Clinical Significance of Probiotics for Children with Idiopathic Nephrotic Syndrome
Abstract
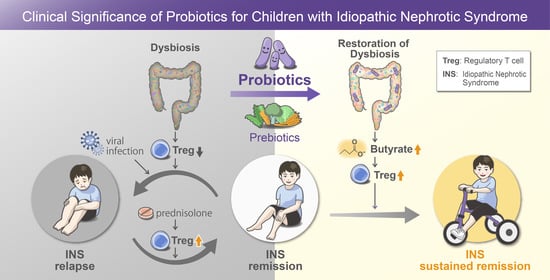
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Fecal Sample Collection and 16S rRNA Sequencing
2.3. Flow Cytometric Analysis of Tregs
2.4. Statistical Analysis
3. Results
3.1. Characteristics of INS Patients
3.2. Composition of the Gut Microbiota in Pediatric INS Patients before and after Probiotic Treatment
3.3. Changes in Numbers of Tregs after Oral Probiotic Treatment in Children with INS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Scarpellini, E.; Ianiro, G.; Attili, F.; Bassanelli, C.; de Santis, A.; Gasbarrini, A. The human gut microbiota and virome: Potential therapeutic implications. Dig. Liver Dis. 2015, 47, 1007–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsuoka, T. Establishment of intestinal bacteriology. Biosci. Microbiota Food Health 2014, 33, 99–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017, 66, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Hoskin-Parr, L.; Teyhan, A.; Blocker, A.; Henderson, A.J. Antibiotic exposure in the first two years of life and development of asthma and other allergic diseases by 7.5 yr: A dose-dependent relationship. Pediatr. Allergy Immunol. 2013, 24, 762–771. [Google Scholar] [CrossRef] [Green Version]
- Rusconi, F.; Zugna, D.; Annesi-Maesano, I.; Baiz, N.; Barros, H.; Correia, S.; Duijts, L.; Forastiere, F.; Inskip, H.; Kelleher, C.C.; et al. Mode of delivery and asthma at school age in 9 European Birth Cohorts. Am. J. Epidemiol. 2017, 185, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Patrick, D.M.; Sbihi, H.; Dai, D.L.Y.; Al Mamun, A.; Rasali, D.; Rose, C.; Marra, F.; Boutin, R.C.T.; Petersen, C.; Stiemsma, L.T.; et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: Evidence from population-based and prospective cohort studies. Lancet Respir. Med. 2020, 8, 1094–1105. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, S.; Suruda, C.; Hashiyada, M.; Kimata, T.; Yamanouchi, S.; Kitao, T.; Kino, J.; Akane, A.; Kaneko, K. Gut microbiota dysbiosis in children with relapsing idiopathic nephrotic syndrome. Am. J. Nephrol. 2018, 47, 164–170. [Google Scholar] [CrossRef]
- Tsuji, S.; Akagawa, S.; Akagawa, Y.; Yamaguchi, T.; Kino, J.; Yamanouchi, S.; Kimata, T.; Hashiyada, M.; Akane, A.; Kaneko, K. Idiopathic nephrotic syndrome in children: Role of regulatory T cells and gut microbiota. Pediatr. Res. 2020. [Google Scholar] [CrossRef]
- Kang, Y.; Feng, D.; Law, H.K.; Qu, W.; Wu, Y.; Zhu, G.H.; Huang, W.Y. Compositional alterations of gut microbiota in children with primary nephrotic syndrome after initial therapy. BMC Nephrol. 2019, 20, 434. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.A.; Symons, J.M. Nephrotic syndrome in childhood. Lancet 2003, 362, 629–639. [Google Scholar] [CrossRef]
- Tsuji, S.; Kimata, T.; Yamanouchi, S.; Kitao, T.; Kino, J.; Suruda, C.; Kaneko, K. Regulatory T cells and CTLA-4 in idiopathic nephrotic syndrome. Pediatr. Int. 2017, 59, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Riviere, A.; Selak, M.; Lantin, D.; Leroy, F.; de Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [Green Version]
- Tarshish, P.; Tobin, J.N.; Bernstein, J.; Edelmann, C.M., Jr. Prognostic significance of the early course of minimal change nephrotic syndrome: Report of the International Study of Kidney Disease in Children. J. Am. Soc. Nephrol. 1997, 8, 769–776. [Google Scholar]
- Chapter 3: Steroid-sensitive nephrotic syndrome in children. Kidney Int. Suppl. 2012, 2, 163–171. [CrossRef] [Green Version]
- Lombel, R.M.; Gipson, D.S.; Hodson, E.M.; Kidney Disease: Improving Global Outcomes. Treatment of steroid-sensitive nephrotic syndrome: New guidelines from KDIGO. Pediatr. Nephrol. 2013, 28, 415–426. [Google Scholar] [CrossRef]
- Seki, H.; Shiohara, M.; Matsumura, T.; Miyagawa, N.; Tanaka, M.; Komiyama, A.; Kurata, S. Prevention of antibiotic-associated diarrhea in children by Clostridium butyricum MIYAIRI. Pediatr. Int. 2003, 45, 86–90. [Google Scholar] [CrossRef]
- Aljutaily, T.; Huarte, E.; Martinez-Monteagudo, S.; Gonzalez-Hernandez, J.L.; Rovai, M.; Sergeev, I.N. Probiotic-enriched milk and dairy products increase gut microbiota diversity: A comparative study. Nutr. Res. 2020, 82, 25–33. [Google Scholar] [CrossRef]
- Haak, B.W.; Littmann, E.R.; Chaubard, J.L.; Pickard, A.J.; Fontana, E.; Adhi, F.; Gyaltshen, Y.; Ling, L.; Morjaria, S.M.; Peled, J.U.; et al. Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood 2018, 131, 2978–2986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalhoub, R.J. Pathogenesis of lipoid nephrosis: A disorder of T-cell function. Lancet 1974, 2, 556–560. [Google Scholar] [CrossRef]
- Araya, C.; Diaz, L.; Wasserfall, C.; Atkinson, M.; Mu, W.; Johnson, R.; Garin, E. T regulatory cell function in idiopathic minimal lesion nephrotic syndrome. Pediatr. Nephrol. 2009, 24, 1691–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, X.S.; Yang, X.Q.; Zhao, X.D.; Li, Q.; Xie, Y.Y.; Wang, X.G.; Wang, M.; Zhang, W. The prevalence of Th17 cells and FOXP3 regulate T cells (Treg) in children with primary nephrotic syndrome. Pediatr. Nephrol. 2009, 24, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Prasad, N.; Agarwal, V.; Yadav, B.; Tripathy, D.; Rai, M.; Nath, M.; Sharma, R.K.; Modi, D.R. Regulatory and effector T cells changes in remission and resistant state of childhood nephrotic syndrome. Indian J. Nephrol. 2014, 24, 349–355. [Google Scholar]
- Prasad, N.; Jaiswal, A.K.; Agarwal, V.; Yadav, B.; Sharma, R.K.; Rai, M.; Singh, H.; Chaturvedi, S.; Singh, A. Differential alteration in peripheral T-regulatory and T-effector cells with change in P-glycoprotein expression in Childhood Nephrotic Syndrome: A longitudinal study. Cytokine 2015, 72, 190–196. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wei, S.G.; Zhao, X.; Jia, Y.Z.; Zhang, Y.F.; Sun, S.Z. Th17/Treg cell expression in children with primary nephritic syndrome and the effects of ox-LDL on Th17/Treg cells. Genet. Mol. Res. 2016, 15, gmr7669. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, J.; Yang, B.; Zhang, G.; Wang, M.; Dong, S.; Liu, W.; Yang, H.; Li, Q. IL-23 Activated gammadelta T Cells Affect Th17 cells and regulatory T cells by secreting IL-21 in Children with Primary Nephrotic Syndrome. Scand. J. Immunol. 2018, 87, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Eroglu, F.K.; Orhan, D.; Inozu, M.; Duzova, A.; Gulhan, B.; Ozaltin, F.; Topaloglu, R. CD80 expression and infiltrating regulatory T cells in idiopathic nephrotic syndrome of childhood. Pediatr. Int. 2019, 61, 1250–1256. [Google Scholar] [CrossRef]
- Takahashi, M.; Taguchi, H.; Yamaguchi, H.; Osaki, T.; Kamiya, S. Studies of the effect of Clostridium butyricum on Helicobacter pylori in several test models including gnotobiotic mice. J. Med. Microbiol. 2000, 49, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Guo, Y.; Liu, H.; Gao, J.; Nie, W. Clostridium butyricum reduce lipogenesis through bacterial wall components and butyrate. Appl. Microbiol. Biotechnol. 2014, 98, 7549–7557. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Z.; Yang, L.T.; Qiu, S.Q.; Yang, G.; Luo, X.Q.; Miao, B.P.; Geng, X.R.; Liu, Z.Q.; Liu, J.; Wen, Z.; et al. Combination of specific allergen and probiotics induces specific regulatory B cells and enhances specific immunotherapy effect on allergic rhinitis. Oncotarget 2016, 7, 54360–54369. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Pan, Q.; Liu, X.L.; Yang, R.X.; Chen, Y.W.; Liu, C.; Fan, J.G. Clostridium butyricum B1 alleviates high-fat diet-induced steatohepatitis in mice via enterohepatic immunoregulation. J. Gastroenterol. Hepatol. 2017, 32, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Xu, B.; Qin, Y.; Fan, L.; Chen, J.; Zheng, P.; Gong, X.; Wang, H.; Bai, M.; Pu, J.; et al. Clostridium butyricum miyairi 588 has preventive effects on chronic social defeat stress-induced depressive-like behaviour and modulates microglial activation in mice. Biochem. Biophys. Res. Commun. 2019, 516, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, I.; Morita, R.; Schichita, T.; Komai, K.; Saeki, K.; Matsumoto, M.; Takeda, K.; Nomura, M.; Hayashi, A.; Kanai, T.; et al. Smad2 and Smad3 inversely regulate TGF-beta autoinduction in Clostridium butyricum-activated dendritic cells. Immunity 2015, 43, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Li, D.; Feng, N.; Shamoon, M.; Sun, Z.; Ding, L.; Zhang, H.; Chen, W.; Sun, J.; Chen, Y.Q. Anti-diabetic effects of Clostridium butyricum CGMCC0313.1 through promoting the growth of gut butyrate-producing Bacteria in Type 2 Diabetic Mice. Sci. Rep. 2017, 7, 7046. [Google Scholar] [CrossRef]




| Probiotic Treatment Group | Non-Probiotic Treatment Group | p-Value | |
|---|---|---|---|
| No. of patients | 10 | 10 | |
| Age, years | 6.4 (3.7–10.6) | 4.7 (3.5–7.8) | 0.85 |
| Sex (male/female) | 9/1 | 6/4 | 0.30 |
| Observation period (months) * | 42.5 (36.8–50.5) | 52.5 (37.3–57.3) | 0.73 |
| Urinary protein, g/g Cr | 9.7 (5.8–11.6) | 13.8 (8.8–27.2) | 0.19 |
| Serum albumin, g/dL | 1.0 (0.7–1.5) | 1.3 (1.0–1.85) | 0.44 |
| Consumption of dairy foods † | 6 (60%) | 7 (70%) | 0.64 |
| Confirmed period of probiotic compliance (months) | 25 (7–46) | Not applicable | |
| Total dose of prednisolone (mg/kg) | 180.2 (83.8–230.5) | 270.9 (194.2–294.2) | 0.06 |
| No. of INS relapses per year | 1.0 (0–1.5) | 1.8 (1.5–2.0) | 0.016 |
| Use of immunosuppressive drugs | |||
| Mizoribine | 7 (70%) | 7 (70%) | 1.0 |
| Cyclosporin | 6 (60%) | 9 (90%) | 0.12 |
| Rituximab | 2 (20%) | 7 (70%) | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, T.; Tsuji, S.; Akagawa, S.; Akagawa, Y.; Kino, J.; Yamanouchi, S.; Kimata, T.; Hashiyada, M.; Akane, A.; Kaneko, K. Clinical Significance of Probiotics for Children with Idiopathic Nephrotic Syndrome. Nutrients 2021, 13, 365. https://doi.org/10.3390/nu13020365
Yamaguchi T, Tsuji S, Akagawa S, Akagawa Y, Kino J, Yamanouchi S, Kimata T, Hashiyada M, Akane A, Kaneko K. Clinical Significance of Probiotics for Children with Idiopathic Nephrotic Syndrome. Nutrients. 2021; 13(2):365. https://doi.org/10.3390/nu13020365
Chicago/Turabian StyleYamaguchi, Tadashi, Shoji Tsuji, Shohei Akagawa, Yuko Akagawa, Jiro Kino, Sohsaku Yamanouchi, Takahisa Kimata, Masaki Hashiyada, Atsushi Akane, and Kazunari Kaneko. 2021. "Clinical Significance of Probiotics for Children with Idiopathic Nephrotic Syndrome" Nutrients 13, no. 2: 365. https://doi.org/10.3390/nu13020365
APA StyleYamaguchi, T., Tsuji, S., Akagawa, S., Akagawa, Y., Kino, J., Yamanouchi, S., Kimata, T., Hashiyada, M., Akane, A., & Kaneko, K. (2021). Clinical Significance of Probiotics for Children with Idiopathic Nephrotic Syndrome. Nutrients, 13(2), 365. https://doi.org/10.3390/nu13020365