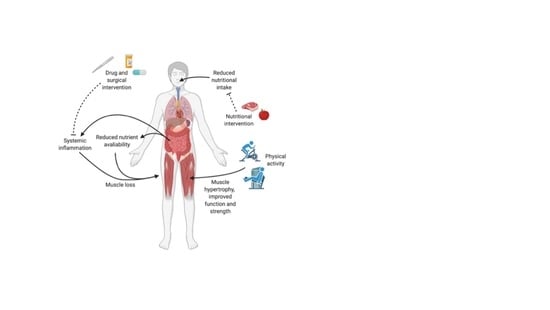
Sarcopenia in Inflammatory Bowel Disease: A Narrative Overview
Abstract
:1. Introduction
2. Methodology
3. Assessment of Sarcopenia
3.1. Muscle Mass
3.2. Physical Function
3.3. Nutrition
4. Mechanisms Driving Sarcopenia in IBD
4.1. Chronic Inflammation
4.2. Vitamin D Deficiency
4.3. Adiposity
4.4. Malabsorption
4.5. Muscle Microbiome Axis
5. The Management of Sarcopenia
5.1. Nutrition
5.2. Supplementation
5.3. Protein
5.4. Vitamin D
5.5. Omega 3 Polyunsaturated Fatty Acids
5.6. Physical Activity
5.7. Pharmacological Treatment
5.8. Surgical Intervention
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Summary comments. Am. J. Clin. Nutr. 1989, 50, 1231–1233. [Google Scholar] [CrossRef]
- Morley, J.E.; Anker, S.D.; Von Haehling, S. Prevalence, incidence, and clinical impact of sarcopenia: Facts, numbers, and epidemiology-update 2014. J. Cachex-Sarcopenia Muscle 2014, 5, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres., J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef] [Green Version]
- Scaldaferri, F.; Pizzoferrato, M.; Lopetuso, L.R.; Musca, T.; Ingravalle, F.; Sicignano, L.L.; Mentella, M.; Miggiano, G.; Mele, M.C.; Gaetani, E.; et al. Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide. Gastroenterol. Res. Pract. 2017, 2017, 8646495. [Google Scholar] [CrossRef]
- Zhang, T.; Ding, C.; Xie, T.; Yang, J.; Dai, X.; Lv, T.; Li, Y.; Gu, L.; Wei, Y.; Gong, J.; et al. Skeletal muscle depletion correlates with disease activity in ulcerative colitis and is reversed after colectomy. Clin. Nutr. 2017, 36, 1586–1592. [Google Scholar] [CrossRef]
- Ryan, E.; McNicholas, D.; Creavin, B.; Kelly, M.E.; Walsh, T.; Beddy, D. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2018, 25, 67–73. [Google Scholar] [CrossRef]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef] [Green Version]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.P.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. 3), s1–s106. [Google Scholar] [CrossRef] [Green Version]
- Pizzoferrato, M.; De Sire, R.; Ingravalle, F.; Mentella, M.C.; Petito, V.; Martone, A.M.; Landi, F.; Miggiano, G.A.D.; Mele, M.C.; Lopetuso, L.R.; et al. Characterization of Sarcopenia in an IBD Population Attending an Italian Gastroenterology Tertiary Center. Nutrients 2019, 11, 2281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhaliwal, A.; Armstrong, M.J. Sarcopenia in cirrhosis: A practical overview. Clin. Med. 2020, 20, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Carey, E.J.; Lai, J.C.; Sonnenday, C.; Tapper, E.B.; Tandon, P.; Duarte-Rojo, A.; Dunn, M.A.; Tsien, C.; Kallwitz, E.R.; Ng, V.; et al. A North American Expert Opinion Statement on Sarcopenia in Liver Transplantation. Hepatology 2019, 70, 1816–1829. [Google Scholar] [CrossRef] [PubMed]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Masuko, K. Rheumatoid Cachexia Revisited: A Metabolic Co-Morbidity in Rheumatoid Arthritis. Front. Nutr. 2014, 1. [Google Scholar] [CrossRef] [Green Version]
- Demiris, G.; Oliver, D.P.; Washington, K.T. Chapter 3—Defining and Analyzing the Problem. In Behavioral Intervention Research in Hospice and Palliative Care; Demiris, G., Oliver, D.P., Washington, K.T., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 27–39. [Google Scholar]
- Green, B.N.; Johnson, C.D.; Adams, A. Writing narrative literature reviews for peer-reviewed journals: Secrets of the trade. J. Chiropr. Med. 2006, 5, 101–117. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Yoon, H.; Oh, D.J.; Lee, J.M.; Choi, Y.J.; Shin, C.M.; Park, Y.S.; Kim, N.; Lee, D.H.; Kim, J.S. The prevalence of sarcopenia and its effect on prognosis in patients with Crohn’s disease. Intest. Res. 2020, 18, 79–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Cao, L.; Cao, T.; Yang, J.; Gong, J.; Zhu, W.; Li, N.; Li, J. Prevalence of Sarcopenia and Its Impact on Postoperative Outcome in Patients With Crohn’s Disease Undergoing Bowel Resection. J. Parenter. Enter. Nutr. 2017, 41, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Cromwell, J.; Nau, P. Sarcopenia is a Predictor of Surgical Morbidity in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1867–1872. [Google Scholar] [CrossRef]
- Bryant, R.V.; Ooi, S.; Schultz, C.G.; Goess, C.; Grafton, R.; Hughes, J.; Lim, A.; Bartholomeusz, F.D.; Andrews, J.M. Low muscle mass and sarcopenia: Common and predictive of osteopenia in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015, 41, 895–906. [Google Scholar] [CrossRef]
- Bamba, S.; Sasaki, M.; Takaoka, A.; Takahashi, K.; Imaeda, H.; Nishida, A.; Inatomi, O.; Sugimoto, M.; Andoh, A. Sarcopenia is a predictive factor for intestinal resection in admitted patients with Crohn’s disease. PLoS ONE 2017, 12, e0180036. [Google Scholar] [CrossRef]
- Adams, D.W.; Gurwara, S.; Silver, H.J.; Horst, S.N.; Beaulieu, D.B.; Schwartz, D.A.; Seidner, D.L. Sarcopenia Is Common in Overweight Patients with Inflammatory Bowel Disease and May Predict Need for Surgery. Inflamm. Bowel Dis. 2017, 23, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.M.; Al-Jaouni, R.; Filippi, J.; Wiroth, J.B.; Zeanandin, G.; Arab, K. Sarcopenia is prevalent in patients with Crohn’s disease in clinical remission. Inflamm. Bowel Dis. 2008, 14, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.V.; Trott, M.J.; Bartholomeusz, F.D.; Andrews, J.M. Systematic review: Body composition in adults with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013, 38, 213–225. [Google Scholar] [CrossRef] [PubMed]
- A Churchward-Venne, T.; A Burd, N.; Phillips, S.M. Nutritional regulation of muscle protein synthesis with resistance exercise: Strategies to enhance anabolism. Nutr. Metab. 2012, 9, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Ney, M.; Eslamparast, T.; VanderMeer, B.; Ismond, K.P.; Kroeker, K.; Halloran, B.; Raman, M.; Tandon, P. Systematic review of nutrition screening and assessment in inflammatory bowel disease. World J. Gastroenterol. 2019, 25, 3823–3837. [Google Scholar] [CrossRef] [PubMed]
- Haskey, N.; Peña-Sánchez, J.N.; Jones, J.L.; Fowler, S.A. Development of a screening tool to detect nutrition risk in patients with inflammatory bowel disease. Asia Pac. J. Clin. Nutr. 2018, 27, 756–762. [Google Scholar] [PubMed]
- Morgan, P.S.B.; Breen, L. Exploring the Impact of Obesity on Skeletal Muscle Function in Older Age. Front. Nutr. 2020, 7, 569904. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M. Symposium on “The challenge of translating nutrition research into public health nutrition”. Session 3: Joint Nutrition Society and Irish Nutrition and Dietetic Institute Symposium on “Nutrition and autoimmune disease”. Nutrition in Crohn’s disease. Proc. Nutr. Soc. 2009, 68, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Dulai, P.S.; Zarrinpar, A.; Ramamoorthy, S.; Sandborn, W.J. Obesity in IBD: Epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 110–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budui, S.L.; Rossi, A.P.; Zamboni, M. The pathogenetic bases of sarcopenia. Clin. Cases Miner. Bone Metab. 2015, 12, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Späte, U.; Schulze, P.C. Proinflammatory cytokines and skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Reid, M.B. NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1165–R1170. [Google Scholar] [CrossRef]
- Frost, R.A.; Lang, C.H. mTor Signaling in Skeletal Muscle During Sepsis and Inflammation: Where Does It All Go Wrong? Physiology 2011, 26, 83–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, S.; Nikolaus, S.; Hampe, J. Activation of nuclear factor kappa B in inflammatory bowel disease. Gut 1998, 42, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.J.; Wackerhage, H.; Spangenburg, E.E.; Booth, F.W. Control of the size of the human muscle mass. Annu. Rev. Physiol. 2004, 66, 799–828. [Google Scholar] [CrossRef] [PubMed]
- Remels, A.H.V.; Langen, R.C.J.; Gosker, H.R.; Russell, A.P.; Spaapen, F.; Voncken, J.W. PPARγ inhibits NF-κB-dependent transcriptional activation in skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, E174–E183. [Google Scholar] [CrossRef]
- Hassan-Smith, Z.K.; Morgan, S.A.; Sherlock, M.; Hughes, B.; Taylor, A.E.; Lavery, G.G.; Tomlinson, J.W.; Stewart, P.M. Gender-Specific Differences in Skeletal Muscle 11β-HSD1 Expression Across Healthy Aging. J. Clin. Endocrinol. Metab. 2015, 100, 2673–2681. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Ahima, R.S.; Park, H.-K. Connecting Myokines and Metabolism. Endocrinol. Metab. 2015, 30, 235–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiFedele, L.M.; He, J.; Bonkowski, E.L.; Han, X.; Held, M.A.; Bohan, A.; Menon, R.K.; Denson, L.A. Tumor Necrosis Factor α Blockade Restores Growth Hormone Signaling in Murine Colitis. Gastroenterol. 2005, 128, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchitomi, R.; Oyabu, M.; Kamei, Y. Vitamin D and Sarcopenia: Potential of Vitamin D Supplementation in Sarcopenia Prevention and Treatment. Nutrients 2020, 12, 3189. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D, Calcium. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Dawson-Hughes, B.; Mithal, A.; Bonjour, J.-P.; Boonen, S.; Burckhardt, P.; Fuleihan, G.E.-H.; Josse, R.G.; Lips, P.; Morales-Torres, J.; Yoshimura, N. IOF position statement: Vitamin D recommendations for older adults. Osteoporos. Int. 2010, 21, 1151–1154. [Google Scholar] [CrossRef]
- Hanley, D.A.; Cranney, A.; Jones, G.; Whiting, S.J.; Leslie, W.D.; Cole, D.E.C. Vitamin D in adult health and disease: A review and guideline statement from Osteoporosis Canada. CMAJ 2010, 182, E610–E618. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, J.; Cooper, S.C.; Ghosh, S.; Hewison, M. The Role of Vitamin D in Inflammatory Bowel Disease: Mechanism to Management. Nutrients 2019, 11, 1019. [Google Scholar] [CrossRef] [Green Version]
- Bass, J.J.; Kazi, A.A.; Deane, C.S.; Nakhuda, A.; Ashcroft, S.P.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; et al. The mechanisms of skeletal muscle atrophy in response to transient knockdown of the vitamin D receptor in vivo. J. Physiol. 2021, 599, 963–979. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.J.; Nakhuda, A.; Deane, C.S.; Brook, M.S.; Wilkinson, D.J.; Phillips, B.E.; Philp, A.; Tarum, J.; Kadi, F.; Andersen, D.; et al. Overexpression of the vitamin D receptor (VDR) induces skeletal muscle hypertrophy. Mol. Metab. 2020, 42, 101059. [Google Scholar] [CrossRef]
- Ashcroft, S.P.; Bass, J.J.; Kazi, A.A.; Atherton, P.J.; Philp, A. The vitamin D receptor regulates mitochondrial function in C2C12 myoblasts. Am. J. Physiol. Physiol. 2020, 318, C536–C541. [Google Scholar] [CrossRef] [PubMed]
- Coen, P.M.; Musci, R.V.; Hinkley, J.M.; Miller, B.F. Mitochondria as a Target for Mitigating Sarcopenia. Front. Physiol. 2019, 9, 1883. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, D.K.; Yin, K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Olivier, I.; Théodorou, V.; Valet, P.; Castan-Laurell, I.; Guillou, H.; Bertrand-Michel, J. Is Crohn’s creeping fat an adipose tissue? Inflamm. Bowel Dis. 2011, 17, 747–757. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, P.; Magro, F.; Martel, F. Metabolic inflammation in inflammatory bowel disease: Crosstalk between adipose tissue and bowel. Inflamm. Bowel Dis. 2015, 21, 453–467. [Google Scholar] [CrossRef]
- Gambero, A.; Maróstica, M.; Saad, M.J.A.; Pedrazzoli, J. Mesenteric adipose tissue alterations resulting from experimental reactivated colitis. Inflamm. Bowel Dis. 2007, 13, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, J.R.; Russell, R.M. Nutritional consequences of intestinal bacterial overgrowth. Compr. Ther. 1994, 20, 523–530. [Google Scholar]
- Balestrieri, P.; Ribolsi, M.; Guarino, M.P.L.; Emerenziani, S.; Altomare, A.; Cicala, M. Nutritional Aspects in Inflammatory Bowel Diseases. Nutrients 2020, 12, 372. [Google Scholar] [CrossRef] [Green Version]
- Lucendo, A.J.; De Rezende, L.C. Importance of nutrition in inflammatory bowel disease. World J. Gastroenterol. 2009, 15, 2081–2088. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nat. Cell Biol. 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gaulke, C.A.; Sharpton, T.J. The influence of ethnicity and geography on human gut microbiome composition. Nat. Med. 2018, 24, 1495–1496. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, F.; Van Sinderen, D.; O’Toole, P.W.; Stanton, C. Feeding the microbiota: Transducer of nutrient signals for the host. Gut 2017, 66, 1709–1717. [Google Scholar] [CrossRef] [Green Version]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut–Muscle Axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017, 35, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Ni Lochlainn, M.; Bowyer, R.C.E.; Steves, C.J. Dietary Protein and Muscle in Aging People: The Potential Role of the Gut Microbiome. Nutrients 2018, 10, 929. [Google Scholar] [CrossRef] [Green Version]
- Dukes, A.; Davis, C.; El Refaey, M.; Upadhyay, S.; Mork, S.; Arounleut, P. The aromatic amino acid tryptophan stimulates skeletal muscle IGF1/p70s6k/mTor signaling in vivo and the expression of myogenic genes in vitro. Nutrients 2015, 31, 1018–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, R.; Liu, W.; Piao, M.; Zhu, H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef]
- Picca, A.; Fanelli, F.; Calvani, R.; Mulè, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut Dysbiosis and Muscle Aging: Searching for Novel Targets against Sarcopenia. Mediat. Inflamm. 2018, 2018, 7026198. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Breen, L.; Phillips, S.M. Alterations in human muscle protein metabolism with aging: Protein and exercise as countermeasures to offset sarcopenia. BioFactors 2014, 40, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Smeuninx, B.; Greig, C.A.; Breen, L. Amount, Source and Pattern of Dietary Protein Intake Across the Adult Lifespan: A Cross-Sectional Study. Front. Nutr. 2020, 7, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rondanelli, M.; Faliva, M.; Monteferrario, F.; Peroni, G.; Repaci, E.; Allieri, F.; Perna, S. Novel Insights on Nutrient Management of Sarcopenia in Elderly. BioMed Res. Int. 2015, 2015, 524948. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef] [Green Version]
- Witard, O.C.; Wardle, S.L.; Macnaughton, L.S.; Hodgson, A.B.; Tipton, K.D. Protein Considerations for Optimising Skeletal Muscle Mass in Healthy Young and Older Adults. Nutrients 2016, 8, 181. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper From the PROT-AGE Study Group. J. Am. Med Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Breen, L.; Phillips, S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr. Metab. 2011, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Brook, M.S.; Wilkinson, D.J.; Mitchell, W.K.; Lund, J.N.; Phillips, B.E.; Szewczyk, N.J.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J. Physiol. 2016, 594, 7399–7417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Langenberg, D.R.; Della Gatta, P.; Hill, B.; Zacharewicz, E.; Gibson, P.R.; Russell, A.P. Delving into disability in Crohn’s disease: Dysregulation of molecular pathways may explain skeletal muscle loss in Crohn’s disease. J. Crohns Colitis 2014, 8, 626–634. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, C.; Fares, S.; Chardigny, J.M.; Boirie, Y.; Walrand, S. Vitamin D supplementation and muscle strength in pre-sarcopenic elderly Lebanese people: A randomized controlled trial. Arch. Osteoporos. 2018, 14, 4. [Google Scholar] [CrossRef]
- Tomlinson, P.B.; Joseph, C.; Angioi, M. Effects of vitamin D supplementation on upper and lower body muscle strength levels in healthy individuals. A systematic review with meta-analysis. J. Sci. Med. Sport 2015, 18, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Hallajzadeh, J.; Mirhosseini, N.; Lankarani, K.B.; Maharlouei, N.; Akbari, M.; Asemi, Z. The effects of vitamin D supplementation on muscle function among postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. EXCLI J. 2019, 18, 591–603. [Google Scholar]
- Hradsky, O.; Soucek, O.; Maratova, K.; Matyskova, J.; Copova, I.; Zarubova, K.; Bronsky, J.; Sumnik, Z. Supplementation with 2000 IU of Cholecalciferol Is Associated with Improvement of Trabecular Bone Mineral Density and Muscle Power in Pediatric Patients with IBD. Inflamm. Bowel Dis. 2017, 23, 514–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritsche, K. Fatty Acids as Modulators of the Immune Response. Annu. Rev. Nutr. 2006, 26, 45–73. [Google Scholar] [CrossRef] [Green Version]
- Dubuquoy, L.; Jansson, E.A.; Deeb, S.; Rakotobe, S.; Karoui, M.; Colombel, J.F. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology 2003, 124, 1265–1276. [Google Scholar] [CrossRef]
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 1998, 115, 357–369. [Google Scholar] [CrossRef]
- McGlory, C.; Calder, P.C.; Nunes, E.A. The Influence of Omega-3 Fatty Acids on Skeletal Muscle Protein Turnover in Health, Disuse, and Disease. Front. Nutr. 2019, 6, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelhamid, A.; the PUFAH Group; Hooper, L.; Sivakaran, R.; Hayhoe, R.P.G.; Welch, A. The Relationship Between Omega-3, Omega-6 and Total Polyunsaturated Fat and Musculoskeletal Health and Functional Status in Adults: A Systematic Review and Meta-analysis of RCTs. Calcif. Tissue Int. 2019, 105, 353–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, M.G.; Ellison, G.M.; Cable, N.T. Basic science behind the cardiovascular benefits of exercise. Br. J. Sports Med. 2016, 50, 93–99. [Google Scholar] [CrossRef]
- Lopez, P.; Pinto, R.S.; Radaelli, R.; Rech, A.; Grazioli, R.; Izquierdo, M.; Cadore, E.L. Benefits of resistance training in physically frail elderly: A systematic review. Aging Clin. Exp. Res. 2018, 30, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Baillet, A.; Vaillant, M.; Guinot, M.; Juvin, R.; Gaudin, P. Efficacy of resistance exercises in rheumatoid arthritis: Meta-analysis of randomized controlled trials. Rheumatology 2012, 51, 519–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, F.R.; Berzigotti, A.; Lord, J.M.; Lai, J.C.; Armstrong, M.J. Review article: Impact of exercise on physical frailty in patients with chronic liver disease. Aliment. Pharmacol. Ther. 2019, 50, 988–1000. [Google Scholar] [CrossRef]
- Engels, M.; Cross, R.K.; Long, M.D. Exercise in patients with inflammatory bowel diseases: Current perspectives. Clin. Exp. Gastroenterol. 2017, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bilski, J.; Mazur-Bialy, A.; Brzozowski, B.; Magierowski, M.; Zahradnik-Bilska, J.; Wójcik, D.; Magierowska, K.; Kwiecien, S.; Mach, T.; Brzozowski, T. Can exercise affect the course of inflammatory bowel disease? Experimental and clinical evidence. Pharmacol. Rep. 2016, 68, 827–836. [Google Scholar] [CrossRef]
- Murphy, R.M.; Watt, M.J.; Febbraio, M.A. Metabolic communication during exercise. Nat. Metab. 2020, 2, 805–816. [Google Scholar] [CrossRef]
- Das, D.K.; Graham, Z.A.; Cardozo, C.P. Myokines in skeletal muscle physiology and metabolism: Recent advances and future perspectives. Acta Physiol. 2020, 228, e13367. [Google Scholar] [CrossRef]
- Chan, D.; Robbins, H.; Rogers, S.; Clark, S.; Poullis, A. Inflammatory bowel disease and exercise: Results of a Crohn’s and Colitis UK survey. Frontline Gastroenterol. 2014, 5, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Klare, P.; Nigg, J.; Nold, J.; Haller, B.; Krug, A.B.; Mair, S.; Thoeringer, C.K.; Christle, J.W.; Schmid, R.M.; Halle, M.; et al. The Impact of a Ten-Week Physical Exercise Program on Health-Related Quality of Life in Patients with Inflammatory Bowel Disease: A Prospective Randomized Controlled Trial. Digestion 2015, 91, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Ploeger, H.; Obeid, J.; Nguyen, T.; Takken, T.; Issenman, R.; de Greef, M. Exercise and inflammation in pediatric Crohn’s disease. Int. J. Sports Med. 2012, 33, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.; Millard, W.; Lebrun, C.; Howard, J. Low-intensity exercise improves quality of life in patients with Crohn’s disease. Clin. J. Sport Med. 2007, 17, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Elsenbruch, S.; Langhorst, J.; Popkirowa, K.; Müller, T.; Luedtke, R.; Franken, U.; Paul, A.; Spahn, G.; Michalsen, A.; Janssen, O.E.; et al. Effects of Mind-Body Therapy on Quality of Life and Neuroendocrine and Cellular Immune Functions in Patients with Ulcerative Colitis. Psychother. Psychosom. 2005, 74, 277–287. [Google Scholar] [CrossRef] [PubMed]
- D’Incà, R.; Varnier, M.; Mestriner, C.; Martines, D.; D’Odorico, A.; Sturniolo, G.C. Effect of moderate exercise on Crohn’s disease patients in remission. Ital. J. Gastroenterol. Hepatol. 1999, 31, 205–210. [Google Scholar] [PubMed]
- Gupta, N.; Khera, S.; Vempati, R.P.; Sharma, R.; Bijlani, R.L. Effect of yoga based lifestyle intervention on state and trait anxiety. Indian J. Physiol. Pharmacol. 2006, 50, 41–47. [Google Scholar]
- Loudon, C.P.; Corroll, V.; Butcher, J.; Rawsthorne, P.; Bernstein, C.N. The effects of physical exercise on patients with Crohn’s disease. Am. J. Gastroenterol. 1999, 94, 697–703. [Google Scholar] [CrossRef]
- Grgic, J.; Garofolini, A.; Orazem, J.; Sabol, F.; Schoenfeld, B.J.; Pedisic, Z. Effects of Resistance Training on Muscle Size and Strength in Very Elderly Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sports Med. 2020, 50, 1983–1999. [Google Scholar] [CrossRef]
- Robinson, R.; Krzywicki, T.; Almond, L.; al-Azzawi, F.; Abrams, K.; Iqbal, S.J. Effect of a low-impact exercise program on bone mineral density in Crohn’s disease: A randomized controlled trial. Gastroenterology 1998, 115, 36–41. [Google Scholar] [CrossRef]
- Danese, S.; Vuitton, L.; Peyrin-Biroulet, L. Biologic agents for IBD: Practical insights. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Adegbola, S.O.; Sahnan, K.; Warusavitarne, J.; Hart, A.; Tozer, P. Anti-TNF Therapy in Crohn’s Disease. Int. J. Mol. Sci. 2018, 19, 2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.C.; Jeen, Y.T. Anti-integrin therapy for inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 1868–1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramaniam, K.; Fallon, K.; Ruut, T.; Lane, D.; McKay, R.; Shadbolt, B. Infliximab reverses inflammatory muscle wasting (sarcopenia) in Crohn’s disease. Aliment. Pharmacol. Ther. 2015, 41, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Csontos, Á.A.; Molnár, A.; Piri, Z.; Katona, B.; Dakó, S.; Pálfi, E. The Effect of anti-TNFα Induction Therapy on the Nutritional Status and Dietary Intake in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2016, 25, 49–56. [Google Scholar]
- Briot, K.; Garnero, P.; Le Henanff, A.; Dougados, M.; Roux, C. Body weight, body composition, and bone turnover changes in patients with spondyloarthropathy receiving anti-tumour necrosis factor α treatment. Ann. Rheum. Dis. 2005, 64, 1137–1140. [Google Scholar] [CrossRef] [Green Version]
- Barros, M.; Saad, C.; Takayama, L.; Moraes, J.; Medeiros, A.; Bonfa, E.; Pereira, R. THU0266 Sarcopenia reversal in ankylosing spondylitis (AS) under anti-tnf therapy: A 24-month longitudinal analysis. Ann. Rheum. Dis. 2013, 71, 244–245. [Google Scholar] [CrossRef]
- Brown, R.A.; Spina, D.; Butt, S.; Summers, G.D. Long-term effects of anti-tumour necrosis factor therapy on weight in patients with rheumatoid arthritis. Clin. Rheumatol. 2011, 31, 455–461. [Google Scholar] [CrossRef]
- Florin, V.; Cottencin, A.; Delaporte, E.; Staumont-Salle, D. Body weight increment in patients treated with infliximab for plaque psoriasis. J. Eur. Acad. Dermatol. Venereol. 2012, 27, e186–e190. [Google Scholar] [CrossRef]
- Renzo, L.D.; Saraceno, R.; Schipani, C.; Rizzo, M.; Bianchi, A.; Noce, A.; Esposito, M.; Tiberti, S.; Chimenti, S.; De Lorenzo, A. Prospective assessment of body weight and body composition changes in patients with psoriasis receiving anti-TNF-α treatment. Dermatol. Ther. 2011, 24, 446–451. [Google Scholar] [CrossRef] [Green Version]
- Engvall, I.-L.; Tengstrand, B.; Brismar, K.; Hafström, I. Infliximab therapy increases body fat mass in early rheumatoid arthritis independently of changes in disease activity and levels of leptin and adiponectin: A randomised study over 21 months. Arthritis Res. 2010, 12, R197. [Google Scholar] [CrossRef] [Green Version]
- Ebadi, M.; Bhanji, R.A.; Mazurak, V.C.; Montano-Loza, A.J. Sarcopenia in cirrhosis: From pathogenesis to interventions. J. Gastroenterol. 2019, 54, 845–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanada, M.; Sakamoto, N.; Ishimatsu, Y.; Kakugawa, T.; Obase, Y.; Kozu, R.; Senjyu, H.; Izumikawa, K.; Mukae, H.; Kohno, S. Effect of long-term treatment with corticosteroids on skeletal muscle strength, functional exercise capacity and health status in patients with interstitial lung disease. Respirology 2016, 21, 1088–1093. [Google Scholar] [CrossRef]
- Braun, T.P.; Marks, D.L. The regulation of muscle mass by endogenous glucocorticoids. Front. Physiol. 2015, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, J.; Lussiez, A.; Sullivan, J.; Wang, S.; Englesbe, M. Implications of Sarcopenia in Major Surgery. Nutr. Clin. Pract. 2015, 30, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Erős, A.; Soós, A.; Hegyi, P.; Szakács, Z.; Benke, M.; Szűcs, Á.; Hartmann, P.; Erőss, B.; Sarlós, P. Sarcopenia as an independent predictor of the surgical outcomes of patients with inflammatory bowel disease: A meta-analysis. Surg. Today 2019, 50, 1138–1150. [Google Scholar] [CrossRef] [Green Version]
- Galata, C.; Hodapp, J.; Weiß, C.; Karampinis, I.; Vassilev, G.; Reißfelder, C. Skeletal Muscle Mass Index Predicts Postoperative Complications in Intestinal Surgery for Crohn’s Disease. J. Parenter. Enteral. Nutr. 2020, 44, 714–721. [Google Scholar] [CrossRef]
- Fujikawa, H.; Araki, T.; Okita, Y.; Kondo, S.; Kawamura, M.; Hiro, J.; Toiyama, Y.; Kobayashi, M.; Tanaka, K.; Inoue, Y.; et al. Impact of sarcopenia on surgical site infection after restorative proctocolectomy for ulcerative colitis. Surg. Today 2017, 47, 92–98. [Google Scholar] [CrossRef]
- Grillot, J.; D’Engremont, C.; Parmentier, A.L.; Lakkis, Z.; Pitonm, G.; Cazaux, D. Sarcopenia and visceral obesity assessed by computed tomography are associated with adverse outcomes in patients with Crohn’s disease. Clin. Nutr. 2020, 39, 3024–3030. [Google Scholar] [CrossRef]
- O’Brien, S.; Kavanagh, R.G.; Carey, B.W.; Maher, M.M.; O’Connor, O.J.; Andrews, E.J. The impact of sarcopenia and myosteatosis on postoperative outcomes in patients with inflammatory bowel disease. Eur. Radiol. Exp. 2018, 2, 1–10. [Google Scholar] [CrossRef]


| Component | Muscle Mass | Muscle Function | Nutritional Assessment Tools |
|---|---|---|---|
| Validated tools | CT L3 CSA CT L3 SMI DXA ASMI | Nil | Nil |
| Future research | MRI Ultrasound | HGS Gait speed Timed up and go | MUST NRS 2002 NRI MIRT SaskIBD-NRT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhaliwal, A.; Quinlan, J.I.; Overthrow, K.; Greig, C.; Lord, J.M.; Armstrong, M.J.; Cooper, S.C. Sarcopenia in Inflammatory Bowel Disease: A Narrative Overview. Nutrients 2021, 13, 656. https://doi.org/10.3390/nu13020656
Dhaliwal A, Quinlan JI, Overthrow K, Greig C, Lord JM, Armstrong MJ, Cooper SC. Sarcopenia in Inflammatory Bowel Disease: A Narrative Overview. Nutrients. 2021; 13(2):656. https://doi.org/10.3390/nu13020656
Chicago/Turabian StyleDhaliwal, Amritpal, Jonathan I. Quinlan, Kellie Overthrow, Carolyn Greig, Janet M. Lord, Matthew J. Armstrong, and Sheldon C. Cooper. 2021. "Sarcopenia in Inflammatory Bowel Disease: A Narrative Overview" Nutrients 13, no. 2: 656. https://doi.org/10.3390/nu13020656
APA StyleDhaliwal, A., Quinlan, J. I., Overthrow, K., Greig, C., Lord, J. M., Armstrong, M. J., & Cooper, S. C. (2021). Sarcopenia in Inflammatory Bowel Disease: A Narrative Overview. Nutrients, 13(2), 656. https://doi.org/10.3390/nu13020656