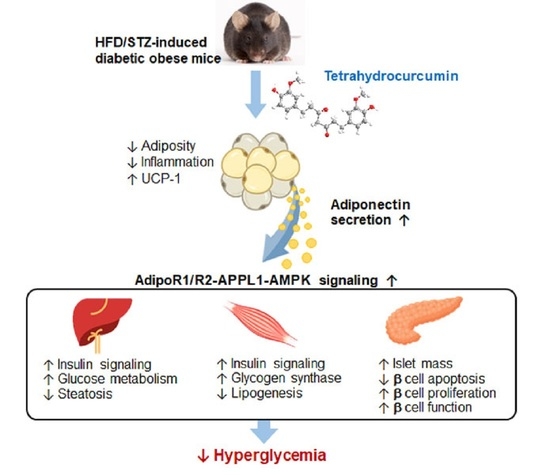
Tetrahydrocurcumin Upregulates the Adiponectin-AdipoR Pathway and Improves Insulin Signaling and Pancreatic β-Cell Function in High-Fat Diet/Streptozotocin-Induced Diabetic Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal and Treatments
2.3. Oral Glucose Tolerance Test, Serum Parameters, and Hepatic Triacylglycerol
2.4. Metabolic Measurement
2.5. Histological, Immunohistochemical and Immunofluorescence Analysis
2.6. Western Blot Analysis
2.7. Reverse Transcription PCR
2.8. Statistical Analysis
3. Results
3.1. THC Ameliorates Weight Gain, Adiposity, and Dyslipidemia in Diabetic Obese Mice
3.2. THC Relieves Hyperglycemia, Hyperinsulinemia, Improves Glucose Tolerance, and Energy Metabolism in Diabetic Obese Mice
3.3. THC Alleviates Adipose Chronic Inflammation, Improves Adiponectin Secretion and Glut4 Levels in Diabetic Obese Mice
3.4. THC Ameliorates Steatosis, Insulin Signaling and Upregulates AdipoR-APPL1 Signaling in the Liver and Muscle of Diabetic Obese Mice
3.5. THC Restores β-Cell Function in Diabetic Obese Mice
3.6. THC Resorted AdipoR1 Expression in Diabetic Obese Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rao, G.; Jensen, E.T. Type 2 Diabetes in Youth. Glob. Pediatr. Health 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic beta-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shul-man, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Barnett, A.H.; Bailey, C.J. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 566–592. [Google Scholar] [CrossRef] [Green Version]
- Maruthur, N.M.; Gribble, M.O.; Bennett, W.L.; Bolen, S.; Wilson, L.M.; Balakrishnan, P.; Sahu, A.; Bass, E.; Kao, W.H.; Clark, J.M. The pharmacogenetics of type 2 diabetes: A systematic review. Diabetes Care 2014, 37, 876–886. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Rupasinghe, T.W.; Tull, D.L.; Boughton, B.; Oliver, C.; McSweeny, C.; Gras, S.L.; Augustin, M.A. Degradation of curcuminoids by in vitro pure culture fermentation. J. Agric. Food Chem. 2014, 62, 11005–11015. [Google Scholar] [CrossRef]
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Holder, G.M.; Plummer, J.L.; Ryan, A.J. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica 1978, 8, 761–768. [Google Scholar] [CrossRef]
- Han, J.S.; Lee, S.; Kim, H.Y.; Lee, C.H. MS-Based Metabolite Profiling of Aboveground and Root Components of Zingiber mioga and Officinale. Molecules. 2015, 20, 16170–16185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Zhou, C.; Huang, K.; Song, L.; Zheng, Q.; Yu, R.; Zhang, R.; Wu, Y.; Zeng, S.; Cheng, C.H.; et al. Antioxidative and cytotoxic properties of diarylheptanoids isolated from Zingiber officinale. China J. Chin. Mater. Med. 2009, 34, 319–323. [Google Scholar]
- Lai, C.S.; Ho, C.T.; Pan, M.H. The Cancer Chemopreventive and Therapeutic Potential of Tetrahydrocurcumin. Biomolecules 2020, 10, 831. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Tsai, M.L.; Lai, C.S.; Wang, Y.J.; Ho, C.T.; Pan, M.H. Chemopreventative effects of tetrahydrocurcumin on human diseases. Food Funct. 2014, 5, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Murugan, P. Antihyperlipidemic effect of curcumin and tetrahydrocurcumin in experimental type 2 diabetic rats. Ren. Fail. 2007, 29, 881–889. [Google Scholar] [CrossRef] [Green Version]
- Murugan, P.; Pari, L. Antioxidant effect of tetrahydrocurcumin in streptozotocin-nicotinamide induced diabetic rats. Life Sci. 2006, 79, 1720–1728. [Google Scholar] [CrossRef]
- Li, K.; Zhai, M.; Jiang, L.; Song, F.; Zhang, B.; Li, J.; Li, H.; Li, B.; Xia, L.; Xu, L.; et al. Tetrahydrocurcumin ameliorates diabetic cardiomyopathy by attenuating high glucose-induced oxidative stress and fibrosis via activating the SIRT1 pathway. Oxid. Med. Cell. Longev. 2019, 2019, 6746907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugan, P.; Pari, L. Effect of tetrahydrocurcumin on plasma antioxidants in streptozotocin-nicotinamide experimental diabetes. J. Basic Clin. Physiol. Pharmacol. 2006, 17, 231–244. [Google Scholar] [CrossRef]
- Pari, L.; Murugan, P. Effect of tetrahydrocurcumin on blood glucose, plasma insulin and hepatic key enzymes in streptozotocin induced diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2005, 16, 257–274. [Google Scholar] [CrossRef]
- Yuan, T.; Yin, Z.; Yan, Z.; Hao, Q.; Zeng, J.; Li, L.; Zhao, J. Tetrahydrocurcumin ameliorates diabetes profiles of db/db mice by altering the composition of gut microbiota and up-regulating the expression of GLP-1 in the pancreas. Fitoterapia 2020, 146, 104665. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Chen, J.W.; Kong, Z.L.; Wu, J.C.; Ho, C.T.; Lai, C.S. Attenuation by Tetrahydrocurcumin of Adiposity and Hepatic Steatosis in Mice with High-Fat-Diet-Induced Obesity. J. Agric. Food Chem. 2018, 66, 12685–12695. [Google Scholar] [CrossRef]
- Chen, J.W.; Kong, Z.L.; Tsai, M.L.; Lo, C.Y.; Ho, C.T.; Lai, C.S. Tetrahydrocurcumin ameliorates free fatty acid-induced hepatic steatosis and improves insulin resistance in HepG2 cells. J. Food Drug Anal. 2018, 26, 1075–1085. [Google Scholar] [CrossRef]
- Friedman, J.M. Leptin and the endocrine control of energy balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Straub, L.G.; Scherer, P.E. Metabolic Messengers: Adiponectin. Nat. Metab. 2019, 1, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- Dei, C.M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar]
- Okada, K.; Wangpoengtrakul, C.; Tanaka, T.; Toyokuni, S.; Uchida, K.; Osawa, T. Curcumin and especially tetrahydrocurcumin ameliorate oxidative stress-induced renal injury in mice. J. Nutr. 2001, 131, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Begum, A.N.; Jones, M.R.; Lim, G.P.; Morihara, T.; Kim, P.; Heath, D.D.; Rock, C.L.; Pruitt, M.A.; Yang, F.; Hudspeth, B.; et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 196–208. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar] [PubMed]
- Vijaya Saradhi, U.V.; Ling, Y.; Wang, J.; Chiu, M.; Schwartz, E.B.; Fuchs, J.R.; Chan, K.K.; Liu, Z. A liquid chromatography-tandem mass spectrometric method for quantification of curcuminoids in cell medium and mouse plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 3045–3051. [Google Scholar] [CrossRef] [Green Version]
- Somparn, P.; Phisalaphong, C.; Nakornchai, S.; Unchern, S.; Morales, N.P. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol. Pharm. Bull. 2007, 30, 74–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Q.F.; Cheng, J.J.; Chen, J.F.; Feng, Y.C.; Lin, G.S.; Xu, Y. Comparation of Anti-Inflammatory and Antioxidantactivities of Curcumin, Tetrahydrocurcuminand Octahydrocurcuminin LPS-Stimulated RAW264.7 Macrophages. Evid. Based Complement. Alternat. Med. 2020, 2020, 8856135. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Kunwar, A.; Mishra, B.; Priyadarsini, K.I. Concentration dependent antioxidant/pro-oxidant activity of curcumin studies from AAPH induced hemolysis of RBCs. Chem. Biol. Interact. 2008, 174, 134–139. [Google Scholar] [CrossRef]
- Sandur, S.K.; Ichikawa, H.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Sethi, G.; Aggarwal, B.B. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane). Free. Radic. Biol. Med. 2007, 43, 568–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atsumi, T.; Fujisawa, S.; Tonosaki, K. Relationship between intracellular ROS production and membrane mobility in curcumin- and tetrahydrocurcumin-treated human gingival fibroblasts and human submandibular gland carcinoma cells. Oral Dis. 2005, 11, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.K.; Panda, P.; Sethi, K.K.; Gangwar, M.; Mondal, S.C.; Jana, S. Solid and liquid state characterization of tetrahydrocurcumin using XRPD, FT-IR, DSC, TGA, LC-MS, GC-MS, and NMR and its biological activities. J. Pharm. Anal. 2020, 10, 334–345. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Iglesias, M.A.; Lam, K.S.; Wang, Y.; Sweeney, G.; Zhu, W.; Vanhoutte, P.M.; Kraegen, E.W.; Xu, A. APPL1 potentiates insulin-mediated inhibition of hepatic glucose production and alleviates diabetes via Akt activation in mice. Cell Metab. 2009, 9, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.; Galan, A.K.; Xin, X.; Dong, F.; Abdul-Ghani, M.A.; Zhou, L.; Wang, C.; Li, C.; Holmes, B.M.; Sloane, L.B.; et al. APPL1 potentiates insulin sensitivity by facilitating the binding of IRS1/2 to the insulin receptor. Cell Rep. 2014, 7, 1227–1238. [Google Scholar] [CrossRef] [Green Version]
- Karthikesan, K.; Pari, L.; Menon, V.P. Protective effect of tetrahydrocurcumin and chlorogenic acid against streptozotocin–nicotinamide generated oxidative stress induced diabetes. J. Funct. Foods 2020, 2, 134–142. [Google Scholar] [CrossRef]
- Ahlgren, U.; Jonsson, J.; Jonsson, L.; Simu, K.; Edlund, H. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998, 12, 1763–1768. [Google Scholar] [CrossRef] [Green Version]
- Kharroubi, I.; Rasschaert, J.; Eizirik, D.L.; Cnop, M. Expression of adiponectin receptors in pancreatic beta cells. Biochem. Biophys. Res. Commun. 2003, 312, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, N.; Krishnamurthy, M.; Bhattacharjee, A.; Suhail, A.; Sweeney, G.; Wheeler, M.B. Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J. Biol. Chem. 2010, 285, 33623–33631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, K.K.; Lam, K.S.; Wu, D.; Wang, Y.; Sweeney, G.; Hoo, R.L.; Zhang, J.; Xu, A. APPL1 potentiates insulin secretion in pancreatic beta cells by enhancing protein kinase Akt-dependent expression of SNARE proteins in mice. Proc. Natl. Acad. Sci. USA 2012, 109, 8919–8924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Zhou, Y.; Wu, K.K.; Chen, Z.; Xu, A.; Cheng, K.K. APPL1 prevents pancreatic beta cell death and inflammation by dampening NFkappaB activation in a mouse model of type 1 diabetes. Diabetologia 2017, 60, 464–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majeed, M.; Natarajan, S.; Pandey, A.; Bani, S.; Mundkur, L. Subchronic and Reproductive/Developmental Toxicity Studies of Tetrahydrocurcumin in Rats. Toxicol. Res. 2019, 35, 65–74. [Google Scholar] [CrossRef] [PubMed]







| ND | HFD/STZ | HFD/STZ + THC 20 mg/kg | HFD/STZ + THC 100 mg/kg | ND + THC 100 mg/kg | |
|---|---|---|---|---|---|
| Body weight gain and food intake | |||||
| Initial body weight (g) | 25.94 ± 1.50 bc | 28.21 ± 0.63 a | 27.38 ± 1.24 ab | 28.25 ± 0.71 a | 25.11 ± 0.38 c |
| Final body weight (g) | 30.32 ± 1.40 bc | 37.49 ± 1.06 a | 32.24 ± 1.77 b | 32.66 ± 0.52 b | 29.67 ± 0.97 c |
| Body weight gain (g) | 4.38 ± 0.37 b | 9.28 ± 1.07 a | 4.86 ± 1.01 b | 4.42 ± 0.66 b | 4.60 ± 0.58 b |
| Food intake (g/mouse/day) | 3.39 ± 0.09 a | 3.08 ± 0.18 c | 3.21 ± 0.05 b | 2.92 ± 0.10 d | 3.48 ± 0.03 a |
| Relative organ weights | |||||
| Liver (%) | 4.11 ± 0.17 b | 4.85 ± 0.51 a | 4.96 ± 0.40 a | 5.36 ± 0.55 a | 4.11 ± 0.11 b |
| Kidney (%) | 1.47 ± 0.09 a | 1.35 ± 0. 19 a | 1.48 ± 0. 15 a | 1.38 ± 0.07 a | 1.46 ± 0.06 a |
| Spleen (%) | 0.21 ± 0.02 b | 0.21 ± 0.03 b | 0.32 ± 0.03 a | 0.30 ± 0.03 a | 0.22 ± 0.04 b |
| Pancreas (%) | 0.56 ± 0.05 a | 0.42 ± 0.06 b | 0.48 ± 0.04 ab | 0.49 ± 0.07 ab | 0.54 ± 0.04 a |
| Fasting serum biochemical parameters | |||||
| TG (mg/dL) | 61.67 ± 3.73 b | 92.00 ± 18.33 a | 41.67 ± 10.67 c | 40.00 ± 8.94 c | 55.00 ± 5.00 b,c |
| TCHO (mg/dL) | 78.75 ± 3.31 c | 130.00 ± 7.07 a | 95.00 ± 5.00 b | 105.00 ±12.58 b | 56.67 ± 4.71 d |
| Cholesterol/HDL-C | 1.31 ± 0.03 a | 1.38 ± 0.09 a | 1.38 ± 0.10 a | 1.36 ± 0.08 a | 1.30 ± 0.14 a |
| GOT (U/L) | 71.43 ± 8.33 bc | 90.00 ± 12.65 a | 78.00 ± 7.48 ab | 63.33 ± 7.45 cd | 56.67 ± 4.71 d |
| GPT (U/L) | 31.43 ± 8.33 c | 72.00 ± 7.48 a | 48.00 ± 9.80 b | 38.00 ± 14.70 bc | 27.50 ± 4.33 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-Z.; Tsai, M.-L.; Hsu, L.-Y.; Ho, C.-T.; Lai, C.-S. Tetrahydrocurcumin Upregulates the Adiponectin-AdipoR Pathway and Improves Insulin Signaling and Pancreatic β-Cell Function in High-Fat Diet/Streptozotocin-Induced Diabetic Obese Mice. Nutrients 2021, 13, 4552. https://doi.org/10.3390/nu13124552
Tsai Y-Z, Tsai M-L, Hsu L-Y, Ho C-T, Lai C-S. Tetrahydrocurcumin Upregulates the Adiponectin-AdipoR Pathway and Improves Insulin Signaling and Pancreatic β-Cell Function in High-Fat Diet/Streptozotocin-Induced Diabetic Obese Mice. Nutrients. 2021; 13(12):4552. https://doi.org/10.3390/nu13124552
Chicago/Turabian StyleTsai, Yi-Zhen, Mei-Ling Tsai, Li-Yin Hsu, Chi-Tang Ho, and Ching-Shu Lai. 2021. "Tetrahydrocurcumin Upregulates the Adiponectin-AdipoR Pathway and Improves Insulin Signaling and Pancreatic β-Cell Function in High-Fat Diet/Streptozotocin-Induced Diabetic Obese Mice" Nutrients 13, no. 12: 4552. https://doi.org/10.3390/nu13124552
APA StyleTsai, Y. -Z., Tsai, M. -L., Hsu, L. -Y., Ho, C. -T., & Lai, C. -S. (2021). Tetrahydrocurcumin Upregulates the Adiponectin-AdipoR Pathway and Improves Insulin Signaling and Pancreatic β-Cell Function in High-Fat Diet/Streptozotocin-Induced Diabetic Obese Mice. Nutrients, 13(12), 4552. https://doi.org/10.3390/nu13124552