Impact of Micronutrients on Hypertension: Evidence from Clinical Trials with a Special Focus on Meta-Analysis
Abstract
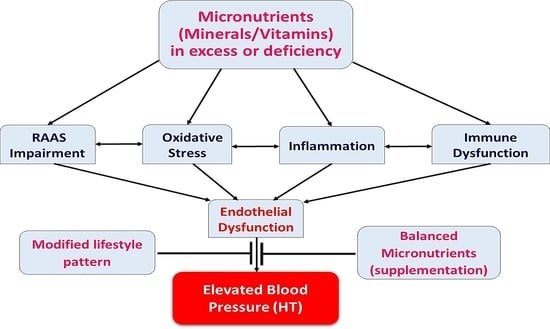
:1. Introduction
2. Relationship with Different Minerals with HT
2.1. Sodium (Na+)
2.1.1. BP Regulating Mechanism ((Na+/Salt) Induced HT)
2.1.2. Clinical Evidence
2.2. Potassium (K+)
2.2.1. BP Regulating Mechanism
2.2.2. Clinical Evidence
2.3. Calcium (Ca2+)
2.3.1. BP Regulating Mechanism
2.3.2. Clinical Evidence
2.4. Magnesium (Mg2+)
2.4.1. BP Regulating Mechanism
2.4.2. Clinical Evidence
2.5. Zinc (Zn2+)
2.5.1. BP Regulating Mechanism
2.5.2. Clinical Evidence
2.6. Selenium (Se2−)
2.6.1. BP Regulating Mechanism
2.6.2. Clinical Evidence
2.7. Copper (Cu2+)
2.7.1. BP Regulating Mechanism
2.7.2. Clinical Evidence
3. Relationship with Different Vitamins on HT
3.1. Vitamin C or Ascorbate
3.1.1. BP Regulating Mechanism
3.1.2. Clinical Evidence
3.2. Vitamin D or Cholecalciferol
3.2.1. BP Regulating Mechanism
3.2.2. Clinical Evidence
3.3. Vitamin E or Tocopherol
3.3.1. BP Regulating Mechanism
3.3.2. Clinical Evidence
3.4. Vitamin B Complex
3.4.1. BP Regulating Mechanism
3.4.2. Clinical Evidence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giles, T.D.; Materson, B.J.; Cohn, J.N.; Kostis, J.B. Definition and Classification of Hypertension: An Update. J. Clin. Hypertens. 2009, 11, 611–614. [Google Scholar] [CrossRef]
- Venkatakrishnan, K.; Chiu, H.F.; Wang, C.K. Impact of Functional Foods and Nutraceuticals on High Blood Pressure with a Special Focus on Meta-Analysis: Review from a Public Health Perspective. Food Funct. 2020, 11, 2792–2804. [Google Scholar] [CrossRef] [PubMed]
- Adrogué, H.J.; Madias, N.E. Sodium and Potassium in the Pathogenesis of Hypertension. N. Engl. J. Med. 2007, 356, 1966–1978. [Google Scholar] [CrossRef] [Green Version]
- Beunza, J.J.; Martínez-González, M.A.; Ebrahim, S.; Bes-Rastrollo, M.; Núñez, J.; Martínez, J.A.; Alonso, A. Sedentary Behaviors and the Risk of Incident Hypertension: The SUN Cohort. Am. J. Hypertens. 2007, 20, 1156–1162. [Google Scholar]
- Nguyen, H.; Odelola, O.A.; Rangaswami, J.; Amanullah, A. A Review of Nutritional Factors in Hypertension Management. Int. J. Hypertens. 2013, 2013, 698940. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Damasceno, A. Hypertension in Developing Countries. Lancet 2012, 380, 611–619. [Google Scholar] [CrossRef]
- Darroudi, S.; Saberi-Karimian, M.; Tayefi, M.; Tayefi, B.; Khashyarmanesh, Z.; Fereydouni, N.; Haghighi, H.M.; Mahmoudi, A.A.; Kharazmi-Khorassani, J.; Gonoodi, K.; et al. Association between Hypertension in Healthy Participants and Zinc and Copper Status: A Population-Based Study. Biol. Trace Elem. Res. 2019, 190, 38–44. [Google Scholar] [CrossRef]
- Kitt, J.; Fox, R.; Tucker, K.L.; McManus, R.J. New Approaches in Hypertension Management: A Review of Current and Developing Technologies and Their Potential Impact on Hypertension Care. Curr. Hypertens. Rep. 2019, 21, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawes, C.M.M.; Vander Hoorn, S.; Rodgers, A.; International Society of Hypertension. Global Burden of Blood-Pressure-Related Disease, 2001. Lancet 2008, 371, 1513–1518. [Google Scholar] [CrossRef]
- Iqbal, S.; Klammer, N.; Ekmekcioglu, C. The Effect of Electrolytes on Blood Pressure: A Brief Summary of Meta-Analyses. Nutrients 2019, 11, 1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suliburska, J.; Skrypnik, K.; Szulińska, M.; Kupsz, J.; Bogdański, P. Effect of Hypotensive Therapy Combined with Modified Diet or Zinc Supplementation on Biochemical Parameters and Mineral Status in Hypertensive Patients. J. Trace Elem. Med. Biol. 2018, 47, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Slimko, M.L.; Mensah, G.A. The Role of Diets, Food, and Nutrients in the Prevention and Control of Hypertension and Prehypertension. Cardiol. Clin. 2010, 28, 665–674. [Google Scholar] [CrossRef]
- Savica, V.; Bellinghieri, G.; Kopple, J.D. The Effect of Nutrition on Blood Pressure. Annu. Rev. Nutr. 2010, 30, 365–401. [Google Scholar] [CrossRef] [Green Version]
- Houston, M.C. The Role of Nutrition, Nutraceuticals, Vitamins, Antioxidants, and Minerals in the Prevention and Treatment of Hypertension. Altern. Ther. Health Med. 2013, 19 (Suppl. S1), 32–49. [Google Scholar] [PubMed]
- Kim, S.Y.; Kang, M.H.; Choi, M.K. Relationship between Five Serum Minerals (Na, K, Cl, Ca, P) and Blood Pressure and Biomarkers in Healthy Adults. Trace Elem. Electrolytes 2016, 33, 47–54. [Google Scholar] [CrossRef]
- Godswill, A.G.; Somtochukwu, I.V.; Ikechukwu, A.O.; Kate, E.C. Health benefits of micronutrients (vitamins and minerals) and their Associated Deficiency Diseases: A systematic review. Int. J. Food Sci. 2020, 3, 1–32. [Google Scholar]
- Ravi, S.; Bermudez, O.I.; Harivanzan, V.; Kenneth Chui, K.H.; Vasudevan, P.; Must, A.; Thanikachalam, S.; Thanikachalam, M. Sodium Intake, Blood Pressure, and Dietary Sources of Sodium in an Adult South Indian Population. Ann. Glob. Health 2016, 82, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, J.L.; Dunford, E.K.; Neal, B.C. A Systematic Survey of the Sodium Contents of Processed Foods. Am. J. Clin. Nutr. 2010, 91, 413–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karppanen, H.; Mervaala, E. Sodium Intake and Hypertension. Prog. Cardiovasc. Dis. 2006, 49, 59–75. [Google Scholar] [CrossRef]
- Haddy, F.J. Role of Dietary Salt in Hypertension. Life Sci. 2006, 79, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Intersalt Cooperative Research Group. Intersalt: An International Study of Electrolyte Excretion and Blood Pressure. Results for 24 Hour Urinary Sodium and Potassium Excretion. BMJ 1988, 297, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, H.F.; Venkatakrishnan, K.; Wang, C.K. Nutraceuticals and Functional Foods in the Prevention of Hypertension Induced by Excessive Intake of Dietary Salt. In Dietary Sugar, Salt and Fat in Human Health; Academic Press: Cambridge, MA, USA, 2020; pp. 423–450. [Google Scholar]
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium Intake and Hypertension. Nutrients 2019, 11, 1970. [Google Scholar] [CrossRef] [Green Version]
- Jaitovich, A.; Bertorello, A.M. Salt, Na, K-ATPase and Hypertension. Life Sci. 2010, 86, 73–78. [Google Scholar] [CrossRef]
- Paczula, A.; Wiecek, A.; Piecha, G. Cardiotonic Steroids-A Possible Link between High-Salt Diet and Organ Damage. Int. J. Mol. Sci. 2019, 20, 590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, E.J.; Bauer, J.D.; Hawley, C.M.; Isbel, N.M.; Stowasser, M.; Johnson, D.W.; Campbell, K.L. A Randomized Trial of Dietary Sodium Restriction in CKD. J. Am. Soc. Nephrol. 2013, 24, 2096–2103. [Google Scholar] [CrossRef] [Green Version]
- He, F.J.; Marciniak, M.; Visagie, E.; Markandu, N.D.; Anand, V.; Dalton, R.N.; MacGregor, G.A. Effect of Modest Salt Reduction on Blood Pressure, Urinary Albumin, and Pulse Wave Velocity in White, Black, and Asian Mild Hypertensives. Hypertension 2009, 54, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.S.; Ashton, K.E.; Moxham, T.; Hooper, L.; Ebrahim, S. Reduced Dietary Salt for the Prevention of Cardiovascular Disease: A Meta-Analysis of Randomized Controlled Trials (Cochrane Review). Am. J. Hypertens. 2011, 24, 843–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aburto, N.J.; Hanson, S.; Gutierrez, H.; Hooper, L.; Elliott, P.; Cappuccio, F.P. Effect of Increased Potassium Intake on Cardiovascular Risk Factors and Disease: Systematic Review and Meta-Analyses. BMJ 2013, 346, f1378. [Google Scholar] [CrossRef] [Green Version]
- Suckling, R.J.; He, F.J.; Markandu, N.D.; MacGregor, G.A. Modest Salt Reduction Lowers Blood Pressure and Albumin Excretion in Impaired Glucose Tolerance and Type 2 Diabetes Mellitus: A Randomized Double-Blind Trial. Hypertension 2016, 67, 1189–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Elia, L.; Galletti, F.; La Fata, E.; Sabino, P.; Strazzullo, P. Effect of Dietary Sodium Restriction on Arterial Stiffness: Systematic Review and Meta-Analysis of the Randomized Controlled Trials. J. Hypertens. 2018, 36, 734–743. [Google Scholar] [CrossRef]
- Suter, P.M. Potassium and Hypertension. Nutr. Rev. 1998, 56 Pt 1, 151–153. [Google Scholar] [CrossRef] [Green Version]
- Filippini, T.; Naska, A.; Kasdagli, M.I.; Torres, D.; Lopes, C.; Carvalho, C.; Moreira, P.; Malavolti, M.; Orsini, N.; Whelton, P.K.; et al. Potassium Intake and Blood Pressure: A Dose-Response Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2020, 9, e015719. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. Nutrition and Nutraceutical Supplements in the Treatment of Hypertension. Expert Rev. Cardiovasc. Ther. 2010, 8, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Hao, Y.C.; Liu, J.; Wang, W.; Wang, M.; Li, G.Q.; Qi, Y.; Zhao, F.; Xie, W.X.; Li, Y.; et al. Associations between Serum Potassium and Sodium Levels and Risk of Hypertension: A Community-Based Cohort Study. J. Geriatr. Cardiol. 2015, 12, 119–126. [Google Scholar] [PubMed]
- Dickinson, H.O.; Nicolson, D.J.; Campbell, F.; Beyer, F.R.; Mason, J. Potassium Supplementation for the Management of Primary Hypertension in Adults. Cochrane Database Syst. Rev. 2006, 3, CD004641. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C. Treatment of Hypertension with Nutrition and Nutraceutical Supplements: Part 1. Altern. Complement. Ther. 2018, 24, 260–275. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; MacGregor, G.A. Does Potassium Supplementation Lower Blood Pressure? A Meta-Analysis of Published Trials. J. Hypertens. 1991, 9, 465–473. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Kok, F.J.; Grobbee, D.E. Blood Pressure Response to Changes in Sodium and Potassium Intake: A Metaregression Analysis of Randomised Trials. J. Hum. Hypertens. 2003, 17, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Filippini, T.; Violi, F.; D’Amico, R.; Vinceti, M. The Effect of Potassium Supplementation on Blood Pressure in Hypertensive Subjects: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2017, 230, 127–135. [Google Scholar] [CrossRef]
- Cormick, G.; Ciapponi, A.; Cafferata, M.L.; Belizán, J.M. Calcium Supplementation for Prevention of Primary Hypertension. Cochrane Database Syst. Rev. 2015, 6, CD010037. [Google Scholar] [CrossRef]
- Imdad, A.; Jabeen, A.; Bhutta, Z.A. Role of Calcium Supplementation during Pregnancy in Reducing Risk of Developing Gestational Hypertensive Disorders: A Meta-Analysis of Studies from Developing Countries. BMC Public Health 2011, 11 (Suppl. 3), S18. [Google Scholar] [CrossRef] [Green Version]
- Van Mierlo, L.A.; Arends, L.R.; Streppel, M.T.; Zeegers, M.P.A.; Kok, F.J.; Grobbee, D.E.; Geleijnse, J.M. Blood Pressure Response to Calcium Supplementation: A Meta-Analysis of Randomized Controlled Trials. J. Hum. Hypertens. 2006, 20, 571–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, L.E.; Guyatt, G.H.; Cook, R.J.; Bucher, H.C.; Cook, D.J. The Influence of Dietary and Nondietary Calcium Supplementation on Blood Pressure: An Updated Metaanalysis of Randomized Controlled Trials. Am. J. Hypertens. 1999, 12 Pt 1, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Khanam, F.; Hossain, B.; Mistry, S.K.; Mitra, D.K.; Raza, W.A.; Rifat, M.; Afsana, K.; Rahman, M. The Association between Daily 500 Mg Calcium Supplementation and Lower Pregnancy-Induced Hypertension Risk in Bangladesh. BMC Pregnancy Childbirth 2018, 18, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunutsor, S.K.; Laukkanen, J.A. Circulating Active Serum Calcium Reduces the Risk of Hypertension. Eur. J. Prev. Cardiol. 2017, 24, 239–243. [Google Scholar] [CrossRef]
- Garland, C.J.; Bagher, P.; Powell, C.; Ye, X.; Lemmey, H.A.L.; Borysova, L.; Dora, K.A. Voltage-Dependent Ca2+ Entry into Smooth Muscle during Contraction Promotes Endothelium-Mediated Feedback Vasodilation in Arterioles. Sci. Signal. 2017, 10, eaal3806. [Google Scholar] [CrossRef] [Green Version]
- Hatton, D.C.; McCarron, D.A. Dietary Calcium and Blood Pressure in Experimental Models of Hypertension. A Review. Hypertension 1994, 23, 513–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucher, H.C.; Cook, R.J.; Guyatt, G.H.; Lang, J.D.; Cook, D.J.; Hatala, R.; Hunt, D.L. Effects of Dietary Calcium Supplementation on Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. JAMA 1996, 275, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Del Gobbo, L.C.; Rosanoff, A.; Wang, J.; Zhang, W.; Song, Y. Effects of Magnesium Supplementation on Blood Pressure: A Meta-Analysis of Randomized Double-Blind Placebo-Controlled Trials: A Meta-Analysis of Randomized Double-Blind Placebo-Controlled Trials. Hypertension 2016, 68, 324–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, Y.; Matsuoka, H.; Takishita, S.; Omae, T. Effects of Magnesium Supplementation in Hypertensive Patients: Assessment by Office, Home, and Ambulatory Blood Pressures. Hypertension 1998, 32, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Jee, S.H.; Miller, E.R., 3rd; Guallar, E.; Singh, V.K.; Appel, L.J.; Klag, M.J. The Effect of Magnesium Supplementation on Blood Pressure: A Meta-Analysis of Randomized Clinical Trials. Am. J. Hypertens. 2002, 15, 691–696. [Google Scholar] [CrossRef] [Green Version]
- Geleijnse, J.M.; Witteman, J.C.; den Breeijen, J.H.; Hofman, A.; de Jong, P.T.; Pols, H.A.; Grobbee, D.E. Dietary Electrolyte Intake and Blood Pressure in Older Subjects: The Rotterdam Study. J. Hypertens. 1996, 14, 737–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taneja, S.K.; Mandal, R. Mineral Factors Controlling Essential Hypertension—A Study in the Chandigarh, India Population. Biol. Trace Elem. Res. 2007, 120, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Houston, M. The Role of Magnesium in Hypertension and Cardiovascular Disease: Magnesium, Hypertension, and Cardiovascular Disease. J. Clin. Hypertens. (Greenwich) 2011, 13, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, S.; Cappuccio, F.P.; Nichols, R.; Elliott, P. Dietary Magnesium Intake and Blood Pressure: A Qualitative Overview of the Observational Studies. J. Hum. Hypertens. 1998, 12, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Romani, A.M.P. Beneficial Role of Mg2+ in Prevention and Treatment of Hypertension. Int. J. Hypertens. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sontia, B.; Touyz, R.M. Role of Magnesium in Hypertension. Arch. Biochem. Biophys. 2007, 458, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kass, L.; Weekes, J.; Carpenter, L. Effect of Magnesium Supplementation on Blood Pressure: A Meta-Analysis. Eur. J. Clin. Nutr. 2012, 66, 411–418. [Google Scholar] [CrossRef]
- Verma, H.; Garg, R. Effect of Magnesium Supplementation on Type 2 Diabetes Associated Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. J. Hum. Nutr. Diet. 2017, 30, 621–633. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and Human Health: An Update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Nevárez-López, S.C.; Simental-Mendía, L.E.; Guerrero-Romero, F.; Burciaga-Nava, J.A. Zinc Deficiency Is an Independent Risk Factor for Prehypertension in Healthy Subjects. Int. J. Vitam. Nutr. Res. 2019, 91, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Wathurapatha, W.S.; Galappatthy, P.; Katulanda, P.; Jayawardena, R.; Constantine, G.R. Zinc Supplementation in Prediabetes: A Randomized Double-Blind Placebo-Controlled Clinical Trial. J. Diabetes 2018, 10, 386–397. [Google Scholar] [CrossRef]
- Çıkım, G.; İzgi, K.; Aksu, E. The Levels of Trace Elements and Homocysteine in Arterial Hypertension. Trace Elem. Electrolytes 2017, 34, 34–39. [Google Scholar] [CrossRef]
- Afridi, H.I.; Kazi, T.G.; Talpur, F.N.; Kazi, A.; Arain, S.S.; Arain, S.A.; Brahman, K.D.; Panhwar, A.H.; Shezadi, M.; Ali, J. Interaction between Essential Elements Selenium and Zinc with Cadmium and Mercury in Samples from Hypertensive Patients. Biol. Trace Elem. Res. 2014, 160, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Canatan, H.; Bakan, I.; Akbulut, M.; Halifeoglu, I.; Cikim, G.; Baydas, G.; Kilic, N. Relationship among Levels of Leptin and Zinc, Copper, and Zinc/Copper Ratio in Plasma of Patients with Essential Hypertension and Healthy Normotensive Subjects. Biol. Trace Elem. Res. 2004, 100, 117–123. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Laukkanen, J.A. Serum Zinc Concentrations and Incident Hypertension: New Findings from a Population-Based Cohort Study. J. Hypertens. 2016, 34, 1055–1061. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Hu, P.; Zhang, D. Associations between Copper and Zinc and Risk of Hypertension in US Adults. Biol. Trace Elem. Res. 2018, 186, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Dietary Zinc Intake Is Inversely Associated with Systolic Blood Pressure in Young Obese Women. Nutr. Res. Pract. 2013, 7, 380–384. [Google Scholar] [CrossRef]
- Sentürk, U.K.; Kaputlu, I.; Gündüz, F.; Kuru, O.; Gökalp, O. Tissue and Blood Levels of Zinc, Copper, and Magnesium in Nitric Oxide Synthase Blockade-Induced Hypertension. Biol. Trace Elem. Res. 2000, 77, 97–106. [Google Scholar] [CrossRef]
- Riordan, J.F. Angiotensin-I-Converting Enzyme and Its Relatives. Genome Biol. 2003, 4, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, S.M.; Mofrad, M.D.; do Nascimento, I.J.B.; Milajerdi, A.; Mokhtari, T.; Esmaillzadeh, A. The Effect of Zinc Supplementation on Blood Pressure: A Systematic Review and Dose-Response Meta-Analysis of Randomized-Controlled Trials. Eur. J. Nutr. 2020, 59, 1815–1827. [Google Scholar] [CrossRef]
- McDaid, O.; Stewart-Knox, B.; Parr, H.; Simpson, E. Dietary Zinc Intake and Sex Differences in Taste Acuity in Healthy Young Adults. J. Hum. Nutr. Diet. 2007, 20, 103–110. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Liu, H.; Li, S.; Zhang, D. The Association of Serum Zinc and Copper with Hypertension: A Meta-Analysis. J. Trace Elem. Med. Biol. 2019, 53, 41–48. [Google Scholar] [CrossRef]
- Drake, E.N. Cancer Chemoprevention: Selenium as a Prooxidant, Not an Antioxidant. Med. Hypotheses 2006, 67, 318–322. [Google Scholar] [CrossRef]
- Vinceti, M.; Chawla, R.; Filippini, T.; Dutt, C.; Cilloni, S.; Loomba, R.; Bargellini, A.; Orsini, N.; Dhillon, K.S.; Whelton, P. Blood Pressure Levels and Hypertension Prevalence in a High Selenium Environment: Results from a Cross-Sectional Study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 398–408. [Google Scholar] [CrossRef]
- Jossa, F.; Trevisan, M.; Krogh, V.; Farinaro, E.; Giumetti, D.; Fusco, G.; Galasso, R.; Panico, S.; Frascatore, S.; Mellone, C. Serum Selenium and Coronary Heart Disease Risk Factors in Southern Italian Men. Atherosclerosis 1991, 87, 129–134. [Google Scholar] [CrossRef]
- Virtamo, J.; Valkeila, E.; Alfthan, G.; Punsar, S.; Huttunen, J.K.; Karvonen, M.J. Serum Selenium and the Risk of Coronary Heart Disease and Stroke. Am. J. Epidemiol. 1985, 122, 276–282. [Google Scholar] [CrossRef]
- Nawrot, T.S.; Staessen, J.A.; Roels, H.A.; Den Hond, E.; Thijs, L.; Fagard, R.H.; Dominiczak, A.F.; Struijker-Boudier, H.A. Blood Pressure and Blood Selenium: A Cross-Sectional and Longitudinal Population Study. Eur. Heart J. 2007, 28, 628–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastola, M.M.; Locatis, C.; Maisiak, R.; Fontelo, P. Selenium, Copper, Zinc and Hypertension: An Analysis of the National Health and Nutrition Examination Survey (2011–2016). BMC Cardiovasc. Disord. 2020, 20, 45. [Google Scholar] [CrossRef] [Green Version]
- Berthold, H.K.; Michalke, B.; Krone, W.; Guallar, E.; Gouni-Berthold, I. Influence of Serum Selenium Concentrations on Hypertension: The Lipid Analytic Cologne Cross-Sectional Study. J. Hypertens. 2012, 30, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Costa, S.M.; Valerio, E.G.; Ramos, J.G.L. Comparison of Serum Selenium Levels among Hypertensive and Normotensive Pregnant Women from Southern Brazil: A Case Control-Study. Pregnancy Hypertens. 2016, 6, 213. [Google Scholar] [CrossRef]
- Kuruppu, D.; Hendrie, H.C.; Yang, L.; Gao, S. Selenium Levels and Hypertension: A Systematic Review of the Literature. Public Health Nutr. 2014, 17, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Hashiura, Y.; Matsumura, K.; Matsukawa, T.; Shinohara, A.; Furuta, N. Dynamic Pathways of Selenium Metabolism and Excretion in Mice under Different Selenium Nutritional Statuses. Metallomics 2010, 2, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, W.E.; Lam, D.; Toney, G.M.; Weintraub, N.L.; Qin, Z. Zinc, Copper, and Blood Pressure: Human Population Studies. Med. Sci. Monit. 2013, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chiplonkar, S.A.; Agte, V.V.; Tarwadi, K.V.; Paknikar, K.M.; Diwate, U.P. Micronutrient Deficiencies as Predisposing Factors for Hypertension in Lacto-Vegetarian Indian Adults. J. Am. Coll. Nutr. 2004, 23, 239–247. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.; Liao, Y.; Zhu, Z.; Yang, L.; Zhang, Q. The Association between Serum Copper Concentrations and Elevated Blood Pressure in US children and adolescents: National Health and Nutrition Examination Survey 2011–2016. BMC Cardiovascular Disorders 2020, 21, 1–7. [Google Scholar]
- Loyke, H.F. Copper and Zinc in Experimental Hypertension. Biol. Trace Elem. Res. 1991, 29, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Saltman, P. Trace Elements and Blood Pressure. Ann. Intern. Med. 1983, 98 Pt 2, 823–827. [Google Scholar] [CrossRef]
- Filetti, F.M.; Vassallo, D.V.; Fioresi, M.; Simões, M.R. Reactive Oxygen Species Impair the Excitation-Contraction Coupling of Papillary Muscles after Acute Exposure to a High Copper Concentration. Toxicol. In Vitro 2018, 51, 106–113. [Google Scholar] [CrossRef]
- Ghayour-Mobarhan, M.; Shapouri-Moghaddam, A.; Azimi-Nezhad, M.; Esmaeili, H.; Parizadeh, S.M.R.; Safarian, M.; Kazemi-Bajestani, S.M.R.; Khodaei, G.H.; Hosseini, S.J.; Parizadeh, S.M.J.; et al. The Relationship between Established Coronary Risk Factors and Serum Copper and Zinc Concentrations in a Large Persian Cohort. J. Trace Elem. Med. Biol. 2009, 23, 167–175. [Google Scholar] [CrossRef]
- Maqbool, M.A.; Aslam, M.; Akbar, W.; Iqbal, Z. Biological Importance of Vitamins for Human Health: A Review. J. Agric. Basic Sci. 2018, 2, 50–58. [Google Scholar]
- Czernichow, S.; Blacher, J.; Hercberg, S. Antioxidant Vitamins and Blood Pressure. Curr. Hypertens. Rep. 2004, 6, 27–30. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Hamm, L.; Batuman, V.; Whelton, P.K. Serum Antioxidant Vitamins and Blood Pressure in the United States Population. Hypertension 2002, 40, 810–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walingo, K.M. Role of Vitamin C (Ascorbic Acid) on Human Health-a Review. Afr. J. Food Agric. Nutr. Dev. 2005, 5. [Google Scholar]
- Mahajan, A.S.; Babbar, R.; Kansal, N.; Agarwal, S.K.; Ray, P.C. Antihypertensive and Antioxidant Action of Amlodipine and Vitamin C in Patients of Essential Hypertension. J. Clin. Biochem. Nutr. 2007, 40, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ness, A.R.; Chee, D.; Elliott, P. Vitamin C and Blood Pressure—An Overview. J. Hum. Hypertens. 1997, 11, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Plantinga, Y.; Ghiadoni, L.; Magagna, A.; Giannarelli, C.; Franzoni, F.; Taddei, S.; Salvetti, A. Supplementation with Vitamins C and E Improves Arterial Stiffness and Endothelial Function in Essential Hypertensive Patients. Am. J. Hypertens. 2007, 20, 392–397. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Grassi, D.; Tocci, G.; Galletti, F.; Borghi, C.; Ferri, C. Nutrients and Nutraceuticals for the Management of High Normal Blood Pressure: An Evidence‑based Consensus Document. Hypertension 2019, 0, 27–41. [Google Scholar] [CrossRef] [Green Version]
- Duffy, S.; Gokce, N.; Holbrook, M.; Huang, A.; Frei, B.; Keaney, J.F., Jr.; Vita, J.A. Treatment of hypertension with ascorbic acid. Lancet 1999, 354, 2048–2049. [Google Scholar] [CrossRef]
- McRae, M.P. Is Vitamin C an Effective Antihypertensive Supplement? A Review and Analysis of the Literature. J. Chiropr. Med. 2006, 5, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Juraschek, S.P.; Guallar, E.; Appel, L.J.; Miller, E.R., 3rd. Effects of Vitamin C Supplementation on Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2012, 95, 1079–1088. [Google Scholar] [CrossRef]
- Ashor, A.W.; Lara, J.; Mathers, J.C.; Siervo, M. Effect of Vitamin C on Endothelial Function in Health and Disease: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Atherosclerosis 2014, 235, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Dai, P.; Wang, H. Effects of Vitamin C Supplementation on Essential Hypertension: A Systematic Review and Meta-Analysis: A Systematic Review and Meta-Analysis. Medicine 2020, 99, e19274. [Google Scholar] [CrossRef]
- Zmijewski, M.A. Vitamin D and Human Health. Int. J. Mol. Sci. 2019, 20, 145. [Google Scholar] [CrossRef] [Green Version]
- Kunutsor, S.K.; Apekey, T.A.; Steur, M. Vitamin D and Risk of Future Hypertension: Meta-Analysis of 283,537 Participants. Eur. J. Epidemiol. 2013, 28, 205–221. [Google Scholar] [CrossRef]
- Judd, S.E.; Nanes, M.S.; Ziegler, T.R.; Wilson, P.W.F.; Tangpricha, V. Optimal Vitamin D Status Attenuates the Age-Associated Increase in Systolic Blood Pressure in White Americans: Results from the Third National Health and Nutrition Examination Survey. Am. J. Clin. Nutr. 2008, 87, 136–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Morvaridzadeh, M.; Sepidarkish, M.; Fazelian, S.; Rahimlou, M.; Omidi, A.; Ardehali, S.H.; Sanoobar, M.; Heshmati, J. Effect of Calcium and Vitamin D Co-Supplementation on Blood Pressure: A Systematic Review and Meta-Analysis. Clin. Ther. 2020, 42, e45–e63. [Google Scholar] [CrossRef]
- Lee, J.H.; O’Keefe, J.H.; Bell, D.; Hensrud, D.D.; Holick, M.F. Vitamin D Deficiency: An Important, Common, and Easily Treatable Cardiovascular Risk Factor? J. Am. Coll. Cardiol. 2008, 52, 1949–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugden, J.A.; Davies, J.I.; Witham, M.D.; Morris, A.D.; Struthers, A.D. Vitamin D Improves Endothelial Function in Patients with Type 2 Diabetes Mellitus and Low Vitamin D Levels. Diabet. Med. 2008, 25, 320–325. [Google Scholar] [CrossRef]
- Yuan, W.; Pan, W.; Kong, J.; Zheng, W.; Szeto, F.L.; Wong, K.E.; Cohen, R.; Klopot, A.; Zhang, Z.; Li, Y.C. 1,25-Dihydroxyvitamin D3 Suppresses Renin Gene Transcription by Blocking the Activity of the Cyclic AMP Response Element in the Renin Gene Promoter. J. Biol. Chem. 2007, 282, 29821–29830. [Google Scholar] [CrossRef] [Green Version]
- Witham, M.D.; Nadir, M.A.; Struthers, A.D. Effect of Vitamin D on Blood Pressure: A Systematic Review and Meta-Analysis. J. Hypertens. 2009, 27, 1948–1954. [Google Scholar] [CrossRef]
- Burgaz, A.; Orsini, N.; Larsson, S.C.; Wolk, A. Blood 25-Hydroxyvitamin D Concentration and Hypertension: A Meta-Analysis. J. Hypertens. 2011, 29, 636–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beveridge, L.A.; Struthers, A.D.; Khan, F.; Jorde, R.; Scragg, R.; Macdonald, H.M.; Alvarez, J.A.; Boxer, R.S.; Dalbeni, A.; Gepner, A.D.; et al. Effect of Vitamin D Supplementation on Blood Pressure: A Systematic Review and Meta-Analysis Incorporating Individual Patient Data. JAMA Intern. Med. 2015, 175, 745–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golzarand, M.; Shab-Bidar, S.; Koochakpoor, G.; Speakman, J.R.; Djafarian, K. Effect of Vitamin D3 Supplementation on Blood Pressure in Adults: An Updated Meta-Analysis. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 663–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Hao, X. The Effect of Vitamin D3 on Blood Pressure in People with Vitamin D Deficiency: A System Review and Meta-Analysis. Medicine (Baltimore) 2019, 98, e15284. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The Role of Vitamin e in Human Health and Some Diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar]
- Boshtam, M.; Rafiei, M.; Sadeghi, K.; Sarraf-Zadegan, N. Vitamin E can reduce blood pressure in mild hypertensives. Int. J. Vitam. Nutr. Res. 2002, 72, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Emami, M.R.; Safabakhsh, M.; Alizadeh, S.; Asbaghi, O.; Khosroshahi, M.Z. Effect of Vitamin E Supplementation on Blood Pressure: A Systematic Review and Meta-Analysis. J. Hum. Hypertens. 2019, 33, 499–507. [Google Scholar] [CrossRef]
- Palumbo, G.; Avanzini, F.; Alli, C.; Roncaglioni, M.C.; Ronchi, E.; Cristofari, M.; Capra, A.; Rossi, S.; Nosotti, L.; Costantini, C.; et al. Effects of Vitamin E on Clinic and Ambulatory Blood Pressure in Treated Hypertensive Patients. Collaborative Group of the Primary Prevention Project (PPP)—Hypertension Study. Am. J. Hypertens. 2000, 13 Pt 1, 564–567. [Google Scholar] [CrossRef] [Green Version]
- Mottram, P.; Shige, H.; Nestel, P. Vitamin E Improves Arterial Compliance in Middle-Aged Men and Women. Atherosclerosis 1999, 145, 399–404. [Google Scholar] [CrossRef]
- Dakshinamurti, K.; Dakshinamurti, S. Blood Pressure Regulation and Micronutrients. Nutr. Res. Rev. 2001, 14, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.D.; Wechter, W.J.; Kantoci, D.; Wang, W.H.; Pham, T.; Quiggle, D.D.; Anner, B.M. Endogenous Natriuretic Factors 7: Biospecificity of a Natriuretic γ-Tocopherol Metabolite LLU-α. J. Pharmacol. Exp. Ther. 1997, 282, 657–662. [Google Scholar]
- Gratacós, E.; Casals, E.; Deulofeu, R.; Cararach, V.; Alonso, P.L.; Fortuny, A. Lipid Peroxide and Vitamin E Patterns in Pregnant Women with Different Types of Hypertension in Pregnancy. Am. J. Obstet. Gynecol. 1998, 178, 1072–1076. [Google Scholar] [CrossRef]
- Dakshinamurti, K.; Sharma, S.K.; Geiger, J.D. Neuroprotective Actions of Pyridoxine. Biochim. Biophys. Acta Proteins Proteom. 2003, 1647, 225–229. [Google Scholar] [CrossRef]
- Lal, K.J.; Dakshinamurti, K.; Thliveris, J. The Effect of Vitamin B6 on the Systolic Blood Pressure of Rats in Various Animal Models of Hypertension. J. Hypertens. 1996, 14, 355–363. [Google Scholar] [CrossRef]
- Aybak, M.; Sermet, A.; Ayyildiz, M.O.; Karakilçik, A.Z. Effect of Oral Pyridoxine Hydrochloride Supplementation on Arterial Blood Pressure in Patients with Essential Hypertension. Arzneimittelforschung 1995, 45, 1271–1273. [Google Scholar] [PubMed]
- Dakshinamurti, K.; Lal, K.J.; Ganguly, P.K. Hypertension, Calcium Channel and Pyridoxine (Vitamin B6); Springer: Boston, MA, USA, 1998; pp. 137–148. [Google Scholar]
- Menzel, D.; Haller, H.; Wilhelm, M.; Robenek, H. L-Arginine and B Vitamins Improve Endothelial Function in Subjects with Mild to Moderate Blood Pressure Elevation. Eur. J. Nutr. 2018, 57, 557–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousa, A.S.; Mousa, S.A. Cellular Effects of Garlic Supplements and Antioxidant Vitamins in Lowering Marginally High Blood Pressure in Humans: Pilot Study. Nutr. Res. 2007, 27, 119–123. [Google Scholar] [CrossRef]
- Ghaffari, S.; Roshanravan, N. The Role of Nutraceuticals in Prevention and Treatment of Hypertension: An Updated Review of the Literature. Food Res. Int. 2020, 128, 108749. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Cicero, A.F.G. Nutraceuticals with a Clinically Detectable Blood Pressure-Lowering Effect: A Review of Available Randomized Clinical Trials and Their Meta-Analyses. Br. J. Clin. Pharmacol. 2017, 83, 163–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Micronutrients | Source | RDA/RDI (Dietary Reference) Value/Day * |
|---|---|---|
| Sodium | Table salt, processed food, seaweed, cheese | >1500 mg |
| Potassium | Grains, lean meat, milk, potato, banana, avocado | 4700–4800 mg |
| Magnesium | Nuts, grains, pumpkin seed, avocado, spinach | 350–420 mg |
| Zinc | Grains, red meat, egg, salmon, oyster, crab | 8–12 mg |
| Calcium | Milk, tofu, okra, thyme, soybean, salmon, kale | 1000–1300 mg |
| Selenium | Brazil nuts, seafood, organ meat, grains, egg | 50–55 µg |
| Copper | Nuts, organ meat, oyster, grains, beans | 1–1.8 mg |
| Vitamin B6 | Milk, egg, lean meat, tuna, pistachios | 1.3–1.8 mg |
| Vitamin B12 | Liver, egg, salmon, mussels, red meat | 1.5–2.4 µg |
| Vitamin C | Citrus fruits, guava, kiwi fruit, broccoli, pepper | 70–90 mg |
| Vitamin D | Salmon, tuna, herring, egg, mushroom, meat | 10–20 µg |
| Vitamin E | Spinach, avocado, nuts, seeds oil, bell pepper | 10–15 mg |
| BP Regulating Mechanism | Micronutrients |
|---|---|
| Diuretics or natriuresis | Calcium, Potassium, Magnesium, Zinc, Vitamin C, Vitamin B6 |
| SNS Modulators | Sodium (increase SNS), Potassium, Calcium, Zinc, Copper, Vitamin B6, Vitamin C |
| RAAS and ACEI/ARB | Sodium, Potassium, Calcium, Zinc, Vitamin B6, Vitamin C, Vitamin D |
| Vascular Modulator (Vasodilators) | Sodium, Potassium, Calcium, Magnesium, Zinc, Selenium, Vitamin C, Vitamin E |
| Calcium Channel Blockers (Ca antagonist) | Magnesium, Calcium, Vitamin B6, Vitamin C, Vitamin D, Vitamin E |
| Antioxidant/Anti-inflammatory activities | Potassium, Selenium, Copper, Zinc, Vitamin C, Vitamin E, Vitamin B6/D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, H.-F.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.-K. Impact of Micronutrients on Hypertension: Evidence from Clinical Trials with a Special Focus on Meta-Analysis. Nutrients 2021, 13, 588. https://doi.org/10.3390/nu13020588
Chiu H-F, Venkatakrishnan K, Golovinskaia O, Wang C-K. Impact of Micronutrients on Hypertension: Evidence from Clinical Trials with a Special Focus on Meta-Analysis. Nutrients. 2021; 13(2):588. https://doi.org/10.3390/nu13020588
Chicago/Turabian StyleChiu, Hui-Fang, Kamesh Venkatakrishnan, Oksana Golovinskaia, and Chin-Kun Wang. 2021. "Impact of Micronutrients on Hypertension: Evidence from Clinical Trials with a Special Focus on Meta-Analysis" Nutrients 13, no. 2: 588. https://doi.org/10.3390/nu13020588
APA StyleChiu, H. -F., Venkatakrishnan, K., Golovinskaia, O., & Wang, C. -K. (2021). Impact of Micronutrients on Hypertension: Evidence from Clinical Trials with a Special Focus on Meta-Analysis. Nutrients, 13(2), 588. https://doi.org/10.3390/nu13020588