers’ perspectives of psychopharmacological interventions targeting anorexia nervosa symptoms. In Anorexia and Bulimia Nervosa; IntechOpen: London, UK, 2019; p. 103. [Google Scholar]
- Harding, F.; Seynaeve, M.; Keeler, J.; Himmerich, H.; Treasure, J.; Kan, C. Perspectives on psychedelic treatment and research in eating disorders: A web-based questionnaire study of people with eating disorders. J. Int. Neurosci. 2021, 20, 551–560. [Google Scholar]
- Dalton, B.; Lewis, Y.D.; Bartholdy, S.; Kekic, M.; McClelland, J.; Campbell, I.C.; Schmidt, U. Repetitive transcranial magnetic stimulation treatment in severe, enduring anorexia nervosa: An open longer-term follow-up. Eur. Eat. Disord. Rev. 2020, 28, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Arroteia, I.F.; Husch, A.; Baniasadi, M.; Hertel, F. Impressive weight gain after deep brain stimulation of nucleus accumbens in treatment-resistant bulimic anorexia nervosa. BMJ Case Rep. 2020, 13, e239316. [Google Scholar] [CrossRef] [PubMed]
- Lipsman, N.; Lam, E.; Volpini, M.; Sutandar, K.; Twose, R.; Giacobbe, P.; Sodums, D.J.; Smith, G.S.; Woodside, D.B.; Lozano, A.M. Deep brain stimulation of the subcallosal cingulate for treatment-refractory anorexia nervosa: 1 year follow-up of an open-label trial. Lancet Psychiatry 2017, 4, 285–294. [Google Scholar] [CrossRef]
- Krystal, J.H.; Abdallah, C.G.; Sanacora, G.; Charney, D.S.; Duman, R.S. Ketamine: A paradigm shift for depression research and treatment. Neuron 2019, 101, 774–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazdin, A.E. Mediators and mechanisms of change in psychotherapy research. Annu. Rev. Clin. Psychol. 2007, 3, 1–27. [Google Scholar] [CrossRef] [Green Version]

| Parameter | Administration Route | ||
|---|---|---|---|
| Intravenous | Sublingual | Oral | |
| Dosage (mg) | 10 | 25 | 25 |
| Cmax (μg/L) M ± SD | 156 ± 161 | 28.6 ± 6.6 | 22.8 ± 12.8 |
| Tmax (h) M ± SD | 0.24 ± 0.29 | 0.76 ± 0.51 | 0.96 ± 0.8 |
| AUC/dose (μg.h/L.mg) M ± SD | 13.4 ± 2.4 | 4.0 ± 1.9 | 3.1 ± 0.7 |
| Study [Ref] | Study Design | N | Diagnosis | Administration Route | Dosage | Main Findings |
|---|---|---|---|---|---|---|
| Dechant et al. [171] | Case study | 1 | SE-AN and MDD | IV R-Ketamine | 9 × 0.5 mg/kg over 40 min | Reduction in depression and suicidality. |
| Mills et al. [172] | Case series | 15 | SE-AN | IV Ketamine | 2–15 × 20 mg/h for 10 h | 9/15 responded to treatment, with reductions in depression. and compulsive starving/eating. |
| Schwartz et al. [173] | Case series | 4 | SE-ED and TRD | IM/IV Ketamine | 5–9 × 0.4–0.5 mg/kg | Improvements in depression, anxiety and eating disorder psychopathology over approx. days. |
| Scolnick et al. [174] | Case study | 1 | SE-AN and MDD | IV R-Ketamine | 4 × 0.75 mg/kg over 40 min | Reduction in “anorexic voice” and depression and full and sustained remission. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keeler, J.L.; Treasure, J.; Juruena, M.F.; Kan, C.; Himmerich, H. Ketamine as a Treatment for Anorexia Nervosa: A Narrative Review. Nutrients 2021, 13, 4158. https://doi.org/10.3390/nu13114158
Keeler JL, Treasure J, Juruena MF, Kan C, Himmerich H. Ketamine as a Treatment for Anorexia Nervosa: A Narrative Review. Nutrients. 2021; 13(11):4158. https://doi.org/10.3390/nu13114158
Chicago/Turabian StyleKeeler, Johanna Louise, Janet Treasure, Mario F. Juruena, Carol Kan, and Hubertus Himmerich. 2021. "Ketamine as a Treatment for Anorexia Nervosa: A Narrative Review" Nutrients 13, no. 11: 4158. https://doi.org/10.3390/nu13114158
APA StyleKeeler, J. L., Treasure, J., Juruena, M. F., Kan, C., & Himmerich, H. (2021). Ketamine as a Treatment for Anorexia Nervosa: A Narrative Review. Nutrients, 13(11), 4158. https://doi.org/10.3390/nu13114158