Changes in Circulating Metabolites during Weight Loss and Weight Loss Maintenance in Relation to Cardiometabolic Risk
Abstract
:1. Introduction
2. Subjects and Methods
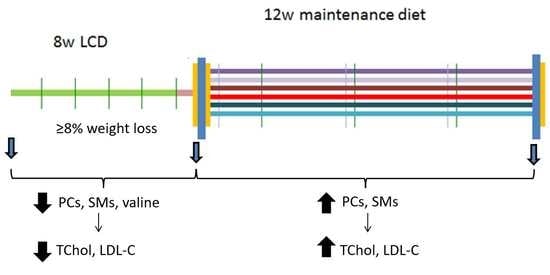
2.1. Study Design and Participants
2.2. Assessment of Energy Intake and Physical Activity
2.3. Anthropometric and Biochemical Measurements
2.4. Multiplatform Targeted Metabolomics
2.5. Statistical Analyses
3. Results
3.1. Characteristics of the Study Participants
3.2. Association between Changes in Metabolite Concentrations and Changes in Cardiometabolic Parameters over the 8-Week LCD
3.3. Association between Changes in Metabolite Concentrations and Changes in Cardiometabolic Parameters over the 12-Week Weight Loss Maintenance Period
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aslibekyan, S.; Garvey, W.T. Obesity: Obesity and cardiometabolic disease—More than meets the eye. Nat. Rev. Endocrinol. 2017, 13, 566–568. [Google Scholar] [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006, 113, 898–918. [Google Scholar]
- Franz, M.J.; VanWormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet. Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Lagerros, Y.T.; Rössner, S. Obesity management: What brings success? Ther. Adv. Gastroenterol. 2013, 6, 77–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magkos, F.; Fraterrigo, G.; Yoshino, J.; Luecking, C.; Kirbach, K.; Kelly, S.C.; De Las Fuentes, L.; He, S.; Okunade, A.L.; Patterson, B.W.; et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016, 23, 591–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wing, R.R.; Espeland, M.A.; Clark, J.M.; Hazuda, H.P.; Knowler, W.C.; Pownall, H.J.; Unick, J.; Wadden, T.; Wagenknecht, L.; for the Action for Health in Diabetes (Look AHEAD) Study Group. Association of Weight Loss Maintenance and Weight Regain on 4-Year Changes in CVD Risk Factors: The Action for Health in Diabetes (Look AHEAD) Clinical Trial. Diabetes Care. 2016, 39, 1345–1355. [Google Scholar] [CrossRef] [Green Version]
- Geidenstam, N.; Al-Majdoub, M.; Ekman, M.; Spégel, P.; Ridderstråle, M. Metabolite profiling of obese individuals before and after a one year weight loss program. Int. J. Obes. 2017, 41, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Sun, D.; Li, X.; DiDonato, J.A.; Bray, G.A.; Sacks, F.M.; Qi, L. Gut microbiota metabolites, amino acid metabolites and improvements in insulin sensitivity and glucose metabolism: The POUNDS Lost trial. Gut. 2019, 68, 263–270. [Google Scholar] [CrossRef]
- Papandreou, C.; García-Gavilán, J.; Camacho-Barcia, L.; Hansen, T.T.; Harrold, J.A.; Sjödin, A.; Halford, J.C.G.; Bulló, M. Changes in Circulating Metabolites During Weight Loss are Associated with Adiposity Improvement, and Body Weight and Adiposity Regain During Weight Loss Maintenance: The SATIN Study. Mol. Nutr. Food Res. 2021, 65, 2001154. [Google Scholar] [CrossRef]
- Hansen, T.T.; Hjorth, M.F.; Sandby, K.; Andersen, S.V.; Astrup, A.; Ritz, C.; Bulló, M.; Camacho-Barcía, M.L.; García-Gavilán, J.F.; Salas-Salvadó, J.; et al. Predictors of successful weight loss with relative maintenance of fat-free mass in individuals with overweight and obesity on an 8-week low-energy diet. Br. J. Nutr. 2019, 122, 468–479. [Google Scholar] [CrossRef]
- Hansen, T.T.; Mead, B.R.; García-Gavilán, J.F.; Korndal, S.K.; Harrold, J.A.; Camacho-Barcía, L.; Ritz, C.; Christiansen, P.; Salas-Salvadó, J.; Hjorth, M.F.; et al. Is reduction in appetite beneficial for body weight management in the context of overweight and obesity? Yes, according to the SATIN (Satiety Innovation) study. J. Nutr. Sci. 2019, 8, e39. [Google Scholar] [CrossRef] [Green Version]
- Moreiras, C.; Cabrera, O.; Cuadrado, L. Tablas de Composición de Alimentos. (Spanish Food Composition Tables), 17th ed.; Pirámide: Madrid, Spain, 2015. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: In-sulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijlsma, S.; Bobeldijk, I.; Verheij, E.R.; Ramaker, R.; Kochhar, S.; Macdonald, I.; van Ommen, B.; Smilde, A.K. Large-Scale Human Metabolomics Studies: A Strategy for Data (Pre-) Processing and Validation. Anal. Chem. 2006, 78, 567–574. [Google Scholar] [CrossRef]
- Montani, J.-P.; Viecelli, A.K.; Prévot, A.; Dulloo, A. Weight cycling during growth and beyond as a risk factor for later cardiovascular diseases: The ‘repeated overshoot’ theory. Int. J. Obes. 2006, 30 (Suppl. S4), S58–S66. [Google Scholar] [CrossRef] [Green Version]
- Beavers, D.P.; Beavers, K.M.; Lyles, M.F.; Nicklas, B.J. Cardiometabolic Risk After Weight Loss and Subsequent Weight Regain in Overweight and Obese Postmenopausal Women. J. Gerontol. Ser. A Boil. Sci. Med Sci. 2013, 68, 691–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graessler, J.; Schwudke, D.; Schwarz, P.E.H.; Herzog, R.; Shevchenko, A.; Bornstein, S.R. Top-Down Lipidomics Reveals Ether Lipid Deficiency in Blood Plasma of Hypertensive Patients. PLoS ONE. 2009, 4, e6261. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; García-Gavilán, J.; Camacho-Barcia, L.; Hansen, T.; Sjödin, A.; Harrold, J.; Halford, J.; Bulló, M. Circulating Metabolites Associated with Body Fat and Lean Mass in Adults with Overweight/Obesity. Metabolites. 2021, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Schlitt, A.; Blankenberg, S.; Yan, D.; von Gizycki, H.; Buerke, M.; Werdan, K.; Bickel, C.; Lackner, K.J.; Meyer, J.; Rupprecht, H.J.; et al. Further evaluation of plasma sphingomyelin levels as a risk factor for coronary artery disease. Nutr. Metab. 2006, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Stroeve, J.H.; Saccenti, E.; Bouwman, J.; Dane, A.; Strassburg, K.; Vervoort, J.; Hankemeier, T.; Astrup, A.; Smilde, A.K.; Van Ommen, B.; et al. Weight loss predictability by plasma metabolic signatures in adults with obesity and morbid obesity of the DiOGenes study. Obesity 2016, 24, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Avenell, A.; Bolland, M.; Hudson, J.; Stewart, F.; Robertson, C.; Sharma, P.; Fraser, C.; MacLennan, G. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: Systematic review and meta-analysis. BMJ. 2017, 359, j4849. [Google Scholar] [CrossRef] [Green Version]
- Dashti, M.; Kulik, W.; Hoek, F.; Veerman, E.C.; Peppelenbosch, M.; Rezaee, F. A Phospholipidomic Analysis of All Defined Human Plasma Lipoproteins. Sci. Rep. 2011, 1, 139. [Google Scholar] [CrossRef] [Green Version]
- Meikle, P.; Summers, S. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat. Rev. Endocrinol. 2017, 13, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Han, X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016, 12, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Leal-Witt, M.J.; Ramon-Krauel, M.; Samino, S.; Llobet, M.; Cuadras, D.; Jimenez-Chillaron, J.C.; Yanes, O.; Lerin, C. Untargeted metabolomics identifies a plasma sphingolipid-related signature associated with lifestyle intervention in prepubertal children with obesity. Int. J. Obes. 2017, 42, 72–78. [Google Scholar] [CrossRef]
- Almanza-Aguilera, E.; Brunius, C.; Bernal-Lopez, M.R.; Garcia-Aloy, M.; Madrid-Gambin, F.; Tinahones, F.J.; Gómez-Huelgas, R.; Landberg, R.; Andres-Lacueva, C. Impact in Plasma Metabolome as Effect of Lifestyle Intervention for Weight-Loss Reveals Metabolic Benefits in Metabolically Healthy Obese Women. J. Proteome Res. 2018, 17, 2600–2610. [Google Scholar] [CrossRef]
- Yu, Z.; Peng, Q.; Huang, Y. Potential therapeutic targets for atherosclerosis in sphingolipid metabolism. Clin. Sci. 2019, 133, 763–776. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, M.; Lewis, G.D.; Ericson, U.; Orho-Melander, M.; Hedblad, B.; Engström, G.; Östling, G.; Clish, C.; Wang, T.J.; Gerszten, R.E.; et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur. Heart J. 2013, 34, 1982–1989. [Google Scholar] [CrossRef] [Green Version]
- Tobias, D.K.; Lawler, P.R.; Harada, P.H.; Demler, O.V.; Ridker, P.M.; Manson, J.E.; Cheng, S.; Mora, S. Circulating Branched-Chain Amino Acids and Incident Cardiovascular Disease in a Prospective Cohort of US Women. Circ. Genom. Precis. Med. 2018, 11, e002157. [Google Scholar] [CrossRef] [Green Version]
- Yamakado, M.; Nagao, K.; Imaizumi, A.; Tani, M.; Toda, A.; Tanaka, T.; Jinzu, H.; Miyano, H.; Yamamoto, H.; Daimon, T.; et al. Plasma Free Amino Acid Profiles Predict Four-Year Risk of Developing Diabetes, Metabolic Syndrome, Dyslipidemia and Hypertension in Japanese Population. Sci. Rep. 2015, 5, 11918. [Google Scholar] [CrossRef]
- Li, X.; Sun, D.; Zhou, T.; Ma, H.; Heianza, Y.; Liang, Z.; Bray, G.A.; Sacks, F.M.; Qi, L. Changes of Branched-Chain Amino Acids and Ectopic Fat in Response to Weight-loss Diets: The POUNDS Lost Trial. J. Clin. Endocrinol. Metab. 2020, 105, e3747–e3756. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ceglarek, U.; Huang, T.; Li, L.; Rood, J.; Ryan, D.H.; Bray, G.A.; Sacks, F.M.; Schwarzfuchs, D.; Thiery, J.; et al. Weight-loss diets and 2-y changes in circulating amino acids in 2 randomized intervention trials1–3. Am. J. Clin. Nutr. 2016, 103, 505–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halama, A.; Horsch, M.; Kastenmüller, G.; Möller, G.; Kumar, P.; Prehn, C.; Laumen, H.; Hauner, H.; de Angelis, M.H.; Beckers, J.; et al. Metabolic switch during adipogenesis: From branched chain amino acid catabolism to lipid synthesis. Arch. Biochem. Biophys. 2016, 589, 93–107. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, K.; Harada, S.; Takeuchi, A.; Kurihara, A.; Iida, M.; Fukai, K.; Kuwabara, K.; Kato, S.; Matsumoto, M.; Hirata, A.; et al. Association between dyslipidemia and plasma levels of branched-chain amino acids in the Japanese population without diabetes mellitus. J. Clin. Lipidol. 2019, 13, 932–939.e2. [Google Scholar] [CrossRef] [Green Version]
- Linna, M.S.; Borg, P.; Kukkonen-Harjula, K.; Fogelholm, M.; Nenonen, A.; Ahotupa, M.; Vasankari, T.J. Successful weight maintenance preserves lower levels of oxidized LDL achieved by weight reduction in obese men. Int. J. Obes. 2006, 31, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, T.R.; Warner, S.O.; Dellsperger, K.C.; Hinton, P.S.; Whaley-Connell, A.T.; Rector, R.S.; Liu, Y.; Linden, M.A.; Chockalingam, A.; Thyfault, J.P.; et al. Exercise and the metabolic syndrome with weight regain. J. Appl. Physiol. 2010, 109, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Delbridge, E.A.; Prendergast, L.; Pritchard, J.E.; Proietto, J.W. One-year weight maintenance after significant weight loss in healthy overweight and obese subjects: Does diet composition matter? Am. J. Clin. Nutr. 2009, 90, 1203–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, T.; Kato, Y.; Murotake, Y.; Kim, M.-K.; Unno, H.; Tanaka, K. An increase in high-density lipoprotein cholesterol after weight loss intervention is associated with long-term maintenance of reduced visceral abdominal fat. Int. J. Obes. 2010, 34, 1742–1751. [Google Scholar] [CrossRef] [Green Version]
- Quinn, W.J.; Wan, M.; Shewale, S.V.; Gelfer, R.; Rader, D.J.; Birnbaum, M.J.; Titchenell, P.M. mTORC1 stimulates phosphatidylcholine synthesis to promote triglyceride secretion. J. Clin. Investig. 2017, 127, 4207–4215. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Baseline (n = 162) | 8 Weeks Change (n = 162) | Weight Loss Maintenance Change (n = 155) | p Value for 8 Weeks Change | p Value for Weight Loss Maintenance Change | |
|---|---|---|---|---|---|
| Sex (%Women) | 75.0 | NA | NA | NA | NA |
| Age (years) | 47.5 ± 9.9 | NA | NA | NA | NA |
| Height (m) | 1.68 ± 0.09 | NA | NA | NA | NA |
| Weight (kg) | 88.1 ± 10.7 | −9.7 (−10.2, −9.2) | 1.0 (0.6, 1.4) | <0.001 | <0.001 |
| BMI (kg/m2) | 30.9 ± 2.0 | −3.4 (−3.5, −3.2) | 0.3 (0.2, 0.5) | <0.001 | <0.001 |
| Sagittal diameter (cm) | 23.2 ± 2.4 | −3.1 (−3.3, −2.8) | 0.6 (0.4, 0.8) | <0.001 | <0.001 |
| Glucose (mg/dL) | 94.9 ± 11.2 | −2.9 (−4.1, −1.8) | −0.5 (−1.5, 0.6) | <0.001 | 0.367 |
| Insulin (mcUI/mL) | 9.1 ± 6.4 | −3.2 (−3.9, −2.5) | 0.2 (−0.2, 0.7) | <0.001 | 0.361 |
| HOMA-IR | 2.2 ± 1.7 | −0.8 (−1.0, −0.6) | 0.04 (−0.07, 0.1) | <0.001 | 0.482 |
| TChol (mg/dL) | 197.6 ± 34.9 | −19.0 (−23.4, 16.2) | 15.1 (11.6, 18.6) | <0.001 | <0.001 |
| HDL-C (mg/dL) | 56.3 ± 15.9 | −5.2 (−6.5, −3.8) | 10.3 (8.9, 11.7) | <0.001 | <0.001 |
| LDL-C (mg/dL) | 120.6 ± 30.9 | −12.3 (−15.5, −9.2) | 5.9 (2.9, 8.9) | <0.001 | <0.001 |
| Triglycerides (mg/dL) | 103.1 ± 50.5 | −11.6 (−18.0, −5.1) | −6.5 (−11.6, −1.4) | <0.001 | 0.012 |
| IL-6 (pg/mL) | 2.24 ± 3.43 | −0.33 (−0.75, 0.21) | −0.04 (−0.32, 0.24) | 0.125 | 0.762 |
| CRP (mg/mL) | 1.41 ± 1.61 | −0.34 (−0.57, −0.10) | −0.008 (−0.23, 0.21) | 0.005 | 0.941 |
| EI (Kcal/d) | 1953.5 ± 633.5 | −400.8 (−502.3, −299.2) | 57.5 (−26.7, 141.8) | <0.001 | 0.179 |
| TPA (CPM) | 608.9 ± 187.9 | 47.9 (23.5, 72.4) | −14.9 (−46.4, 16.5) | <0.001 | 0.348 |
| Change in Metabolite between Baseline and 8 Weeks LCD | Change in Glucose (mg/dL) | Change in HOMA-IR | Change in TChol (mg/dL) | Change in LDL-C (mg/dL) | Change in HDL-C (mg/dL) | Change in Triglycerides (mg/dL) | Change in CRP (mg/mL) |
|---|---|---|---|---|---|---|---|
| Free Chol | −0.08 (−1.16, 1.01) | 0.04 (−0.09, 0.17) | NA | NA | NA | 7.93 (2.62, 13.24) | −0.18 (−0.38, 0.03) |
| Esterified Chol | 0.01 (−1.01, 1.03) | 0.06 (−0.06, 0.18) | NA | NA | NA | 6.46 (1.44, 11.49) | −0.21 (−0.40, −0.01) |
| Total Chol | −0.93 (−1.90, 0.05) | −0.05 (−0.17, 0.08) | NA | NA | NA | −1.51 (−6.55, 3.54) | 0.07 (−0.12, 0.26) |
| TG | −0.04 (−1.14, 1.05) | 0.18 (0.04, 0.31) | 11.16 (7.65, 14.67) ** | 6.90 (3.60, 10.20) ** | −0.78 (−2.04, 0.49) | NA | 0.05 (−0.16, 0.26) |
| PC | −1.10 (−1.16, 0.95) | 0.05 (−0.07, 0.19) | 13.55 (10.45, 16.65) ** | 9.85 (6.92, 12.77) ** | 1.82 (0.60, 3.04) | 9.22 (4.09, 14.34) | −0.19 (−0.39, 0.007) |
| LPC | −0.07 (−1.12, 0.98) | 0.08 (−0.05, 0.21) | 13.60 (10.57, 16.63) ** | 9.11 (6.17, 12.06) ** | 2.32 (1.13, 3.51) ** | 10.46 (5.44, 15.49) *** | −0.22 (−0.42, −0.02) |
| SM | −0.39 (−1.49, 0.72) | −0.03 (−0.17, 0.10) | 7.93 (4.04, 11.83) ** | 7.49 (4.14, 10.83) ** | 0.34 (−0.98, 1.66) | 1.18 (−4.46, 6.82) | −0.10 (−0.31, 0.11) |
| FAC | 0.003 (−1.17, 1.18) | 0.11 (−0.02, 0.26) | 14.18 (10.69, 17.67) ** | 9.82 (6.52, 13.11) ** | 0.77 (−0.59, 2.13) | 17.45 (12.31, 22.59) *** | −0.01 (−0.23, 0.21) |
| MUFA | −0.35 (−1.26, 0,56) | −0.005 (−0.12, 0.11) | 8.63 (5.66, 11.59) * | 7.90 (5.32, 10.49) ** | 0.52 (−0.58, 1.64) | 3.75 (−0.87, 8.38) | −0.12 (−0.30, 0.05) |
| LPC 14:0 | 1.17 (−0.13, 2.47) | 0.25 (0.09, 0.41) | 5.96 (1.30, 10.62) | 2.95 (−1.21, 7.10) | 0.85 (−0.74, 2.44) | 8.11 (1.43, 14.78) | −0.14 (−0.40, 0.11) |
| LPC 20:3 | −0.23 (−1.39, 0.93) | 0.13 (−0.01, 0.27) | 5.80 (1.81, 9.78) | 3.61 (0.03, 7.19) | 0.68 (−0.70, 2.07) | 6.96 (1.17, 12.74) | −0.10 (−0.33, 0.12) |
| PC 30:0 | 1.25 (−0.08, 2.58) | 0.25 (0.09, 0.42) | 7.58 (2.91, 12.25) | 3.94 (−0.30, 8.17) | 1.00 (−0.64, 2.64) | 9.84 (3.02, 16.67) | −0.09 (−0.36, 0.17) |
| PC 32:1 | 0.23 (−0.92, 1.38) | 0.18 (0.04, 0.32) | 6.39 (2.43, 10.34) | 2.18 (−1.44, 5.80) | 1.03 (−0.37, 2.44) | 12.89 (7.17, 18.60) *** | −0.06 (−0.28, 0.17) |
| PC 32:2 | 1.42 (0.18, 2.67) | 0.20 (0.04, 0.35) | 7.46 (3.07, 11.85) | 3.47 (−0.50, 7.44) | 1.72 (0.23, 3.21) | 10.39 (4.16, 16.61) | −0.23 (−0.47, 0.01) |
| PC 33:1 | 0.48 (−0.75, 1.71) | 0.14 (−0.01, 0.29) | 8.44 (4.24, 12.64) ** | 5.05 (1.22, 8.88) | 0.20 (−1.32, 1.72) | 13.78 (7.81, 19.74) *** | −0.16 (−0.40, 0.08) |
| PC 34:4 | 1.71 (0.40, 3.01) | 0.25 (0.08, 0.41) | 8.26 (3.63, 12.90) | 3.97 (−0.22, 8.16) | 2.04 (0.47, 3.61) | 10.57 (3.96, 17.18) | −0.18 (−0.44, 0.07) |
| PC 35:1 | 0.47 (−0.75, 1.69) | 0.13 (−0.02, 0.28) | 10.44 (6.35, 14.53) ** | 7.81 (4.16, 11.47) ** | −0.23 (−1.69, 1.23) | 12.36 (6.46, 18.25) *** | −0.22 (−0.45, 0.01) |
| PC 36:1 | 0.18 (−1.05, 1.41) | 0.16 (0.01, 0.31) | 9.56 (5.51, 13.61) ** | 5.95 (2.21, 9.70) | 1.04 (−0.42, 2.51) | 11.28 (5.23, 17.33) *** | −0.08 (−0.32, 0.15) |
| PC 36:4e | 0.50 (−0.82, 1.82) | 0.002 (−0.16, 0.16) | 8.51 (3.99, 13.02) ** | 7.66 (3.72, 11.59) ** | 2.16 (0.62, 3.69) | −6.85 (−13.40, −0.31) | −0.18 (−0.43, 0.07) |
| PC 36:5 | −0.05 (−1.06, 0.96) | 0.03 (−0.09, 0.16) | 4.72 (1.19, 8.24) | 3.05 (−0.08, 6.19) | 0.78 (−0.42, 1.99) | 3.06 (−2.05, 8.17) | −0.04 (−0.23, 0.15) |
| PC 38:3 | 0.73 (−0.35, 1.82) | 0.19 (0.06, 0.32) | 10.14 (6.67, 13.60) ** | 6.81 (3.57, 10.05) ** | 0.71 (−0.59, 2.01) | 13.66 (8.54, 18.78) *** | 0.06 (−0.15, 0.27) |
| PC 38:4 | −0.35 (−1.58, 0.89) | 0.09 (−0.06, 0.23) | 7.50 (3.48, 11.53) ** | 5.92 (2.20, 9.64) | 0.17 (−1.19, 1.53) | 8.32 (2.29, 14.36) | 0.19 (−0.02, 0.40) |
| PC 38:4e | 1.14 (0.01, 2.28) | 0.01 (−0.13, 0.16) | 7.06 (3.06, 11.07) | 6.59 (3.08, 10.09) ** | 1.84 (0.48, 3.20) | −7.56 (−13.36, −1.77) | −0.20 (−0.42, 0.02) |
| PC 40:4 | −0.04 (−1.25, 1.17) | 0.12 (−0.03, 0.27) | 7.44 (3.24, 11.63) | 3.99 (0.18, 7.80) | 1.33 (−0.12, 2.79) | 10.16 (4.14, 16.19) | −0.03 (−0.26, 0.21) |
| PC 40:6 | −0.07 (−1.09, 0.94) | 0.13 (0.004, 0.26) | 8.63 (5.18, 12.09) ** | 5.42 (2.26, 8.57) | 0.83 (−0.41, 2.07) | 10.33 (5.31, 15.34) *** | −0.004 (−0.20, 0.19) |
| PE 36:5e | 0.48 (−0.61, 1.57) | 0.05 (−0.08, 0.19) | 0.08 (−3.82, 3.97) | 0.84 (−2.58, 4.26) | 0.46 (−0.83, 1.76) | −4.87 (−10.34, 0.60) | −0.08 (−0.29, 0.12) |
| PE 38:5e | 0.42 (−0.71, 1.56) | 0.04 (−0.10, 0.19) | 2.88 (−1.17, 6.93) | 3.20 (−0.36, 6.75) | 0.92 (−0.42, 2.27) | −6.06 (−11.75, −0.37) | −0.15 (−0.37, 0.07) |
| PE 38:6e | 0.34 (−0.91, 1.59) | −0.03 (−0.18, 0.13) | 3.58 (−0.89, 8.05) | 3.15 (−0.78, 7.08) | 2.03 (0.56, 3.49) | −6.52 (−12.82, −0.22) | −0.25 (−0.49, −0.01) |
| SM 32:1 | −0.06 (−1.24, 1.11) | 0.01 (−0.13, 0.16) | 14.51 (10.92, 18.10) ** | 11.73 (8.50, 14.97) ** | 2.31 (0.95, 3.67) | 3.08 (-2.89, 9.05) | −0.15 (−0.38, 0.07) |
| SM 32:2 | 0.20 (−1.10, 1.50) | 0.14 (−0.01, 0.30) | 11.92 (7.67, 16.17) ** | 8.48 (4.59, 12.37) ** | 1.76 (0.27, 3.26) | 10.68 (4.27, 17.10) | −0.09 (−0.35, 0.16) |
| SM 33:1 | −0.09 (−1.24, 1.07) | −0.02 (−0.16, 0.12) | 12.66 (9.02, 16.30) ** | 10.98 (7.78, 14.18) ** | 1.86 (0.52, 3.20) | 0.36 (−5.52, 6.24) | −0.20 (−0.42, 0.01) |
| SM 35:1 | −0.10 (−1.17, 0.97) | −0.07 (−0.20, 0.07) | 10.46 (6.93, 13.98) ** | 9.12 (6.04, 12.20) ** | 1.44 (0.18, 2.71) | −0.29 (−5.76, 5.17) | −0.10 (−0.31, 0.10) |
| SM 36:0 | 0.30 (−0.95, 1.56) | −0.03 (−0.18, 0.13) | 3.46 (−1.16, 8.08) | 4.90 (0.95, 8.85) | −0.50 (−1.99, 1.00) | −1.57 (−7.88, 4.75) | 0.06 (−0.17, 0.31) |
| SM 36:1 | −0.41 (−1.50, 0.67) | −0.10 (−0.23, 0.03) | 9.06 (5.34, 12.78) ** | 7.87 (4.66, 11.08) ** | 1.09 (−0.20, 2.37) | 1.10 (−4.43, 6.64) | −0.07 (−0.28, 0.14) |
| SM 38:1 | 0.63 (−0.43, 1.70) | 0.04 (−0.09, 0.17) | 10.64 (7.20, 14.08) ** | 8.19 (5.08, 11.31) ** | 1.39 (0.14, 2.65) | 6.31 (0.97, 11.65) | −0.13 (−0.33, 0.08) |
| SM 40:1 | 0.37 (−0.78, 1.52) | 0.03 (−0.11, 0.18) | 14.71 (11.21, 18.21) ** | 11.92 (8.72, 15.11) ** | 2.09 (0.74, 3.44) | 7.40 (1.62, 13.17) | −0.23 (−0.45, −0.01) |
| SM 40:2 | 0.24 (−0.92, 1.39) | 0.02 (−0.12, 0.17) | 14.63 (11.19, 18.07) ** | 11.45 (8.30, 14.60) *** | 2.55 (1.24, 3.87) ** | 4.53 (−1.32, 10.39) | −0.30 (−0.52, −0.08) |
| SM 41:1 | 0.51 (−0.71, 1.74) | 0.04 (−0.11, 0.19) | 15.29 (11.60, 18.99) ** | 12.51 (9.15, 15.87) ** | 1.87 (0.43, 3.31) | 7.05 (0.91, 13.18) | −0.23 (−0.47, −0.0008) |
| SM 41:2 | −0.20 (−1.69, 1.29) | −0.03 (−0.21, 0.16) | 10.55 (5.45, 15.65) ** | 8.02 (3.46, 12.57) | 1.50 (−0.27, 3.26) | 6.95 (−0.54, 14.45) | −0.37 (−0.65, −0.09) |
| SM 42:1 | 0.62 (−0.63, 1.87) | 0.07 (−0.08, 0.22) | 12.48 (8.39, 16.57) ** | 9.47 (5.72, 13.23) ** | 1.88 (0.41, 3.35) | 9.00 (2.83, 15.18) | −0.19 (−0.43, 0.05) |
| TG 50:2 | 0.16 (−0.92, 1.23) | 0.14 (0.004, 0.27) | 6.92 (3.18, 10.66) ** | 4.09 (0.71, 7.47) | −0.94 (−2.20, 0.31) | NA | 0.06 (−0.15, 0.27) |
| Lactic acid | 0.68 (−0.40, 1.77) | 0.11 (−0.03, 0.24) | 2.73 (−1.17, 6.62) | 1.48 (−1.97, 4.93) | 0.63 (−0.68, 1.94) | 3.30 (−2.25, 8.86) | 0.05 (−0.16, 0.26) |
| Glycolic acid | 0.17 (−1.03, 1.38) | 0.11 (−0.04, 0.26) | 0.16 (−4.16, 4.50) | 0.05 (−3.72, 3.84) | −0.05 (−1.49, 1.39) | −2.90 (−9.13, 3.32) | −0.09 (−0.32, 0.13) |
| Valine | 0.50 (−0.58, 1.58) | 0.11(−0.02, 0.24) | 6.45 (2.73, 10.16) | 5.57 (2.31, 8.83) | −0.63 (−1.93, 0.66) | 6.38 (0.92, 11.83) | −0.06 (−0.27, 0.15) |
| Glutamate | 0.99 (−0.03, 2.01) | 0.12 (−0.01, 0.24) | −0.32 (−4.01, 3.37) | −0.61 (−3.85, 2.63) | −0.38 (−1.62, 0.85) | 3.11 (−2.03, 8.26) | 0.002 (−0.20, 0.20) |
| Glucose | NA | NA | 1.09 (−2.87, 5.06) | 1.47 (−2.02, 4.97) | 0.37 (−0.96, 1.70) | −2.64 (−8.30, 3.02) | −0.02 (−0.24, 0.19) |
| Tyrosine | 1.13 (0.07, 2.19) | 0.18 (0.05, 0.32) | 3.22 (−0.56, 7.01) | 2.09 (−1.26, 5.45) | 0.63 (−0.65, 1.91) | 2.47 (−2.99, 7.92) | −0.13 (−0.34, 0.07) |
| LPC 16:0 | −1.03 (−2.27, 0.21) | 0.03 (−0.13, 0.19) | 6.52 (2.12, 10.92) | 4.77 (0.85, 8.69) | 0.54 (−0.95, 2.04) | 4.95 (−1.42, 11.32) | −0.22 (−0.46, 0.02) |
| LPC 16:1e | −1.05 (−2.07, −0.02) | −0.07 (−0.20, 0.06) | 1.83 (−1.89, 5.54) | 1.59 (−1.67, 4.86) | 0.37 (−0.87, 1.61) | −2.77 (−8.09, 2.56) | −0.24 (−0.44, −0.04) |
| LPC 18:1 | −1.33 (−2.41, −0.25) | 0.01 (−0.12, 0.15) | 3.43 (−0.49, 7.35) | 2.31 (−1.14, 5.77) | 0.61 (−0.70, 1.93) | −1.16 (−6.87, 4.55) | −0.24 (−0.45, −0.03) |
| LPC 18:2 | −0.51 (−1.52, 0.50) | 0.08 (−0.04, 0.21) | 2.56 (−1.05, 6.17) | 1.49 (−1.69, 4.67) | 0.89 (−0.31, 2.09) | −0.64 (−5.80, 4.52) | −0.26 (−0.45, −0.07) |
| LPC 20:0 | 0.09 (−1.17, 1.19) | 0.01 (−0.14, 0.16) | 6.18 (2.05, 10.30) | 4.84 (1.20, 8.49) | 1.36 (−0.03, 2.77) | 0.11 (−5.89, 6.12) | −0.33 (−0.55, −0.11) |
| LPC 20:1 | −1.01 (−2.03, 0.09) | 0.02 (−0.11, 0.15) | 3.37 (−0.33, 7.07) | 3.37 (0.14, 6.60) | −0.41 (−1.65, 0.83) | −0.36 (−5.72, 5.00) | −0.08 (−0.28, 0.12) |
| LPC 20:4 | −0.52 (−1.52, 0.47) | 0.09 (−0.03, 0.22) | 2.30 (−1.28, 5.89) | 1.72 (−1.43, 4.88) | 0.19 (−1.00, 1.38) | 0.98 (−4.13, 6.08) | −0.08 (−0.27, 0.11) |
| LPC 22:6 | −0.44 (−1.35, 0.47) | 0.07 (−0.04, 0.18) | 2.47 (−0.80, 5.75) | 2.07 (−0.82, 4.96) | 0.30 (−0.79, 1.40) | −0.09 (−4.78, 4.59) | −0.02 (−0.20, 0.15) |
| PC 34:2e | −0.49 (−1.63, 0.64) | −0.25 (−0.39, −0.11) * | 5.65 (1.66, 9.65) | 4.42 (0.88, 7.96) | 3.21 (1.93, 4.49) ** | −10.36 (−15.95, −4.77) *** | −0.37 (−0.58, −0.16) |
| PC 42:5e | 0.22 (−0.80, 1.25) | 0.06 (−0.06, 0.19) | 2.20 (−1.48, 5.87) | 2.41 (−0.81, 5.64) | 0.52 (−0.71, 1.75) | −3.41 (−8.62, 1.80) | 0.005 (−0.19, 0.20) |
| SM 42:3 | −0.53 (−1.64, 0.58) | −0.02 (−0.16, 0.11) | 6.89 (3.05, 10.74) | 6.76 (3.47, 10.06) ** | 0.50 (−0.81, 1.81) | −1.69 (−7.31, 3.94) | 0.01 (−0.20, 0.22) |
| Glycine | 0.13 (−0.87, 1.14) | −0.08 (−0.20, 0.05) | −2.91 (−6.51, 0.69) | −1.67 (−4.83, 1.48) | 0.41 (−0.78, 1.60) | −5.11 (−10.23, 0.01) | −0.22 (−0.41, −0.03) |
| Citric acid | −0.80 (−1.93, 0.32) | −0.10 (−0.25, 0.04) | −0.31 (−4.50, 3.87) | 0.73 (−2.95, 4.40) | −0.20 (−1.62, 1.22) | −4.81 (−10.73, 1.11) | −0.17 (−0.40, 0.05) |
| Change in Metabolite between 8 Weeks and 12 Weeks | Change in TChol (mg/dL) | Change in LDL-C (mg/dL) | Change in Triglycerides (mg/dL) |
|---|---|---|---|
| TG | 3.01 (−0.80, 6.81) | 1.54 (−1.59, 4.68) | NA |
| PC | 11.44 (8.13, 14.75) * | 8.92 (6.10, 11.74) * | NA |
| LPC | 11.45 (8.17, 14.74) * | 8.58 (5.74, 11.41) * | 5.66 (0.47, 10.86) |
| SM | 8.30 (4.70, 11.91) * | 8.31 (5.49, 11.13) * | NA |
| FAC | 9.82 (6.47, 13.18) * | 7.45 (4.65, 10.25) * | 10.57 (5.77, 15.36) * |
| MUFA | 6.40 (2.82, 9.97) * | 4.86 (1.91, 7.80) * | NA |
| PC 32:1 | NA | NA | 16.00 (10.85, 21.14) * |
| PC 33:1 | 6.95 (3.34, 10.56) * | NA | 15.30 (10.79, 19.82) * |
| PC 36:1 | 11.28 (7.83, 14.73) * | NA | 8.49 (3.32, 13.67) * |
| PC 36:4e | 8.85 (5.26, 12.44) * | 7.12 (4.21, 10.02) * | NA |
| PC 38:3 | 13.76 (10.42, 17.09) * | 9.84 (6.91, 12.77) * | 7.38 (1.93, 12.83) |
| PC 38:4e | NA | 6.55 (3.56, 9.53) * | NA |
| SM 32:1 | 12.89 (9.37, 16.37) * | 10.08 (7.11, 13.04) * | NA |
| SM 32:2 | 11.55 (7.77, 15.34) * | 8.12 (4.90, 11.34) * | NA |
| SM 33:1 | 10.36 (6.86, 13.86) * | 8.94 (6.11, 11.76) * | NA |
| SM 38:1 | 8.64 (4.98, 12.31) * | 6.71 (3.69, 9.74) * | NA |
| SM 40:1 | 11.07 (7.81, 14.32) * | 9.71 (7.06, 12.35) * | NA |
| SM 40:2 | 11.95 (8.53, 15.37) * | 9.57 (6.68, 12.47) * | NA |
| SM 41:1 | 12.46 (9.16, 15.76) * | 11.17 (8.55, 13.79) * | NA |
| SM 41:2 | 8.43 (4.81, 12.05) * | NA | NA |
| SM 42:1 | 9.88 (6.48, 13.28) * | 8.72 (6.00, 11.45) * | NA |
| SM 42:3 | NA | 7.07 (3.92, 10.22) * | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papandreou, C.; Harrold, J.A.; Hansen, T.T.; Halford, J.C.G.; Sjödin, A.; Bulló, M. Changes in Circulating Metabolites during Weight Loss and Weight Loss Maintenance in Relation to Cardiometabolic Risk. Nutrients 2021, 13, 4289. https://doi.org/10.3390/nu13124289
Papandreou C, Harrold JA, Hansen TT, Halford JCG, Sjödin A, Bulló M. Changes in Circulating Metabolites during Weight Loss and Weight Loss Maintenance in Relation to Cardiometabolic Risk. Nutrients. 2021; 13(12):4289. https://doi.org/10.3390/nu13124289
Chicago/Turabian StylePapandreou, Christopher, Joanne A. Harrold, Thea T. Hansen, Jason C. G. Halford, Anders Sjödin, and Mònica Bulló. 2021. "Changes in Circulating Metabolites during Weight Loss and Weight Loss Maintenance in Relation to Cardiometabolic Risk" Nutrients 13, no. 12: 4289. https://doi.org/10.3390/nu13124289
APA StylePapandreou, C., Harrold, J. A., Hansen, T. T., Halford, J. C. G., Sjödin, A., & Bulló, M. (2021). Changes in Circulating Metabolites during Weight Loss and Weight Loss Maintenance in Relation to Cardiometabolic Risk. Nutrients, 13(12), 4289. https://doi.org/10.3390/nu13124289