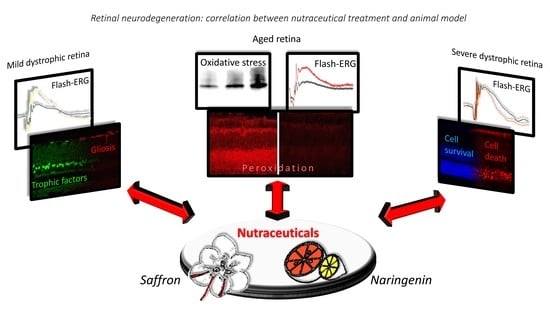
Retinal Neurodegeneration: Correlation between Nutraceutical Treatment and Animal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Nutraceutical Treatments
2.2.1. Naringenin
2.2.2. Saffron
2.3. Electroretinogram (ERG) Recordings
2.3.1. Mice
2.3.2. Rats
2.4. Western Blotting
2.5. Immunohistochemistry
2.6. TUNEL Technique
2.7. Statistical Analysis
3. Results
3.1. Royal College of Surgeons Rat (RCS)
3.2. Fischer-344 Rats (Fischer)
3.3. Aging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourne, R.R.A.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; Limburg, H.; et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e888–e897. [Google Scholar] [CrossRef] [Green Version]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; MPhil, P.A.; Braithwaite, A.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [Green Version]
- Country, M.W. Retinal metabolism: A comparative look at energetics in the retina. Brain Res. 2017, 1672, 50–57. [Google Scholar] [CrossRef]
- Rashid, K.; Akhtar-Schaefer, I.; Langmann, T. Microglia in Retinal Degeneration. Front. Immunol. 2019, 10, 1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, J.; Maslim, J.; Valter-Kocsi, K.; Mervin, K.; Bowers, F.; Chu, Y.; Barnett, N.; Provis, J.; Lewis, G.; Fisher, S.K.; et al. Mechanisms of photoreceptor death and survival in mammalian retina. Prog. Retin. Eye Res. 1999, 18, 689–735. [Google Scholar] [CrossRef]
- Tran, N.M.; Shekhar, K.; Whitney, I.E.; Jacobi, A.; Benhar, I.; Hong, G.; Yan, W.; Adiconis, X.; Arnold, M.E.; Lee, J.M.; et al. Single-Cell Profiles of Retinal Ganglion Cells Differing in Resilience to Injury Reveal Neuroprotective Genes. Neuron 2019, 104, 1039–1055.e12. [Google Scholar] [CrossRef]
- Mirra, S.; Marfany, G. Mitochondrial Gymnastics in Retinal Cells: A Resilience Mechanism Against Oxidative Stress and Neurodegeneration. In Advances in Experimental Medicine and Biology; Springer International Publishing: Berlin, Germany, 2019; Volume 1185, pp. 513–517. [Google Scholar]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Gregory-Roberts, E.; Yamamoto, K.; Blonska, A.; Ghosh, S.K.; Ueda, K.; Zhou, J. The bisretinoids of retinal pigment epithelium. Prog. Retin. Eye Res. 2012, 31, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Sparrrow, R.J.; Hicks, D.; Hamel, C.P. The Retinal Pigment Epithelium in Health and Disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Espinós, C.; Galindo, M.I.; García-Gimeno, M.A.; Ibáñez-Cabellos, J.S.; Martínez-Rubio, D.; Millán, J.M.; Rodrigo, R.; Sanz, P.; Seco-Cervera, M.; Sevilla, T.; et al. Oxidative Stress, a Crossroad Between Rare Diseases and Neurodegeneration. Antioxidants 2020, 9, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, B.; Hawes, N.; Hurd, R.; Davisson, M.; Nusinowitz, S.; Heckenlively, J. Retinal degeneration mutants in the mouse. Vis. Res. 2002, 42, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Gargini, C.; Terzibasi, E.; Mazzoni, F.; Strettoi, E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: A morphological and ERG study. J. Comp. Neurol. 2006, 500, 222–238. [Google Scholar] [CrossRef] [Green Version]
- Komeima, K.; Rogers, B.S.; Campochiaro, P.A. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J. Cell. Physiol. 2007, 213, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Komeima, K.; Usui, S.; Shen, J.; Rogers, B.S.; Campochiaro, P.A. Blockade of neuronal nitric oxide synthase reduces cone cell death in a model of retinitis pigmentosa. Free. Radic. Biol. Med. 2008, 45, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Campochiaro, P.A.; Mir, T.A. The mechanism of cone cell death in Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018, 62, 24–37. [Google Scholar] [CrossRef]
- Piano, I.; D’Antongiovanni, V.; Testai, L.; Calderone, V.; Gargini, C. A Nutraceutical Strategy to Slowing Down the Progression of Cone Death in an Animal Model of Retinitis Pigmentosa. Front. Neurosci. 2019, 13, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, P.; Markey, M.; Rapp, C.M.; Darrow, R.M.; Ziesel, A.; Organisciak, D. Enhancing the efficacy of AREDS antioxidants in light-induced retinal degeneration. Mol. Vis. 2017, 23, 718–739. [Google Scholar] [PubMed]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A.; Srinivasan, B.P. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp. Eye Res. 2014, 125, 193–202. [Google Scholar] [CrossRef]
- Al-Dosari, D.I.; Ahmed, M.M.; Al-Rejaie, S.S.; Alhomida, A.S.; Ola, M.S. Flavonoid Naringenin Attenuates Oxidative Stress, Apoptosis and Improves Neurotrophic Effects in the Diabetic Rat Retina. Nutrients 2017, 9, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef]
- Viswanatha, G.L.; Shylaja, H.; Moolemath, Y. The beneficial role of Naringin- a citrus bioflavonoid, against oxidative stress-induced neurobehavioral disorders and cognitive dysfunction in rodents: A systematic review and meta-analysis. Biomed. Pharmacother. 2017, 94, 909–929. [Google Scholar] [CrossRef]
- Bisti, S.; Maccarone, R.; Falsini, B. Saffron and retina: Neuroprotection and pharmacokinetics. Vis. Neurosci. 2014, 31, 355–361. [Google Scholar] [CrossRef]
- Di Marco, S.; Carnicelli, V.; Franceschini, N.; Di Paolo, M.; Piccardi, M.; Bisti, S.; Falsini, B. Saffron: A Multitask Neuroprotective Agent for Retinal Degenerative Diseases. Antioxidants 2019, 8, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggi, M.A.; Bisti, S.; Picco, C. Saffron: Chemical Composition and Neuroprotective Activity. Molecules 2020, 25, 5618. [Google Scholar] [CrossRef]
- Falsini, B.; Piccardi, M.; Minnella, A.; Savastano, M.C.; Capoluongo, E.; Fadda, A.; Balestrazzi, E.; Maccarone, R.; Bisti, S. Influence of Saffron Supplementation on Retinal Flicker Sensitivity in Early Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6118–6124. [Google Scholar] [CrossRef]
- Piccardi, M.; Marangoni, D.; Minnella, A.M.; Savastano, M.C.; Valentini, P.; Ambrosio, L.; Capoluongo, E.; Maccarone, R.; Bisti, S.; Falsini, B. A Longitudinal Follow-Up Study of Saffron Supplementation in Early Age-Related Macular Degeneration: Sustained Benefits to Central Retinal Function. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Heitmar, R.; Brown, J.; Kyrou, I. Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies. Nutrients 2019, 11, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Albarral, J.A.; De Hoz, R.; Ramírez, A.I.; López-Cuenca, I.; Salobrar-García, E.; Pinazo-Durán, M.D.; Ramírez, J.M.; Salazar, J.J. Beneficial effects of saffron (Crocus sativus L.) in ocular pathologies, particularly neurodegenerative retinal diseases. Neural Regen. Res. 2020, 15, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Piccardi, M.; Fadda, A.; Martelli, F.; Marangoni, D.; Magli, A.; Minnella, A.M.; Bertelli, M.; Di Marco, S.; Bisti, S.; Falsini, B. Antioxidant Saffron and Central Retinal Function in ABCA4-Related Stargardt Macular Dystrophy. Nutrients 2019, 11, 2461. [Google Scholar] [CrossRef] [Green Version]
- Bisti, S.; Di Marco, S.; Maggi, M.A.; Di Paolo, M.; Piccardi, M.; Falsini, B. Saffron Shifts the Degenerative and Inflammatory Phenotype in Photoreceptor Degeneration. In Saffron; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 163–176. [Google Scholar]
- Testai, L.; Da Pozzo, E.; Piano, I.; Pistelli, L.; Gargini, C.; Breschi, M.C.; Braca, A.; Martini, C.; Martelli, A.; Calderone, V. The Citrus Flavanone Naringenin Produces Cardioprotective Effects in Hearts from 1 Year Old Rat, through Activation of mitoBK Channels. Front. Pharmacol. 2017, 8, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef]
- Bostan, H.B.; Mehri, S.; Hosseinzadeh, H. Toxicology effects of saffron and its constituents: A review. Iran J. Basic Med. Sci. 2017, 20, 110–121. [Google Scholar] [PubMed]
- Della Santina, L.; Piano, I.; Cangiano, L.; Caputo, A.; Ludwig, A.; Cervetto, L.; Gargini, C. Processing of Retinal Signals in Normal and HCN Deficient Mice. PLoS ONE 2012, 7, e29812. [Google Scholar] [CrossRef] [PubMed]
- Maccarone, R.; Di Marco, S.; Bisti, S. Saffron Supplement Maintains Morphology and Function after Exposure to Damaging Light in Mammalian Retina. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1254–1261. [Google Scholar] [CrossRef] [Green Version]
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992, 119, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O.; Stumpff, F.; Mergler, S.; Wienrich, M.; Wiederholt, M. The Royal College of Surgeons rat: An animal model for inherited retinal degeneration with a still unknown genetic defect. Acta Anatom 1998, 162, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, D.; Feng, W.; Duncan, J.L.; Yasumura, D.; D’Cruz, P.M.; Chappelow, A.; Matthes, M.T.; Kay, M.A.; Lavail, M.M. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc. Natl. Acad. Sci. USA 2001, 98, 12584–12589. [Google Scholar] [CrossRef] [Green Version]
- Machida, S.; Raz-Prag, D.; Fariss, R.N.; Sieving, P.A.; Bush, R.A. Photopic ERG Negative Response from Amacrine Cell Signaling in RCS Rat Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2008, 49, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Maslim, J.; Valter, K.; Egensperger, R.; Holländer, H.; Stone, J. Tissue oxygen during a critical developmental period controls the death and survival of photoreceptors. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1667–1677. [Google Scholar]
- Ryals, R.C.; Andrews, M.D.; Datta, S.; Coyner, A.S.; Fischer, C.M.; Wen, Y.; Pennesi, M.E.; McGill, T.J. Long-term Characterization of Retinal Degeneration in Royal College of Surgeons Rats Using Spectral-Domain Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1378–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez, J.M.; I Ramírez, A.; Salazar, J.J.; De Hoz, R.; Triviño, A. Changes of Astrocytes in Retinal Ageing and Age-related Macular Degeneration. Exp. Eye Res. 2001, 73, 601–615. [Google Scholar] [CrossRef]
- Zhao, T.T.; Tian, C.Y.; Yin, Z.Q. Activation of Müller Cells Occurs During Retinal Degeneration in RCS Rats. Results Probl. Cell Differ. 2009, 664, 575–583. [Google Scholar] [CrossRef]
- Bruner, R.H.; Keller, W.F.; Stitzel, K.A.; Sauers, L.J.; Reer, P.J.; Long, P.H.; Bruce, R.D.; Alden, C.L. Spontaneous Corneal Dystrophy and Generalized Basement Membrane Changes in Fischer-344 Rats. Toxicol. Pathol. 1992, 20, 357–366. [Google Scholar] [CrossRef]
- Bradley, A.; Bertrand, L.; Rao, D.B.; Hall, D.G.; Sharma, A.K. Brain. In Boorman’s Pathology of the Rat; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 191–215. [Google Scholar]
- Lee, E.W.; Render, J.A.; Garner, C.D.; Brady, A.N.; Li, L.C. Unilateral Degeneration of Retina and Optic Nerve in Fischer-344 Rats. Veter- Pathol. 1990, 27, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.L.; O Jacoby, R.; Jonas, A.M. Age-related and light-associated retinal changes in Fischer rats. Investig. Ophthalmol. Vis. Sci. 1978, 17, 634–638. [Google Scholar]
- DiLoreto, D.; Ison, J.R.; Bowen, G.P.; Cox, C.; Del Cerro, M. A functional analysis of the age-related degeneration in the Fischer 344 rat. Curr. Eye Res. 1995, 14, 303–310. [Google Scholar] [CrossRef]
- Di Marco, F.; Romeo, S.; Nandasena, C.; Purushothuman, S.; Adams, C.; Bisti, S.; Stone, J. The time course of action of two neuroprotectants, dietary saffron and photobiomodulation, assessed in the rat retina. Am. J. Neurodegener. Dis. 2013, 2, 208–220. [Google Scholar]
- Fan, W.; Lin, N.; Sheedlo, H.J.; Turner, J.E. Müller and RPE Cell Response to Photoreceptor Cell Degeneration in Aging Fischer Rats. Exp. Eye Res. 1996, 63, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.M.; Edward, D.P.; Tso, M.O. A comparative study of photic injury in four inbred strains of albino rats. Curr. Eye Res. 1990, 9, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Rutar, M.; Provis, J.M.; Valter, K. Brief Exposure to Damaging Light Causes Focal Recruitment of Macrophages, and Long-Term Destabilization of Photoreceptors in the Albino Rat Retina. Curr. Eye Res. 2010, 35, 631–643. [Google Scholar] [CrossRef]
- Molecular Steps Involved in Light-Induced Oxidative Damage to Retinal Rods-PubMed. Invest Ophthalmol Vis Sci 2421–7 (2002). Available online: https://pubmed.ncbi.nlm.nih.gov/12091446/ (accessed on 3 July 2020).
- Natoli, R.; Zhu, Y.; Valter, K.; Bisti, S.; Eells, J.; Stone, J. Gene and noncoding RNA regulation underlying photoreceptor protection: Microarray study of dietary antioxidant saffron and photobiomodulation in rat retina. Mol. Vis. 2010, 16, 1801–1822. [Google Scholar] [PubMed]
- Corso, L.; Cavallero, A.; Baroni, D.; Garbati, P.; Prestipino, G.; Bisti, S.; Nobile, M.; Picco, C. Saffron reduces ATP-induced retinal cytotoxicity by targeting P2X7 receptors. Purinergic Signal. 2016, 12, 161–174. [Google Scholar] [CrossRef] [Green Version]
- Maccarone, R.; Rapino, C.; Zerti, D.; Di Tommaso, M.; Battista, N.; Di Marco, S.; Bisti, S.; Maccarrone, M. Modulation of Type-1 and Type-2 Cannabinoid Receptors by Saffron in a Rat Model of Retinal Neurodegeneration. PLoS ONE 2016, 11, e0166827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testai, L.; Piragine, E.; Piano, I.; Flori, L.; Da Pozzo, E.; Miragliotta, V.; Pirone, A.; Citi, V.; Mannelli, L.D.C.; Brogi, S.; et al. The Citrus Flavonoid Naringenin Protects the Myocardium from Ageing-Dependent Dysfunction: Potential Role of SIRT1. Oxidative Med. Cell. Longev. 2020, 2020, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Antibody | Company | Work Dilution | Application |
|---|---|---|---|
| Sod 1 | Sigma Aldrich | 1:500 | WB |
| Sirt 1 | Merk Millipore | 1:1000 | WB |
| GAPDH | Sigma Aldrich | 1:5000 | WB |
| Anti-rabbit IgG HRP conjugated | Cell Signaling Technology | 1:5000 | WB |
| Anti-mouse IgG HRP conjugated | Merk Millipore | 1:5000 | WB |
| Antibody | Company | Work Dilution | Application |
|---|---|---|---|
| Anti-acrolein | AbCam | 1:1000 | IH |
| Fibroblast grow factor 2 (FGF2) | Millipore | 1:200 | IH |
| Glial fibrillary acidic protein (GFAP) | Dako | 1:1000 | IH |
| Anti-rabbit Alexa Fluor® 568 | Sigma Aldrich | 1:500 | IH |
| Anti-mouse Alexa Fluor® | Molecular probes, Invitrogen Carlsbad | 1:200 | IH |
| Anti-rabbit Alexa Fluor® | Molecular probes, Invitrogen Carlsbad | 1:200 | IH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piano, I.; Di Paolo, M.; Corsi, F.; Piragine, E.; Bisti, S.; Gargini, C.; Di Marco, S. Retinal Neurodegeneration: Correlation between Nutraceutical Treatment and Animal Model. Nutrients 2021, 13, 770. https://doi.org/10.3390/nu13030770
Piano I, Di Paolo M, Corsi F, Piragine E, Bisti S, Gargini C, Di Marco S. Retinal Neurodegeneration: Correlation between Nutraceutical Treatment and Animal Model. Nutrients. 2021; 13(3):770. https://doi.org/10.3390/nu13030770
Chicago/Turabian StylePiano, Ilaria, Mattia Di Paolo, Francesca Corsi, Eugenia Piragine, Silvia Bisti, Claudia Gargini, and Stefano Di Marco. 2021. "Retinal Neurodegeneration: Correlation between Nutraceutical Treatment and Animal Model" Nutrients 13, no. 3: 770. https://doi.org/10.3390/nu13030770
APA StylePiano, I., Di Paolo, M., Corsi, F., Piragine, E., Bisti, S., Gargini, C., & Di Marco, S. (2021). Retinal Neurodegeneration: Correlation between Nutraceutical Treatment and Animal Model. Nutrients, 13(3), 770. https://doi.org/10.3390/nu13030770