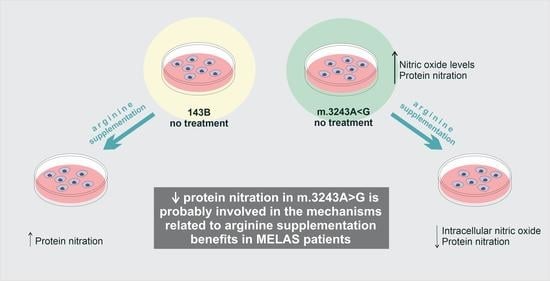
L-Arginine Reduces Nitro-Oxidative Stress in Cultured Cells with Mitochondrial Deficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Respiratory Chain Enzyme Activities
2.2.1. Spectrophometric Assays
2.2.2. Cytochemical Staining
2.3. Expression of Complex II Flavoprotein Subunit (Fp)
2.4. Quantification of mtDNA
2.5. Detection of Nitrated Proteins
2.6. Nitric Oxide Synthesis
2.6.1. Quantification of Extracellular NO
2.6.2. Quantification of Intracellular NO
2.7. Statistical Analysis
3. Results
3.1. L-ARG Had No Effects on Respiratory Chain Enzyme Activities and Mitochondrial Mass
3.2. L-ARG Supplementation Affected NO Synthesis and Protein Nitration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Böger, R.H.; Bode-Böger, S.M. The clinical pharmacology of L-arginine. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 79–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKnight, J.R.; Satterfield, M.C.; Jobgen, W.S.; Smith, S.B.; Spencer, T.E.; Meininger, C.J. Beneficial effects of L-arginine on reducing obesity: Potential mechanisms and important impli-cations for human health. Amino. Acids 2010, 39, 349–357. [Google Scholar] [CrossRef]
- Stancic, A.; Filipovic, M.; Ivanovic-Burmazovic, I.; Masovic, S.; Jankovic, A.; Otasevic, V.; Korac, A.; Buzadzic, B.; Korac, B. Early energy metabolism-related molecular events in skeletal muscle of diabetic rats: The effects of l -arginine and SOD mimic. Chem. Interactions 2017, 272, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Vasilijevic, A.; Vojčić, L.; Dinulovic, I.; Buzadzic, B.; Korać, A.; Petrovic, V.; Jankovic, A.; Korac, B. Expression pattern of thermogenesis-related factors in interscapular brown adipose tissue of alloxan-treated rats: Beneficial effect of l-arginine. Nitric. Oxide 2010, 23, 42–50. [Google Scholar] [CrossRef]
- Desquiret-Dumas, V.; Gueguen, N.; Barth, M.; Chevrollier, A.; Hancock, S.; Wallace, D.C. Metabolically induced heteroplasmy shifting and l-arginine treatment reduce the energet-ic defect in a neuronal-like model of MELAS. Biochim. Biophys. Acta 2012, 1822, 1019–1029. [Google Scholar] [CrossRef] [Green Version]
- El-Hattab, A.W.; Emrick, L.T.; Craigen, W.J.; Scaglia, F. Citrulline and arginine utility in treating nitric oxide deficiency in mitochondrial disorders. Mol. Genet. Metab. 2012, 107, 247–252. [Google Scholar] [CrossRef]
- Fryer, R.H.; Bain, J.M.; De Vivo, D.C. Mitochondrial Encephalomyopathy Lactic Acidosis and Stroke-Like Epi-sodes (MELAS): A Case Report and Critical Reappraisal of Treatment Options. Pediatr. Neurol. 2016, 56, 59–61. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Hsu, J.W.; Emrick, L.T.; Wong LJ, C.; Craigen, W.J.; Jahoor, F.; Scaglia, F. Restoration of impaired nitric oxide production in MELAS syndrome with citrulline and argi-nine supplementation. Mol. Genet. Metab. 2012, 105, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Koga, Y.; Akita, Y.; Junko, N.; Yatsuga, S.; Povalko, N.; Fukiyama, R.; Ishii, M.; Matsuishi, T. Endothelial dysfunction in MELAS improved by l-arginine supplementation. Neurol. 2006, 66, 1766–1769. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Povalko, N.; Nishioka, J.; Katayama, K.; Yatsuga, S.; Matsuishi, T. Molecular pathology of MELAS and l-arginine effects. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 608–614. [Google Scholar] [CrossRef]
- Koga, Y.; Ishibashi, M.; Ueki, I.; Yatsuga, S.; Fukiyama, R.; Akita, Y.; Matsuishi, T. Effects of L-arginine on the acute phase of strokes in three patients with MELAS. Neurology 2002, 58, 827–828. [Google Scholar] [CrossRef] [Green Version]
- El-Hattab, A.W.; Emrick, L.T.; Williamson, K.C.; Craigen, W.J.; Scaglia, F. The effect of citrulline and arginine supplementation on lactic acidemia in MELAS syndrome. Meta Gene. 2013, 1, 8–14. [Google Scholar] [CrossRef]
- Coman, D.J.; Yaplito-Lee, J.; Boneh, A. New indications and controversies in arginine therapy. Clin. Nutr. 2008, 27, 489–496. [Google Scholar] [CrossRef]
- Litvinova, L.; Atochin, D.N.; Efattakhov, N.; Evasilenko, M.; Ezatolokin, P.; Ekirienkova, E. Nitric oxide and mitochondria in metabolic syndrome. Front. Physiol. 2015, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Elms, S.; Chen, F.; Wang, Y.; Qian, J.; Askari, B.; Yu, Y.; Pandey, D.; Iddings, J.A.; Caldwell, R.B.; Fulton, D.J.R. Insights into the arginine paradox: Evidence against the importance of subcellular location of arginase and eNOS. Am. J. Physiol. Circ. Physiol. 2013, 305, H651–H666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popolo, A.; Adesso, S.; Pinto, A.; Autore, G.; Marzocco, S. l-Arginine and its metabolites in kidney and cardiovascular disease. Amino. Acids 2014, 46, 2271–2286. [Google Scholar] [CrossRef]
- Rath, M.; Müller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macro-phages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef] [Green Version]
- Ghafourifar, P.; Asbury, M.L.; Joshi, S.S.; Kincaid, E.D. Determination of Mitochondrial Nitric Oxide Synthase Activity. Methods Enzymol. 2005, 396, 424–444. [Google Scholar] [CrossRef] [PubMed]
- El-Hattab, A.W.; Zarante, A.M.; Almannai, M.; Scaglia, F. Therapies for mitochondrial diseases and current clinical trials. Mol. Genet. Metab. 2017, 122, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bossy-Wetzel, E.; A Lipton, S. Nitric oxide signaling regulates mitochondrial number and function. Cell Death Differ. 2003, 10, 757–760. [Google Scholar] [CrossRef]
- King, M.P.; Koga, Y.; Davidson, M.; Schon, E.A. Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNA(Leu(UUR)) mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike epi-sodes. Mol. Cell Biol. 1992, 12, 489–490. [Google Scholar] [CrossRef] [Green Version]
- Gamba, J.; Gamba, L.T.; Rodrigues, G.S.; Kiyomoto, B.H.; Moraes, C.T.; Tengan, C.H. Nitric Oxide Synthesis Is Increased in Cybrid Cells with m.3243A>G Mutation. Int. J. Mol. Sci. 2012, 14, 394–410. [Google Scholar] [CrossRef] [Green Version]
- Barrientos, A. In vivo and in organello assessment of OXPHOS activities. Methods 2002, 26, 307–316. [Google Scholar] [CrossRef]
- Venegas, V.; Wang, J.; Dimmock, D.; Wong, L.-J.C. Real-Time Quantitative PCR Analysis of Mitochondrial DNA Content. Curr. Protoc. Hum. Genet. 2011, 68, 19-7. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.; Silva, F.D.A.E.; Kurihara, R.S.; Schor, N.; Higa, E.M. Evaluation of the nitric oxide production in rat renal artery smooth muscle cells culture exposed to radiocontrast agents. Kidney Int. 2004, 65, 589–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parikh, S.; Goldstein, A.; Koenig, M.K.; Scaglia, F.; Enns, G.M.; Saneto, R.; Anselm, I.; Cohen, B.H.; Falk, M.J.; Greene, C.; et al. Diagnosis and management of mitochondrial disease: A consensus statement from the Mitochondrial Medicine Society. Genet. Med. 2015, 17, 689–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, M.K.; Emrick, L.; Karaa, A.; Korson, M.; Scaglia, F.; Parikh, S.; Goldstein, A. Recommendations for the Management of Strokelike Episodes in Patients With Mitochondrial Encephalomyopathy, Lactic Acidosis, and Strokelike Episodes. JAMA Neurol. 2016, 73, 591–594. [Google Scholar] [CrossRef]
- Bombicino, S.S.; Iglesias, D.E.; Zaobornyj, T.; Boveris, A.; Valdez, L.B. Mitochondrial nitric oxide production supported by reverse electron transfer. Arch. Biochem. Biophys. 2016, 607, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Tengan, C.H.; Rodrigues, G.S.; Godinho, R.O. Nitric Oxide in Skeletal Muscle: Role on Mitochondrial Biogenesis and Function. Int. J. Mol. Sci. 2012, 13, 17160–17184. [Google Scholar] [CrossRef] [Green Version]
- Koga, Y.; Akita, Y.; Nishioka, J.; Yatsuga, S.; Povalko, N.; Katayama, K.; Matsuishi, T. MELAS and l-arginine therapy. Mitochondrion 2007, 7, 133–139. [Google Scholar] [CrossRef]
- Vattemi, G.; Mechref, Y.; Marini, M.; Tonin, P.; Minuz, P.; Grigoli, L.; Guglielmi, V.; Klouckova, I.; Chiamulera, C.; Meneguzzi, A.; et al. Increased Protein Nitration in Mitochondrial Diseases: Evidence for Vessel Wall Involvement. Mol. Cell. Proteom. 2011, 10, M110-002964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.; Fru, M.F.; Yu, Y.; Montani, J.-P.; Ming, X.-F.; Yang, Z. Long term exposure to L-arginine accelerates endothelial cell senescence through arginase-II and S6K1 signaling. Aging 2014, 6, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacPherson, H.; Noble, B.; Ralston, S. Expression and functional role of nitric oxide synthase isoforms in human osteoblast-like cells. Bone 1999, 24, 179–185. [Google Scholar] [CrossRef]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeu-tic targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Alkaitis, M.S.; Crabtree, M.J. Recoupling the cardiac nitric oxide synthases: Tetrahydrobiopterin synthesis and re-cycling. Curr. Heart Fail Rep. 2012, 9, 200–210. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.; Masuda, M.; Nakayama, R.; Ichikawa, H.; Satow, R.; Shitashige, M. Reduced argininosuccinate synthetase is a predictive biomarker for the development of pulmo-nary metastasis in patients with osteosarcoma. Mol. Cancer Ther. 2010, 9, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Scalera, F.; Closs, E.I.; Flick, E.; Martens-Lobenhoffer, J.; Boissel, J.P.; Lendeckel, U.; Heimburg, A.; Bode-Böger, S.M. Paradoxical effect of l-arginine: Acceleration of endothelial cell senescence. Biochem. Biophys. Res. Commun. 2009, 386, 650–655. [Google Scholar] [CrossRef]
- Li, H.; Meininger, C.J.; Hawker, J.R.; Haynes, T.E.; Kepka-Lenhart, D.; Mistry, S.K.; Morris, S.M.; Wu, G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am. J. Physiol. Metab. 2001, 280, E75–E82. [Google Scholar] [CrossRef] [Green Version]
- Moretto, J.; Guglielmetti, A.-S.; Tournier-Nappey, M.; Martin, H.; Prigent-Tessier, A.; Marie, C.; Demougeot, C. Effects of a chronic l -arginine supplementation on the arginase pathway in aged rats. Exp. Gerontol. 2017, 90, 52–60. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, C.D.S.; Livramento, J.B.; Mouro, M.G.; Higa, E.M.S.; Moraes, C.T.; Tengan, C.H. L-Arginine Reduces Nitro-Oxidative Stress in Cultured Cells with Mitochondrial Deficiency. Nutrients 2021, 13, 534. https://doi.org/10.3390/nu13020534
Barros CDS, Livramento JB, Mouro MG, Higa EMS, Moraes CT, Tengan CH. L-Arginine Reduces Nitro-Oxidative Stress in Cultured Cells with Mitochondrial Deficiency. Nutrients. 2021; 13(2):534. https://doi.org/10.3390/nu13020534
Chicago/Turabian StyleBarros, Camila D. S., Jomênica B. Livramento, Margaret G. Mouro, Elisa Mieko Suemitsu Higa, Carlos T. Moraes, and Celia Harumi Tengan. 2021. "L-Arginine Reduces Nitro-Oxidative Stress in Cultured Cells with Mitochondrial Deficiency" Nutrients 13, no. 2: 534. https://doi.org/10.3390/nu13020534
APA StyleBarros, C. D. S., Livramento, J. B., Mouro, M. G., Higa, E. M. S., Moraes, C. T., & Tengan, C. H. (2021). L-Arginine Reduces Nitro-Oxidative Stress in Cultured Cells with Mitochondrial Deficiency. Nutrients, 13(2), 534. https://doi.org/10.3390/nu13020534