Blueberry Counteracts Prediabetes in a Hypercaloric Diet-Induced Rat Model and Rescues Hepatic Mitochondrial Bioenergetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blueberry Juice Preparation
2.2. Phytochemical Screening of Blueberry Juice
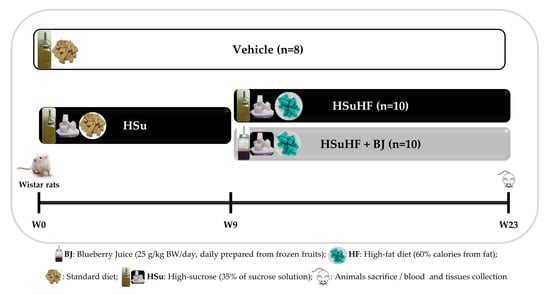
2.3. Animals and Experimental Design
2.4. Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
2.5. In Vivo Hepatic Ultrasonographic Analysis
2.6. Biological Sample Collection
2.7. Determination of Serum Metabolic Parameters
2.8. Evaluation of Serum Redox Status
2.9. 1H Nuclear Magnetic Resonance (NMR) Spectroscopy
2.10. Extraction and Quantification of Gut Microbiota in Feces
2.10.1. DNA Extraction from Stool
2.10.2. Real-Time PCR for Fecal Microbiota Analysis
2.10.3. Quantification of Fecal Short-Chain Fatty Acids (SCFAs) and Organic Acids
2.11. Evaluation of Intestinal Permeability Using FITC-Dextran
2.12. Quantification of Serum Lipopolysaccharide (LPS) Concentration
2.13. Colon and Duodenum Analysis by Transmission Electron Microscopy (TEM)
2.14. Immunohistochemical Staining
2.15. Hepatic Histological Analysis
2.16. Hepatic Triglycerides Quantification
2.17. Hepatic SEM Analysis
2.18. Hepatic Mitochondria Bioenergetics
2.18.1. Mitochondrial Membrane Potential (ΔΨ)
2.18.2. Mitochondrial Respiration (Oxygen Consumption) and Permeability Transition (MPT)
2.19. Gene Expression by Quantitative Real-Time PCR Analysis
2.20. Statistical Analysis
3. Results
3.1. Phenolic Composition of Blueberry Juice
3.2. Effects of BJ on Body Weight and Caloric Intake in HSuHF-Fed Rats
3.3. Effects of BJ on the Glycemic and Insulinemic Profile in HSuHF-Fed Rats
3.4. Effects of BJ on the Serum Lipid Profile and Redox Status Markers in HSuHF-Fed Rats
3.5. Effects of BJ on Gut Microbiota Composition and Intestinal Integrity in HSuHF-Fed Rats
3.6. Effects of BJ on Hepatic Lipid Steatosis in HSuHF-Fed Rats
3.7. Impact of BJ on Hepatic Mitochondrial Function in HSuHF Rats
3.8. Effects of BJ on Hepatic mRNA Expression of Inflammation-Related Genes
3.9. Effects of BJ on Hepatic mRNA Expression of Glucose Metabolism and Insulin Signaling-Related Genes
3.10. Effects of BJ on the Hepatic mRNA Expression of Lipid Metabolism-Associated Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathophysiology of prediabetes. Curr. Diabetes Rep. 2009, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.M.; Cusi, K. Prediabetes. Endocrinol. Metab. Clin. N. Am. 2016, 45, 751–764. [Google Scholar] [CrossRef]
- Hostalek, U. Global epidemiology of prediabetes—Present and future perspectives. Clin. Diabetes Endocrinol. 2019, 5, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.; Kivimaki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C.; Chen, Y.; Ji, L. Human Gut Microbiota Changes Reveal the Progression of Glucose Intolerance. PLoS ONE 2013, 8, e71108. [Google Scholar] [CrossRef]
- Wu, H.; Tremaroli, V.; Schmidt, C.; Lundqvist, A.; Olsson, L.M.; Krämer, M.; Gummesson, A.; Perkins, R.; Bergström, G.; Bäckhed, F. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab. 2020, 32, 379–390.e3. [Google Scholar] [CrossRef]
- Allin, K.H.; The IMI-DIRECT Consortium; Tremaroli, V.; Caesar, R.; Jensen, B.; Damgaard, M.T.F.; Bahl, M.I.; Licht, T.R.; Hansen, T.; Nielsen, T.; et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia 2018, 61, 810–820. [Google Scholar] [CrossRef] [Green Version]
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [Green Version]
- Ferrannini, E.; Gastaldelli, A.; Iozzo, P. Pathophysiology of Prediabetes. Med. Clin. N. Am. 2011, 95, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Burgeiro, A.; Cerqueira, M.; Varela-Rodríguez, B.M.; Nunes, S.; Neto, P.; Pereira, F.C.; Reis, F.; Carvalho, E. Glucose and Lipid Dysmetabolism in a Rat Model of Prediabetes Induced by a High-Sucrose Diet. Nutrients 2017, 9, 638. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alimujiang, M.; Yu, X.; Yu, M.; Hou, W.; Yan, Z.; Yang, Y.; Bao, Y.; Yin, J. Enhanced liver but not muscle OXPHOS in diabetes and reduced glucose output by complex I inhibition. J. Cell. Mol. Med. 2020, 24, 5758–5771. [Google Scholar] [CrossRef]
- Zheng, L.D.; Linarelli, L.E.; Brooke, J.; Smith, C.; Wall, S.S.; Greenawald, M.H.; Seidel, R.W.; Estabrooks, P.A.; Almeida, F.A.; Cheng, Z. Mitochondrial Epigenetic Changes Link to Increased Diabetes Risk and Early-Stage Prediabetes Indicator. Oxidative Med. Cell. Longev. 2016, 2016, 5290638. [Google Scholar] [CrossRef] [PubMed]
- Rabøl, R.; Boushel, R.; Dela, F. Mitochondrial oxidative function and type 2 diabetes. Appl. Physiol. Nutr. Metab. 2006, 31, 675–683. [Google Scholar] [CrossRef]
- Rector, R.S.; Thyfault, J.; Uptergrove, G.M.; Morris, E.M.; Naples, S.P.; Borengasser, S.J.; Mikus, C.R.; Laye, M.; Laughlin, M.H.; Booth, F.W.; et al. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J. Hepatol. 2010, 52, 727–736. [Google Scholar] [CrossRef] [Green Version]
- Sergi, D.; Naumovski, N.N.; Heilbronn, L.H.K.; Abeywardena, M.; O’Callaghan, N.; Lionetti, L.; Luscombe-Marsh, N.L.-M. Mitochondrial (Dys)function and Insulin Resistance: From Pathophysiological Molecular Mechanisms to the Impact of Diet. Front. Physiol. 2019, 10, 532. [Google Scholar] [CrossRef]
- Saltevo, J.; Kautiainen, H.; Vanhala, M. Gender differences in adiponectin and low-grade inflammation among individuals with normal glucose tolerance, prediabetes, and type 2 diabetes. Gend. Med. 2009, 6, 463–470. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and dia-betes. J. Physiol. Pharmacol. 2019, 70, 70. [Google Scholar] [CrossRef]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- Lam, D.W.; LeRoith, D. The worldwide diabetes epidemic. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 93–96. [Google Scholar] [CrossRef]
- Wilson, M.L. Prediabetes. Nurs. Clin. N. Am. 2017, 52, 665–677. [Google Scholar] [CrossRef]
- Glechner, A.; Keuchel, L.; Affengruber, L.; Titscher, V.; Sommer, I.; Matyas, N.; Wagner, G.; Kien, C.; Klerings, I.; Gartlehner, G. Effects of lifestyle changes on adults with prediabetes: A systematic review and meta-analysis. Prim. Care Diabetes 2018, 12, 393–408. [Google Scholar] [CrossRef]
- Basiak-Rasała, A.; Różańska, D.; Zatońska, K. Food groups in dietary prevention of type 2 diabetes. Rocz. Państwowego Zakładu Hig. 2019, 70, 347–357. [Google Scholar] [CrossRef]
- Asif, M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J. Educ. Healh Promot. 2014, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-Y.; Fang, J.-C.; Gao, Z.-H.; Zhang, C.; Xie, S.-Y. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. J. Diabetes Investig. 2016, 7, 56–69. [Google Scholar] [CrossRef]
- McMacken, M.; Shah, S. A plant-based diet for the prevention and treatment of type 2 diabetes. J. Geriatr. Cardiol. 2017, 14, 342–354. [Google Scholar] [CrossRef]
- Yingli, F.; Fan, Y.; Zhang, X.; Hou, W.; Tang, Z. Fruit and vegetable intake and risk of type 2 diabetes mellitus: Meta-analysis of prospective cohort studies. BMJ Open 2014, 4, e005497. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Sun, T.-Y.; He, Y.; Gou, W.; Zuo, L.-S.; Fu, Y.; Miao, Z.; Shuai, M.; Xu, F.; Xiao, C.; et al. Dietary fruit and vegetable intake, gut microbiota, and type 2 diabetes: Results from two large human cohort studies. BMC Med. 2020, 18, 371. [Google Scholar] [CrossRef]
- Cooper, A.J.; Sharp, S.J.; Lentjes, M.A.; Luben, R.N.; Khaw, K.-T.; Wareham, N.J.; Forouhi, N.G. A Prospective Study of the Association Between Quantity and Variety of Fruit and Vegetable Intake and Incident Type 2 Diabetes. Diabetes Care 2012, 35, 1293–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stull, A.J. Blueberries’ Impact on Insulin Resistance and Glucose Intolerance. Antioxidants 2016, 5, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, S.; Costa, E.M.; Veiga, M.; Morais, R.M.; Calhau, C.; Pintado, M. Health promoting properties of blueberries: A review. Crit. Rev. Food Sci. Nutr. 2018, 60, 181–200. [Google Scholar] [CrossRef]
- Nunes, S.; Vieira, P.; Gomes, P.; Viana, S.; Reis, F. Blueberry as an Attractive Functional Fruit to Prevent (Pre)Diabetes Progression. Antioxidants 2021, 10, 1162. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [Green Version]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bujor, O.-C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal variations of the phenolic constituents in bilberry (Vaccinium myrtillus L.) leaves, stems and fruits, and their antioxidant activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Loftus, H.; McAinch, A.; Su, X.Q. Blueberry as a source of bioactive compounds for the treatment of obesity, type 2 diabetes and chronic inflammation. J. Funct. Foods 2017, 30, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Lyons, T.J. Strawberries, Blueberries, and Cranberries in the Metabolic Syndrome: Clinical Perspectives. J. Agric. Food Chem. 2011, 60, 5687–5692. [Google Scholar] [CrossRef]
- Nunes, S.; Viana, S.D.; Preguiça, I.; Alves, A.; Fernandes, R.; Teodoro, J.S.; Figueirinha, A.; Salgueiro, L.; Silva, S.; Jarak, I.; et al. Blueberry Consumption Challenges Hepatic Mitochondrial Bioenergetics and Elicits Transcriptomics Reprogramming in Healthy Wistar Rats. Pharmaceutics 2020, 12, 1094. [Google Scholar] [CrossRef] [PubMed]
- Couto, J.; Figueirinha, A.; Batista, M.T.; Paranhos, A.; Nunes, C.; Gonçalves, L.M.; Marto, J.; Fitas, M.; Pinto, P.; Ribeiro, H.M.; et al. Fragaria vesca L. Extract: A Promising Cosmetic Ingredient with Antioxidant Properties. Antioxidants 2020, 9, 154. [Google Scholar] [CrossRef] [Green Version]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Nunes, S.; Alves, A.; Preguiça, I.; Barbosa, A.; Vieira, P.; Mendes, F.; Martins, D.; Viana, S.D.; Reis, F. Crescent-Like Lesions as an Early Signature of Nephropathy in a Rat Model of Prediabetes Induced by a Hypercaloric Diet. Nutrients 2020, 12, 881. [Google Scholar] [CrossRef] [Green Version]
- Conde, S.V.; Da Silva, T.N.; Gonzalez, C.; Carmo, M.M.; Monteiro, E.C.; Guarino, M.P. Chronic caffeine intake decreases circulating catecholamines and prevents diet-induced insulin resistance and hypertension in rats. Br. J. Nutr. 2011, 107, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, A.C.; Cambri, L.; Botezelli, J.D.; Ribeiro, C.; Dalia, R.A.; De Mello, M.A.R. Metabolic syndrome markers in wistar rats of different ages. Diabetol. Metab. Syndr. 2012, 4, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lessa, A.S.; Paredes, B.D.; Dias, J.V.; Carvalho, A.B.; Quintanilha, L.F.; Takiya, C.M.; Tura, B.R.; Rezende, G.F.; de Carvalho, A.C.C.; MC Resende, C.; et al. Ultrasound imaging in an experimental model of fatty liver disease and cirrhosis in rats. BMC Veter- Res. 2010, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Rodríguez, M.A.; Mendoza-Núñez, V.M. Oxidative Stress Indexes for Diagnosis of Health or Disease in Humans. Oxidative Med. Cell. Longev. 2019, 2019, 4128152. [Google Scholar] [CrossRef]
- Jarak, I.; Almeida, S.; Carvalho, R.; Sousa, M.; Barros, A.; Alves, M.G.; Oliveira, P.F. Senescence and declining reproductive potential: Insight into molecular mechanisms through testicular metabolomics. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 3388–3396. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef]
- Cloarec, O.; Dumas, M.-E.; Craig, A.; Barton, R.H.; Trygg, J.; Hudson, J.; Blancher, C.; Gauguier, D.; Lindon, J.C.; Holmes, A.E.; et al. Statistical Total Correlation Spectroscopy: An Exploratory Approach for Latent Biomarker Identification from Metabolic 1H NMR Data Sets. Anal. Chem. 2005, 77, 1282–1289. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Savorani, F.; Tomasi, G.; Engelsen, S.B. icoshift: A versatile tool for the rapid alignment of 1D NMR spectra. J. Magn. Reson. 2010, 202, 190–202. [Google Scholar] [CrossRef]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. BioSyst. 2014, 11, 13–19. [Google Scholar] [CrossRef]
- Reis, F.; Madureira, A.R.; Nunes, S.; Campos, D.A.; Fernandes, J.C.; Marques, C.; Zuzarte, M.; Gullón, B.; Rodríguez-Alcalá, L.M.; Calhau, C.; et al. Safety profile of solid lipid nanoparticles loaded with rosmarinic acid for oral use: In vitro and animal approaches. Int. J. Nanomed. 2016, 11, 3621–3640. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.-B.; Jia, L.; Yan, Q.-Q.; Deng, Q.; Wei, B. Effect of Clostridium butyricum and Butyrate on Intestinal Barrier Functions: Study of a Rat Model of Severe Acute Pancreatitis With Intra-Abdominal Hypertension. Front. Physiol. 2020, 11, 561061. [Google Scholar] [CrossRef]
- Kumar, A.; Lawrence, J.C.; Jung, D.Y.; Ko, H.J.; Keller, S.R.; Kim, J.K.; Magnuson, M.A.; Harris, T.E. Fat Cell-Specific Ablation of Rictor in Mice Impairs Insulin-Regulated Fat Cell and Whole-Body Glucose and Lipid Metabolism. Diabetes 2010, 59, 1397–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, M.; Mera, P.; Casas, J.; Salvador, J.; Rodríguez, A.; Alonso, S.; Sebastián, D.; Soler-Vázquez, M.C.; Montironi, C.; Recalde, S.; et al. Liver CPT1A gene therapy reduces diet-induced hepatic steatosis in mice and highlights potential lipid biomarkers for human NAFLD. FASEB J. 2020, 34, 11816–11837. [Google Scholar] [CrossRef] [PubMed]
- Rolo, A.P.; Oliveira, P.J.; Moreno, A.J.M.; Palmeira, C.M. Bile acids affect liver mitochondrial bioenergetics: Possible relevance for cholestasis therapy. Toxicol. Sci. 2000, 57, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Varela, A.T.; Simões, A.M.; Teodoro, J.S.; Duarte, F.V.; Gomes, A.P.; Palmeira, C.M.; Rolo, A.P. Indirubin-3′-oxime prevents hepatic I/R damage by inhibiting GSK-3β and mitochondrial permeability transition. Mitochondrion 2010, 10, 456–463. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Teodoro, J.; Duarte, F.V.; Gomes, A.P.; Varela, A.; Peixoto, F.; Rolo, A.; Palmeira, C. Berberine reverts hepatic mitochondrial dysfunction in high-fat fed rats: A possible role for SirT3 activation. Mitochondrion 2013, 13, 637–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2018, 64, 88–100. [Google Scholar] [CrossRef]
- Prior, R.L.; Wilkes, S.E.; Rogers, T.R.; Khanal, R.C.; Wu, X.; Howard, L.R. Purified Blueberry Anthocyanins and Blueberry Juice Alter Development of Obesity in Mice Fed an Obesogenic High-Fat Diet. J. Agric. Food Chem. 2010, 58, 3970–3976. [Google Scholar] [CrossRef]
- Song, Y.; Park, H.J.; Kang, S.N.; Jang, S.-H.; Lee, S.-J.; Ko, Y.-G.; Kim, G.-S.; Cho, J.-H. Blueberry Peel Extracts Inhibit Adipogenesis in 3T3-L1 Cells and Reduce High-Fat Diet-Induced Obesity. PLoS ONE 2013, 8, e69925. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Keirsey, K.I.; Kirkland, R.; Grunewald, Z.I.; Fischer, J.G.; De La Serre, C.B. Blueberry Supplementation Influences the Gut Microbiota, Inflammation, and Insulin Resistance in High-Fat-Diet–Fed Rats. J. Nutr. 2018, 148, 209–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Daza, M.C.; Daoust, L.; Boutkrabt, L.; Pilon, G.; Varin, T.; Dudonné, S.; Levy, É.; Marette, A.; Roy, D.; Desjardins, Y. Wild blueberry proanthocyanidins shape distinct gut microbiota profile and influence glucose homeostasis and intestinal phenotypes in high-fat high-sucrose fed mice. Sci. Rep. 2020, 10, 2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seymour, E.M.; Tanone, I.I.; Urcuyo-Llanes, D.E.; Lewis, S.K.; Kirakosyan, A.; Kondoleon, M.G.; Kaufman, P.B.; Bolling, S.F. Blueberry Intake Alters Skeletal Muscle and Adipose Tissue Peroxisome Proliferator-Activated Receptor Activity and Reduces Insulin Resistance in Obese Rats. J. Med. Food 2011, 14, 1511–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benton, D.; Young, H.A. Role of fruit juice in achieving the 5-a-day recommendation for fruit and vegetable intake. Nutr. Rev. 2019, 77, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.K.H.Y.; Ferruzzi, M.G.; Wightman, J.D. Potential health benefits of (poly)phenols derived from fruit and 100% fruit juice. Nutr. Rev. 2019, 78, 145–174. [Google Scholar] [CrossRef] [PubMed]
- Stribiţcaia, E.; Evans, C.E.L.; Gibbons, C.; Blundell, J.; Sarkar, A. Food texture influences on satiety: Systematic review and meta-analysis. Sci. Rep. 2020, 10, 12929. [Google Scholar] [CrossRef]
- Preguiça, I.; Alves, A.; Nunes, S.; Fernandes, R.; Gomes, P.; Viana, S.D.; Reis, F. Diet-induced rodent models of obesity-related metabolic disorders—A guide to a translational perspective. Obes. Rev. 2020, 21, e13081. [Google Scholar] [CrossRef]
- DeFuria, J.; Bennett, G.; Strissel, K.J.; Perfield, J.W.; Milbury, P.E.; Greenberg, A.S.; Obin, M.S. Dietary Blueberry Attenuates Whole-Body Insulin Resistance in High Fat-Fed Mice by Reducing Adipocyte Death and Its Inflammatory Sequelae. J. Nutr. 2009, 139, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Mykkänen, O.T.; Huotari, A.; Herzig, K.-H.; Dunlop, T.W.; Mykkänen, H.; Kirjavainen, P.V. Wild Blueberries (Vaccinium myrtillus) Alleviate Inflammation and Hypertension Associated with Developing Obesity in Mice Fed with a High-Fat Diet. PLoS ONE 2014, 9, e114790. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Kuhn, P.; Rojo, L.E.; Lila, M.A.; Raskin, I. Blueberry polyphenol-enriched soybean flour reduces hyperglycemia, body weight gain and serum cholesterol in mice. Pharmacol. Res. 2012, 68, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elks, C.M.; Terrebonne, J.D.; Ingram, D.K.; Stephens, J.M. Blueberries improve glucose tolerance without altering body composition in obese postmenopausal mice. Obesity 2015, 23, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Ren, T.; Huang, C.; Cheng, M. Dietary Blueberry and Bifidobacteria Attenuate Nonalcoholic Fatty Liver Disease in Rats by Affecting SIRT1-Mediated Signaling Pathway. Oxidative Med. Cell. Longev. 2014, 2014, 469059. [Google Scholar] [CrossRef]
- Si, X.; Tian, J.; Shu, C.; Wang, Y.; Gong, E.; Zhang, Y.; Zhang, W.; Cui, H.; Li, B. Serum Ceramide Reduction by Blueberry Anthocyanin-Rich Extract Alleviates Insulin Resistance in Hyperlipidemia Mice. J. Agric. Food Chem. 2020, 68, 8185–8194. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.; Ribnicky, D.M.; Kuhn, P.; Poulev, A.; Logendra, S.; Yousef, G.G.; Raskin, I.; Lila, M.A. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine 2009, 16, 406–415. [Google Scholar] [CrossRef] [Green Version]
- Norberto, S.; Silva, S.; Meireles, M.; Faria, A.; Pintado, M.M.; Calhau, C. Blueberry anthocyanins in health promotion: A metabolic overview. J. Funct. Foods 2013, 5, 1518–1528. [Google Scholar] [CrossRef]
- Vuong, T.; Benhaddou-Andaloussi, A.; Brault, A.; Harbilas, D.; Martineau, L.C.; Vallerand, D.; Ramassamy, C.; Matar, C.; Haddad, P.S. Antiobesity and antidiabetic effects of biotransformed blueberry juice in KKAy mice. Int. J. Obes. 2009, 33, 1166–1173. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.E.; Kelly, M.F. Inhibition of lipid peroxidation by anthocyanins, anthocyanidins and their phenolic degradation products. Eur. J. Lipid Sci. Technol. 2007, 109, 66–71. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified Anthocyanin Supplementation Reduces Dyslipidemia, Enhances Antioxidant Capacity, and Prevents Insulin Resistance in Diabetic Patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef]
- Pranprawit, A.; Heyes, J.A.; Molan, A.L.; Kruger, M.C. Antioxidant Activity and Inhibitory Potential of Blueberry Extracts Against Key Enzymes Relevant for Hyperglycemia. J. Food Biochem. 2015, 39, 109–118. [Google Scholar] [CrossRef]
- Wu, T.; Gao, Y.; Guo, X.; Zhang, M.; Gong, L. Blackberry and Blueberry Anthocyanin Supplementation Counteract High-Fat-Diet-Induced Obesity by Alleviating Oxidative Stress and Inflammation and Accelerating Energy Expenditure. Oxidative Med. Cell. Longev. 2018, 2018, 4051232. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Viana, S.; Nunes, S.R.R.P.; Reis, F. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1865, 1876–1897. [Google Scholar] [CrossRef] [PubMed]
- Guirro, M.; Costa, A.; Gual-Grau, A.; Herrero, P.; Torrell, H.; Canela, N.; Arola, L. Effects from diet-induced gut microbiota dysbiosis and obesity can be ameliorated by fecal microbiota transplantation: A multiomics approach. PLoS ONE 2019, 14, e0218143. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W.; Mao, Y.; Zhang, X.; Pang, X.; Wei, C.; et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2009, 4, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Cabral, C.; Kumar, R.; Ganguly, R.; Rana, H.K.; Gupta, A.; Lauro, M.R.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Daza, M.-C.; Roquim, M.; Dudonné, S.; Pilon, G.; Levy, E.; Marette, A.; Roy, D.; Desjardins, Y. Berry Polyphenols and Fibers Modulate Distinct Microbial Metabolic Functions and Gut Microbiota Enterotype-Like Clustering in Obese Mice. Front. Microbiol. 2020, 11, 2032. [Google Scholar] [CrossRef]
- Hereu, M.; Ramos-Romero, S.; Busquets, C.; Atienza, L.; Amézqueta, S.; Miralles-Pérez, B.; Nogués, M.R.; Méndez, L.; Medina, I.; Torres, J.L. Effects of combined D-fagomine and omega-3 PUFAs on gut microbiota subpopulations and diabetes risk factors in rats fed a high-fat diet. Sci. Rep. 2019, 9, 16628. [Google Scholar] [CrossRef]
- Gual-Grau, A.; Guirro, M.; Mayneris-Perxachs, J.; Arola, L.; Boqué, N. Impact of different hypercaloric diets on obesity features in rats: A metagenomics and metabolomics integrative approach. J. Nutr. Biochem. 2019, 71, 122–131. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Shah, V.O.; Lambeth, S.M.; Carson, T.; Lowe, J.; Ramaraj, T.; Leff, J.W.; Luo, L.; Bell, C.J. Composition Diversity and Abundance of Gut Microbiome in Prediabetes and Type 2 Diabetes. J. Diabetes Obes. 2015, 2, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, W.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Liu, R.-X.; Hong, J. Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J. Mol. Endocrinol. 2017, 58, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ni, Y.; Qian, L.; Fang, Q.; Zheng, T.; Zhang, M.; Gao, Q.; Ni, J.; Hou, X.; Bao, Y.; et al. Decreased Abundance of Akkermansia muciniphila Leads to the Impairment of Insulin Secretion and Glucose Homeostasis in Lean Type 2 Diabetes. Adv. Sci. 2021, 8, 2100536. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Wang, Y.; Dilidaxi, D.; Wu, Y.; Sailike, J.; Sun, X.; Nabi, X.-H.; Wang, Y.; Dilidaxi, D.; Wu, Y.; Sailike, J.; et al. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed. Pharmacother. 2020, 125, 109914. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Han, X.; Tan, H.; Huang, W.; You, Y.; Zhan, J. Blueberry Extract Improves Obesity through Regulation of the Gut Microbiota and Bile Acids via Pathways Involving FXR and TGR5. iScience 2019, 19, 676–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ntemiri, A.; Ghosh, T.; Gheller, M.; Tran, T.; Blum, J.; Pellanda, P.; Vlckova, K.; Neto, M.; Howell, A.; Thalacker-Mercer, A.; et al. Whole Blueberry and Isolated Polyphenol-Rich Fractions Modulate Specific Gut Microbes in an In Vitro Colon Model and in a Pilot Study in Human Consumers. Nutrients 2020, 12, 2800. [Google Scholar] [CrossRef]
- Si, X.; Bi, J.; Chen, Q.; Cui, H.; Bao, Y.; Tian, J.; Shu, C.; Wang, Y.; Tan, H.; Zhang, W.; et al. Effect of Blueberry Anthocyanin-Rich Extracts on Peripheral and Hippocampal Antioxidant Defensiveness: The Analysis of the Serum Fatty Acid Species and Gut Microbiota Profile. J. Agric. Food Chem. 2021, 69, 3658–3666. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, F.; Hong, Z.; Wang, S.; Jinjuan, Z.; Han, B.; Xie, R.; Leng, F.; Yang, Q. Blueberry Attenuates Liver Fibrosis, Protects Intestinal Epithelial Barrier, and Maintains Gut Microbiota Homeostasis. Can. J. Gastroenterol. Hepatol. 2019, 2019, 5236149. [Google Scholar] [CrossRef] [Green Version]
- De Vadder, F.; Kovatcheva-Datchary, P.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016, 24, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cladis, D.P.; Simpson, A.M.R.; Cooper, K.J.; Nakatsu, C.H.; Ferruzzi, M.G.; Weaver, C.M. Blueberry polyphenols alter gut microbiota & phenolic metabolism in rats. Food Funct. 2021, 12, 2442–2456. [Google Scholar] [CrossRef]
- Gwiazdowska, D.; Juś, K.; Jasnowska-Małecka, J.; Kluczyńska, K. The impact of polyphenols on Bifidobacterium growth. Acta Biochim. Pol. 2015, 62, 895–901. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Hegde, A.; Yadav, C.; Ahmad, A.; Manjrekar, P.A.; Srikantiah, R.M. Assessment of oxidative stress and inflammation in prediabetes—A hospital based cross-sectional study. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S123–S126. [Google Scholar] [CrossRef]
- Fiorentino, T.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced Oxidative Stress and its Role in Diabetes Mellitus Related Cardiovascular Diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef] [PubMed]
- Jong, C.J.; Azuma, J.; Schaffer, S. Mechanism underlying the antioxidant activity of taurine: Prevention of mitochondrial oxidant production. Amino Acids 2011, 42, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Kim, H.W. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Azuma, J.; Mozaffari, M. Role of antioxidant activity of taurine in diabetesThis article is one of a selection of papers from the NATO Advanced Research Workshop on Translational Knowledge for Heart Health (published in part 1 of a 2-part Special Issue). Can. J. Physiol. Pharmacol. 2009, 87, 91–99. [Google Scholar] [CrossRef]
- Day, C.; Kempson, S.A. Betaine chemistry, roles, and potential use in liver disease. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 1098–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeisel, S.H.; Mar, M.-H.; Howe, J.C.; Holden, J.M. Concentrations of Choline-Containing Compounds and Betaine in Common Foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta (BBA)-Bioenerg. 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Serkova, N.J.; Jackman, M.; Brown, J.L.; Liu, T.; Hirose, R.; Roberts, J.P.; Maher, J.J.; Niemann, C.U. Metabolic profiling of livers and blood from obese Zucker rats. J. Hepatol. 2006, 44, 956–962. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, J.H.; Noh, S.; Hur, H.J.; Sung, M.J.; Hwang, J.-T.; Park, J.H.; Yang, H.J.; Kim, M.-S.; Kwon, D.Y.; et al. Metabolomic Analysis of Livers and Serum from High-Fat Diet Induced Obese Mice. J. Proteome Res. 2010, 10, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Kathirvel, E.; Morgan, K.; Nandgiri, G.; Sandoval, B.C.; Caudill, M.A.; Bottiglieri, T.; French, S.W.; Morgan, T.R. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: A potential mechanism for hepatoprotection by betaine. Am. J. Physiol. Liver Physiol. 2010, 299, G1068–G1077. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-J.; Zhang, H.-W.; Zhou, J.-Y.; Liu, Y.; Yang, Y.; Chen, X.-L.; Zhu, C.-H.; Zheng, R.-D.; Ling, W.-H.; Zhu, H.-L. Betaine attenuates hepatic steatosis by reducing methylation of the MTTP promoter and elevating genomic methylation in mice fed a high-fat diet. J. Nutr. Biochem. 2014, 25, 329–336. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.-W.; Wang, L.-K.; Li, X.; Zhang, H.; Luo, L.-P.; Song, J.-C.; Gong, Z.-J. Betaine Protects Against High-Fat-Diet-Induced Liver Injury by Inhibition of High-Mobility Group Box 1 and Toll-Like Receptor 4 Expression in Rats. Dig. Dis. Sci. 2013, 58, 3198–3206. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, T.; Pini, M.; Zhou, Z.; Fantuzzi, G.; Song, Z. Betaine improved adipose tissue function in mice fed a high-fat diet: A mechanism for hepatoprotective effect of betaine in nonalcoholic fatty liver disease. Am. J. Physiol. Liver Physiol. 2010, 298, G634–G642. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Li, S.; Na, L.; Feng, R.; Liu, L.; Li, Y.; Sun, C. Mangiferin Decreases Plasma Free Fatty Acids through Promoting Its Catabolism in Liver by Activation of AMPK. PLoS ONE 2012, 7, e30782. [Google Scholar] [CrossRef] [Green Version]
- Tranchida, F.; Rakotoniaina, Z.; Shintu, L.; Tchiakpe, L.; Deyris, V.; Yemloul, M.; Stocker, P.; Vidal, N.; Rimet, O.; Hiol, A.; et al. Hepatic metabolic effects of Curcuma longa extract supplement in high-fructose and saturated fat fed rats. Sci. Rep. 2017, 7, 5880. [Google Scholar] [CrossRef] [Green Version]
- Crescenzo, R.; Bianco, F.; Mazzoli, A.; Giacco, A.; Liverini, G.; Iossa, S. A possible link between hepatic mitochondrial dysfunction and diet-induced insulin resistance. Eur. J. Nutr. 2015, 55, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.-S.; Wu, J.-C.; Ho, C.-T.; Pan, M.-H. Chemoprevention of obesity by dietary natural compounds targeting mitochondrial regulation. Mol. Nutr. Food Res. 2016, 61, 1600721. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.C.E.; Cassanyé, A.; Martín-Gari, M.; Granado-Serrano, A.B.; Portero-Otín, M. Effect of Dietary Bioactive Compounds on Mitochondrial and Metabolic Flexibility. Diseases 2016, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Lahouel, M.; Amedah, S.; Zellagui, A.; Touil, A.; Rhouati, S.; Benyache, F.; Leghouchi, E.; Bousseboua, H. The Interaction of New Plant Flavonoids with Rat Liver Mitochondria: Relation between the Anti- and Pro-oxydant Effect and Flavonoids Concentration. Therapies 2006, 61, 347–355. [Google Scholar] [CrossRef]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef]
- Dos Santos, T.W.; Pereira, Q.C.; Teixeira, L.; Gambero, A.; Villena, J.A.; Ribeiro, M.L. Effects of Polyphenols on Thermogenesis and Mitochondrial Biogenesis. Int. J. Mol. Sci. 2018, 19, 2757. [Google Scholar] [CrossRef] [Green Version]
- Schrauwen, P.; Schrauwen-Hinderling, V.; Hoeks, J.; Hesselink, M.K. Mitochondrial dysfunction and lipotoxicity. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2010, 1801, 266–271. [Google Scholar] [CrossRef]
- Diao, L.; Auger, C.; Konoeda, H.; Sadri, A.-R.; Amini-Nik, S.; Jeschke, M.G. Hepatic steatosis associated with decreased β-oxidation and mitochondrial function contributes to cell damage in obese mice after thermal injury. Cell Death Dis. 2018, 9, 530. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, A.R.; Cabral-Costa, J.V.; Kowaltowski, A.J. Effects of a high fat diet on liver mitochondria: Increased ATP-sensitive K+ channel activity and reactive oxygen species generation. J. Bioenerg. Biomembr. 2010, 42, 245–253. [Google Scholar] [CrossRef]
- Ciapaite, J.; Bakker, S.J.; Van Eikenhorst, G.; Wagner, M.J.; Teerlink, T.; Schalkwijk, C.G.; Fodor, M.; Ouwens, D.M.; Diamant, M.; Heine, R.J.; et al. Functioning of oxidative phosphorylation in liver mitochondria of high-fat diet fed rats. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2007, 1772, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franko, A.; von Kleist-Retzow, J.-C.; Neschen, S.; Wu, M.; Schommers, P.; Böse, M.; Kunze, A.; Hartmann, U.; Sanchez-Lasheras, C.; Stoehr, O.; et al. Liver adapts mitochondrial function to insulin resistant and diabetic states in mice. J. Hepatol. 2014, 60, 816–823. [Google Scholar] [CrossRef] [Green Version]
- Holloway, G.P.; Thrush, A.B.; Heigenhauser, G.J.F.; Tandon, N.N.; Dyck, D.J.; Bonen, A.; Spriet, L.L. Skeletal muscle mitochondrial FAT/CD36 content and palmitate oxidation are not decreased in obese women. Am. J. Physiol. Metab. 2007, 292, E1782–E1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, S.H.; Dilthey, B.G.; Liu, X.; Guan, S.; Sims, H.F.; Gross, R.W. High-fat diet activates liver iPLA2γ generating eicosanoids that mediate metabolic stress. J. Lipid Res. 2021, 62, 100052. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Fink, B.D.; Herlein, J.A.; Oltman, C.L.; Lamping, K.G.; Sivitz, W.I. Dietary fat, fatty acid saturation and mitochondrial bioenergetics. J. Bioenerg. Biomembr. 2013, 46, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Park, H.-M.; Ji, H.-S.; Han, J.; Kim, S.-K.; Park, H.-Y.; Jeong, T.-S. Phenolic-enriched blueberry-leaf extract attenuates glucose homeostasis, pancreatic β-cell function, and insulin sensitivity in high-fat diet–induced diabetic mice. Nutr. Res. 2019, 73, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nature 2018, 20, 745–754. [Google Scholar] [CrossRef]
- Koonen, D.P.; Jacobs, R.L.; Febbraio, M.; Young, M.E.; Soltys, C.-L.M.; Ong, H.; Vance, D.E.; Dyck, J.R. Increased Hepatic CD36 Expression Contributes to Dyslipidemia Associated With Diet-Induced Obesity. Diabetes 2007, 56, 2863–2871. [Google Scholar] [CrossRef] [Green Version]
- Aranaz, P.; Romo-Hualde, A.; Zabala, M.; Navarro-Herrera, D.; de Galarreta, M.R.; Gil, A.G.; Martinez, J.A.; Milagro, F.I.; González-Navarro, C.J. Freeze-dried strawberry and blueberry attenuates diet-induced obesity and insulin resistance in rats by inhibiting adipogenesis and lipogenesis. Food Funct. 2017, 8, 3999–4013. [Google Scholar] [CrossRef]
- Nagao, K.; Higa, K.; Shirouchi, B.; Nomura, S.; Inoue, N.; Inafuku, M.; Yanagita, T. Effect ofVaccinium ashei readeLeaves on Lipid Metabolism in Otsuka Long-Evans Tokushima Fatty Rats. Biosci. Biotechnol. Biochem. 2008, 72, 1619–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.-J.; Bian, Y.-P.; Wang, Q.-H.; Yin, F.; Yin, L.; Zhang, Y.-L.; Liu, J.-H. Blueberry-derived exosomes-like nanoparticles ameliorate nonalcoholic fatty liver disease by attenuating mitochondrial oxidative stress. Acta Pharmacol. Sin. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, Q.; Zhang, Y.; Liu, B.; Wu, M. Blueberry polyphenols play a preventive effect on alcoholic fatty liver disease C57BL/6 J mice by promoting autophagy to accelerate lipolysis to eliminate excessive TG accumulation in hepatocytes. Ann. Palliat. Med. 2020, 9, 1045–1054. [Google Scholar] [CrossRef]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary Anthocyanin-Rich Bilberry Extract Ameliorates Hyperglycemia and Insulin Sensitivity via Activation of AMP-Activated Protein Kinase in Diabetic Mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Marsol-Vall, A.; Laaksonen, O.; Kortesniemi, M.; Yang, B. Characterization and Quantification of Nonanthocyanin Phenolic Compounds in White and Blue Bilberry (Vaccinium myrtillus) Juices and Wines Using UHPLC-DAD−ESI-QTOF-MS and UHPLC-DAD. J. Agric. Food Chem. 2020, 68, 7734–7744. [Google Scholar] [CrossRef]
- Ek, S.; Kartimo, H.; Mattila, S.; Tolonen, A. Characterization of Phenolic Compounds from Lingonberry (Vaccinium vitis-idaea). J. Agric. Food Chem. 2006, 54, 9834–9842. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. A comparison of fruit quality parameters of wild bilberry (Vaccinium myrtillus L.) growing at different locations. J. Sci. Food Agric. 2014, 95, 776–785. [Google Scholar] [CrossRef]
- Stanoeva, J.P.; Stefova, M.; Andonovska, K.B.; Vankova, A.; Stafilov, T. Phenolics and mineral content in bilberry and bog bilberry from Macedonia. Int. J. Food Prop. 2017, 20, S863–S883. [Google Scholar] [CrossRef]
- Howard, L.R.; Brownmiller, C.; Mauromoustakos, A.; Prior, R.L. Improved stability of blueberry juice anthocyanins by acidification and refrigeration. J. Berry Res. 2016, 6, 189–201. [Google Scholar] [CrossRef] [Green Version]









| Bacterial Group | Primer Sequence (5′-3′) | PCR Product Size (bp) | AT (°C) |
|---|---|---|---|
| Firmicutes | ATG TGG TTT AAT TCG AAG CA | 126 | 45 |
| AGC TGA CGA CAA CCA TGC AC | |||
| Bacteroidetes | CAT GTG GTT TAA TTC GAT GAT | 126 | 45 |
| AGC TGA CGA CAA CCA TGC AG | |||
| Clostridium | GCA CAA GCA GTG GAG T | 239 | 45 |
| CTT CCT CCG TTT TGT CAA | |||
| Universal | AAA CTC AAA KGA ATT GAC GG | 180 | 45 |
| CTC ACR RCA CGA GCT GAC | |||
| Enterococcus | CCC TTA TTG TTA GTT GCC GCC ATC ATT | 144 | 50 |
| ACTCGT TGT ACT TCC CT TGT | |||
| Prevotella | CAC RGT AAA CGA TGG ATG CC | 513 | 50 |
| GGT CGG GTT GCA GAC C | |||
| Bifidobacterium | CGC GTC YGG TGT GAA AG | 244 | 50 |
| CCC CAC ATC CAG CAT CCA | |||
| Roseburia | TAC TGC ATT GGA AAC TGT CG | 230 | 50 |
| CGG CAC CGA AGA GCA AT | |||
| Lactobacillus | GAG GCA GCA GTA GGG AAT CTT C | 126 | 55 |
| GGC CAG TTA CTA CCT CTA TCC TTC TTC | |||
| Akkermansia | CAG CAC GTG AAG GTG GGG AC | 327 | 55 |
| CCT TGC GGT TGG CTT CAG AT |
| Parameters | CTRL | HSuHF | HSuHF + BJ |
|---|---|---|---|
| Body weight (BW) | |||
| Initial (g) | 285.40 ± 19.80 | 287.30 ± 14.71 | 286.20 ± 16.68 |
| Final (g) | 510.00 ± 20.90 | 602.00 ± 32.89 | 589.40 ± 31.75 |
| Delta BW (g) | 224.60 ± 10.67 | 314.60 ± 23.62 * | 303.20 ± 17.85 * |
| Cumulative intakes | |||
| Total calories (Kcal/rat/week) | 515.50 ± 5.55 | 715.20 ± 10.12 **** | 742.00 ± 16.03 **** |
| Food (g/rat/week) | 163.00 ± 1.77 | 60.44 ± 1.63 **** | 76.42 ± 1.94 ****, ### |
| Drink (mL/rat/week) | 214.10 ± 4.30 | 328.10 ± 7.51 **** | 285.70 ± 12.98 ****, ### |
| Macronutrients cumulative intakes | |||
| Carbohydrates (Kcal/rat/week) | 350.20 ± 3.93 | 573.90 ± 14.01 **** | 548.40 ± 21.73 **** |
| Lipids (Kcal/rat/week) | 44.19 ± 0.47 | 98.73 ± 8.76 | 132.40 ± 12.35 |
| Proteins (Kcal/rat/week) | 121.10 ± 1.30 | 51.92 ± 1.61 **** | 65.84 ± 1.90 ****, #### |
| Parameters | CTRL | HSuHF | HSuHF + BJ |
|---|---|---|---|
| Bacteria groups (Log10 copies /ng of DNA) | |||
| Universal | 6.188 ± 0.317 | 5.941 ± 0.178 | 5.140 ± 0.190 *, # |
| Firmicutes | 7.316 ± 0.175 | 7.043 ± 0.123 | 6.785 ± 0.201 |
| Bacteroidetes | 4.641 ± 0.183 | 4.167 ± 0.262 | 4.142 ± 0.185 |
| Firmicutes/Bacteroidetes | 1.605 ± 0.042 | 1.778 ± 0.086 | 1.643 ± 0.033 |
| Bifidobacterium | 1.706 ± 0.119 | 2.53 ± 0.02 | 1.280 ± 0.156 ## |
| Prevotella | 2.736 ± 0.286 | 2.817 ± 0.286 | 1.820 ± 0.225 # |
| Lactobacillus | 4.087 ± 0.795 | 4.507 ± 0.350 | 4.317 ± 0.169 |
| Akkermansia | 4.823 ± 0.271 | 3.561 ± 0.373 * | 3.859 ± 0.141 |
| Clostridium leptum | 4.817 ± 0.176 | 4.714 ± 0.195 | 4.604 ± 0.138 |
| Roseburia | 3.628 ± 0.277 | 3.829 ± 0.306 | 3.901 ± 0.322 |
| Enterococcus | 2.620 ± 0.494 | 3.096 ± 0. 240 | 2.496 ± 0.230 |
| SCFAs (mg/g feces) | |||
| Acetic acid | 1.24 ± 0.11 | 0.97 ± 0.11 | 0.68 ± 0.07 ** |
| Butyric acid | 0.07 ± 0.01 | 0.02 ± 0.01 ** | 0.02 ± 0.00 ** |
| Propionic acid | 0.03 ± 0.00 | 0.01 ± 0.00 * | 0.01 ± 0.00 * |
| Succinic acid | 7.01 ± 0.79 | 8.86 ± 1.33 | 4.82 ± 0.76 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, S.; Viana, S.D.; Preguiça, I.; Alves, A.; Fernandes, R.; Teodoro, J.S.; Matos, P.; Figueirinha, A.; Salgueiro, L.; André, A.; et al. Blueberry Counteracts Prediabetes in a Hypercaloric Diet-Induced Rat Model and Rescues Hepatic Mitochondrial Bioenergetics. Nutrients 2021, 13, 4192. https://doi.org/10.3390/nu13124192
Nunes S, Viana SD, Preguiça I, Alves A, Fernandes R, Teodoro JS, Matos P, Figueirinha A, Salgueiro L, André A, et al. Blueberry Counteracts Prediabetes in a Hypercaloric Diet-Induced Rat Model and Rescues Hepatic Mitochondrial Bioenergetics. Nutrients. 2021; 13(12):4192. https://doi.org/10.3390/nu13124192
Chicago/Turabian StyleNunes, Sara, Sofia D. Viana, Inês Preguiça, André Alves, Rosa Fernandes, João S. Teodoro, Patrícia Matos, Artur Figueirinha, Lígia Salgueiro, Alexandra André, and et al. 2021. "Blueberry Counteracts Prediabetes in a Hypercaloric Diet-Induced Rat Model and Rescues Hepatic Mitochondrial Bioenergetics" Nutrients 13, no. 12: 4192. https://doi.org/10.3390/nu13124192
APA StyleNunes, S., Viana, S. D., Preguiça, I., Alves, A., Fernandes, R., Teodoro, J. S., Matos, P., Figueirinha, A., Salgueiro, L., André, A., Silva, S., Jarak, I., Carvalho, R. A., Cavadas, C., Rolo, A. P., Palmeira, C. M., Pintado, M. M., & Reis, F. (2021). Blueberry Counteracts Prediabetes in a Hypercaloric Diet-Induced Rat Model and Rescues Hepatic Mitochondrial Bioenergetics. Nutrients, 13(12), 4192. https://doi.org/10.3390/nu13124192