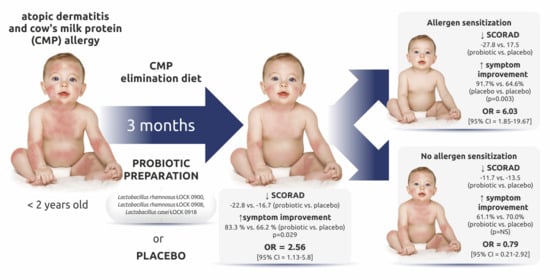
The Effectiveness of Probiotic Lactobacillus rhamnosus and Lactobacillus casei Strains in Children with Atopic Dermatitis and Cow’s Milk Protein Allergy: A Multicenter, Randomized, Double Blind, Placebo Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Design
2.2. Patients
2.3. Probiotic Preparation
2.4. The Study Protocol
2.5. Endpoint Definitions
2.6. Specific and Total IgE
2.7. Statistical Analyses
3. Results
3.1. Subjects
3.2. Changes in the SCORAD Score
3.3. Improvement of AD Symptoms Assessed with the SCORAD Scale
3.4. Secondary Endpoints
3.5. Tolerance of the Probiotic Preparation
4. Discussion
4.1. The Mechanism of Action of ŁOCK Strains
4.2. Limitations and Strengths of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tracy, A.; Bhatti, S.; Eichenfield, L.F. Update on pediatric atopic dermatitis. Cutis 2020, 106, 143–146. [Google Scholar] [CrossRef]
- Dattola, A.; Bennardo, L.; Silvestri, M.; Nisticò, S.P. What’s new in the treatment of atopic dermatitis? Dermatol. Ther. 2019, 32, e12787. [Google Scholar] [CrossRef] [PubMed]
- Ratchataswan, T.; Banzon, T.M.; Thyssen, J.P.; Weidinger, S.; Guttman-Yassky, E.; Phipatanakul, W. Biologics for treatment of atopic dermatitis: Current status and future prospect. J. Allergy Clin. Immunol. Pract. 2021, 9, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Iikura, K.; Morita, H.; Saito, H. Barrier dysfunction in the atopic march-how does atopic dermatitis lead to asthma in children? J. Allergy Clin. Immunol. 2020, 145, 1551–1553. [Google Scholar] [CrossRef]
- Bantz, S.K.; Zhu, Z.; Zheng, T. The atopic march: Progression from atopic dermatitis to allergic rhinitis and asthma. J. Clin. Cell. Immunol. 2014, 5, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, C.; El-Qutob, D.; Martorell, A.; Febrer, I.; Rodriguez, M.; Cedra, J.C.; Felix, R. Sensitization in early age to food allergens in children with atopic dermatitis. Allergol. Immunopathol. 2007, 35, 15–20. [Google Scholar] [CrossRef]
- Bergmann, M.M.; Caubet, J.C.; Boguniewicz, M.; Eigenmann, P.A. Evaluation of food allergy in patients with atopic dermatitis. J. Allergy Clin. Immunol. Pract. 2013, 1, 22–28. [Google Scholar] [CrossRef]
- Cukrowska, B. Microbial and Nutritional Programming-The Importance of the Microbiome and Early Exposure to Potential Food Allergens in the Development of Allergies. Nutrients 2018, 10, 1541. [Google Scholar] [CrossRef] [Green Version]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 2012, 129, 434–440.e1–2. [Google Scholar] [CrossRef] [Green Version]
- Penders, J.; Gerhold, K.; Stobberingh, E.E.; Thijs, C.; Zimmermann, K.; Lau, S.; Hamelmann, E. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J. Allergy Clin. Immunol. 2013, 132, 601–607.e8. [Google Scholar] [CrossRef]
- Berin, M.C. Dysbiosis in food allergy and implications for microbial therapeutics. J. Clin. Investig. 2021, 131, e144994. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Pawankar, R.; Cuello-Garcia, C.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Beyer, K.; Burks, W.; Canonica, G.W.; Ebisawa, M.; et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Probiotics. World Allergy Organ. J. 2015, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Viljanen, M.; Savilahti, E.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; Tuure, T.; Kuitunen, M. Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: A double-blind placebo-controlled trial. Allergy 2005, 60, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Grüber, C.; Wendt, M.; Sulser, C.; Lau, S.; Kulig, M.; Wahn, U.; Werfel, T.; Niggemann, B. Randomized, placebo-controlled trial of Lactobacillus rhamnosus GG as treatment of atopic dermatitis in infancy. Allergy 2007, 62, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Fölster-Holst, R.; Müller, F.; Schnopp, N.; Abeck, D.; Kreiselmaier, I.; Lenz, T.; von Rüden, U.; Schrezenmeir, J.; Christophers, E.; Weichenthal, M. Prospective, randomized controlled trial on Lactobacillus rhamnosus in infants with moderate to severe atopic dermatitis. Br. J. Dermatol. 2006, 155, 1256–1261. [Google Scholar] [CrossRef]
- Brouwer, M.L.; Wolt-Plompen, S.A.; Dubois, A.E.; van der Heide, S.; Jansen, D.F.; Hoijer, M.A.; Kauffman, H.F.; Duiverman, E.J. No effects of probiotics on atopic dermatitis in infancy: A randomized placebo-controlled trial. Clin. Exp. Allerg. 2006, 36, 899–906. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Salminen, S.; Arvilommi, H.; Kero, P.; Koskinen, P.; Isolauri, E. Probiotics in primary prevention of atopic disease: A randomised placebo-controlled trial. Lancet 2001, 357, 1076–1079. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Salminen, S.; Poussa, T.; Arvilommi, H.; Isolauri, E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 2003, 361, 1869–1871. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Salminen, S.; Poussa, T.; Arvilommi, H.; Isolauri, E. Probiotics during the first 7 years of life: A cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2007, 119, 1019–1021. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.V.; Hennemuth, I.; Heinzmann, A.; Urbanek, R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: No clinical effects of Lactobacillus GG supplementation. Pediatric 2008, 121, e850-6. [Google Scholar] [CrossRef] [PubMed]
- Cukrowska, B.; Motyl, I.; Kozáková, H.; Schwarzer, M.; Górecki, R.K.; Klewicka, E.; Slizewska, K.; Libudzisz, Z. Probiotic Lactobacillus strains: In vitro and in vivo studies. Folia Microbiol. 2009, 54, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Cukrowska, B.; Rosiak, I.; Klewicka, E.; Motyl, I.; Schwarzer, M.; Libudzisz, Z.; Kozakova, H. Impact of heat-inactivated Lactobacillus casei and Lactobacillus paracasei strains on cytokine responses in whole blood cell cultures of children with atopic dermatitis. Folia Microbiol. 2010, 55, 277–280. [Google Scholar] [CrossRef]
- Oranje, A.P.; Glazenburg, E.J.; Wolkerstorfer, A.; de Waard-van der Spek, F.B. Practical issues on interpretation of scoring atopic dermatitis: The SCORAD index, objective SCORAD and the three-item severity score. Br. J. Dermatol. 2007, 157, 645–648. [Google Scholar] [CrossRef]
- Hanifin, J.M.; Rajka, G. Diagnostic features of atopic dermatitis. Acta Derm. Venereol. 1980, 92, 44–47. [Google Scholar]
- Aleksandrzak-Piekarczyk, T.; Koryszewska-Bagińska, A.; Grynberg, M.; Nowak, A.; Cukrowska, B.; Kozakova, H.; Bardowski, J. Genomic and Functional Characterization of the Unusual pLOCK 0919 Plasmid Harboring the spaCBA Pili Cluster in Lactobacillus casei LOCK 0919. Genome Biol. Evol. 2015, 8, 202–217. [Google Scholar] [CrossRef] [Green Version]
- Koryszewska-Baginska, A.; Aleksandrzak-Piekarczyk, T.; Bardowski, J. Complete Genome Sequence of the Probiotic Strain Lactobacillus casei (Formerly Lactobacillus paracasei) LOCK919. Genome Announc. 2013, 1, e00758-13. [Google Scholar] [CrossRef] [Green Version]
- Koryszewska-Baginska, A.; Bardowski, J.; Aleksandrzak-Piekarczyk, T. Genome Sequence of the Probiotic Strain Lactobacillus rhamnosus (Formerly Lactobacillus casei) LOCK908. Genome Announc. 2014, 2, e00120-14. [Google Scholar] [CrossRef] [Green Version]
- Konopka, E.; Ceregra, A.; Maciorkowska, E.; Surowska, B.; Trojanowska, I.; Roszko-Kirpsza, I.; Cukrowska, B. Specific IgE Antibodies in Young Children with Atopic Dermatitis—Correlation of Multiple Allergen Simultaneous Immunoblot Test and ImmunoCap System. Clin. Lab. 2016, 62, 815–821. [Google Scholar] [CrossRef]
- Cukrowska, B.; Ceregra, A.; Rosiak, I.; Klewicka, E.; Slizewska, K.; Motyl, I. The influence of probiotic Lactobacillus casei and paracasei strains on clinical status of atopic eczema in children with food allergy on cow’s milk proteins [Wpływ probiotycznych szczepów Lactobacillus casei i paracasei na przebieg kliniczny wyprysku atopowego u dzieci z alergią pokarmową na białka mleka krowiego]. Pediatr. Wspolczesna 2008, 10, 67–70. [Google Scholar]
- Cukrowska, B.; Ceregra, A.; Klewicka, E.; Slizewska, K.; Motyl, I.; Libudzisz, Z. Probiotic lactobacillus casei and lactobacillus paracasei strains in treatment of food allergy in children [Probiotyczne szczepy lactobacillus casei i lactobacillus paracasei w leczeniu alergii pokarmowej u dzieci]. Przegląd Pediatryczny 2010, 40, 21–25. [Google Scholar]
- Makrgeorgou, A.; Leonardi-Bee, J.; Bath-Hextall, F.J.; Murrell, D.F.; Tang, M.L.; Roberts, A.; Boyle, R.J. Probiotics for treating eczema. Cochrane Database Syst. Rev. 2018, 2018, CD006135. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Ni, B.; Liu, Z.; Liu, X.; Xie, W.; Wu, I.X.Y.; Li, X. The Role of Probiotics in the Prevention and Treatment of Atopic Dermatitis in Children: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Paediatr. Drugs 2020, 22, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Shen, C.; Ma, L. Treatment efficacy of probiotics on atopic dermatitis, zooming in on infants: A systematic review and meta-analysis. Int. J. Dermatol. 2018, 57, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Śliżewska, K.; Motyl, I.; Klewicka, E.; Cukrowska, B.; Libudzisz, Z. Effects of probiotic preparation on metabolic activity of enteric microbiota in children with atopic dermatitis. Biotechnol. Biotechnol. Equip. 2009, 23, 885–887. [Google Scholar] [CrossRef]
- Ahn, S.H.; Yoon, W.; Lee, S.Y.; Shin, H.S.; Lim, M.Y.; Nam, Y.D.; Yoo, Y. Effects of Lactobacillus pentosus in Children with Allergen-Sensitized Atopic Dermatitis. J. Korean Med. Sci. 2020, 35, e128. [Google Scholar] [CrossRef]
- Navarro-López, V.; Ramírez-Boscá, A.; Ramón-Vidal, D.; Ruzafa-Costas, B.; Genovés-Martínez, S.; Chenoll-Cuadros, E.; Carrión-Gutiérrez, M.; Horga de la Parte, J.; Prieto-Merino, D.; Codoñer-Cortés, F.M. Effect of Oral Administration of a Mixture of Probiotic Strains on SCORAD Index and Use of Topical Steroids in Young Patients with Moderate Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2018, 154, 37–43. [Google Scholar] [CrossRef]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J. Allergy Clin. Immunol. 2019, 143, 1–11. [Google Scholar] [CrossRef]
- Carlsten, C.; Dimich-Ward, H.; Ferguson, A.; Watson, W.; Rousseau, R.; Dybuncio, A.; Becker, A.; Chan-Yeung, M. Atopic dermatitis in a high-risk cohort: Natural history, associated allergic outcomes, and risk factors. Ann. Allergy Asthma. Immunol. 2013, 110, 18–24. [Google Scholar] [CrossRef]
- van der Hulst, A.E.; Klip, H.; Brand, P.L. Risk of developing asthma in young children with atopic eczema: A systematic review. J. Allergy Clin. Immunol. 2007, 120, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Tsakok, T. Does atopic dermatitis cause food allergy? A systematic review. J. Allergy Clin. Immunol. 2016, 137, 1071–1078. [Google Scholar] [CrossRef] [Green Version]
- Gabet, S.; Rancière, F.; Just, J.; de Blic, J.; Lezmi, G.; Amat, F.; Seta, N.; Momas, I. Asthma and allergic rhinitis risk depends on house dust mite specific IgE levels in PARIS birth cohort children. World Allergy Organ. J. 2019, 12, 100057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elazab, N.; Mendy, A.; Gasana, J.; Vieira, E.R.; Quizon, A.; Forno, E. Probiotic administration in early life, atopy, and asthma: A meta-analysis of clinical trials. Pediatrics 2013, 132, e666–e676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Aa, L.B.; Heymans, H.S.; van Aalderen, W.M.; Sillevis Smitt, J.H.; Knol, J.; Ben Amor, K.; Goossens, D.A.; Sprikkelman, A.B.; Synbad Study Group. Effect of a new synbiotic mixture on atopic dermatitis in infants: A randomized-controlled trial. Clin. Exp. Allergy 2010, 40, 795–804. [Google Scholar] [CrossRef]
- van der Aa, L.B.; van Aalderen, W.M.; Heymans, H.S.; Henk Sillevis Smitt, J.; Nauta, A.J.; Knippels, L.M.; Ben Amor, K.; Sprikkelman, A.B.; Synbad Study Group. Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy 2011, 66, 170–177. [Google Scholar] [CrossRef]
- Canani, B.R.; Di Costanzo, M.; Bedogni, G.; Amoroso, A.; Cosenza, L.; Di Scala, C.; Granata, V.; Nocerino, R. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial. J. Allergy Clin. Immunol. 2017, 139, 1906–1913.e4. [Google Scholar] [CrossRef] [Green Version]
- Kozakova, H.; Schwarzer, M.; Tuckova, L.; Srutkova, D.; Czarnowska, E.; Rosiak, I.; Hudcovic, T.; Schabussova, I.; Hermanova, P.; Zakostelska, Z.; et al. Colonization of germ-free mice with a mixture of three lactobacillus strains enhances the integrity of gut mucosa and ameliorates allergic sensitization. Cell. Mol. Immunol. 2016, 13, 251–262. [Google Scholar] [CrossRef]

| Probiotic Group (n = 66) | Placebo Group (n = 68) | |
|---|---|---|
| Age in months (range) | 8.2 ± 6.1(4–23) | 8.8 ± 6.6 (2–23) |
| Sex—n (%) | ||
| Male | 37 (56.1) | 48 (70.6) |
| Female | 29 (43.9) | 29 (29.4) |
| Weight in kg (range) | 9.55 ± 3.6 (6.7–14.2) | 9.01 ± 3.1 (6.1–13.9) |
| Length/height in cm (range) | 76.95 ± 11.3 (65–89) | 75.8 ± 12.5 (57–92) |
| Family positive history for atopy—n (%) | 53 (80.3) | 52 (76.5) |
| Breastfeeding—n (%) | 10 (15.2) | 11 (16.2) |
| SCORAD score in points (range) | 40.4 ± 20.0 (14–103) | 35.3 ± 17.7 (12–99) |
| AD severity (SCORAD) | ||
| Mild (score <25)—n (%) | 14 (21.2) | 23 (33.3) |
| Moderate (score 25–50)—n (%) | 34 (51.5) | 33 (48.5) |
| Severe (score >50)—n (%) | 18 (27.3) | 12 (17.6) |
| AD type | ||
| Allergen sensitization—n (%) | 48 (72.7) | 48 (70.6) |
| No allergen sensitization—n (%) | 18 (27.3) | 20 (29.4) |
| Allergen | Baseline | 9-Month Follow-Up | ||
|---|---|---|---|---|
| Probiotic Group (n = 66) Number (%) | Placebo Group (n = 68) Number (%) | Probiotic Group (n = 48) Number (%) | Placebo Group (n = 53) Number (%) | |
| Food allergens | ||||
| Egg white | 30 (45.4) | 29 (42.6) | 20 (41.2) | 23 (43.4) |
| Egg yellow | 23 (34.8) | 20 (29.4) | 15 (31.2) | 15 (28.3) |
| Hazelnut | 15 (22.7) | 13 (19.1) | 15 (31.2) | 13 (24.5) |
| Cow’s milk | 14 (21.2) | 12 (17.6) | 7 (14.6) | 7 (13.2) |
| α-lactoalbumin | 9 (13.6) | 8 (11.8) | 4 (8.3) | 6 (11.3) |
| β-lactoglobulin | 6 (9.1) | 5 (7.3) | 3 (6.2) | 5 (9.4) |
| casein | 7 (10.6) | 6 (8.8) | 4 (8.3) | 5 (9.4) |
| Potato | 11 (16.7) | 10 (14.7) | 8 (16.7) | 8 (15.1) |
| Wheat flour | 8 (12.1) | 10 (14.7) | 8 (16.7) | 7 (13.2) |
| Codfish | 8 (12.1) | 7 (10.3) | 6 (12.5) | 7 (13.2) |
| Soybean | 7 (10.6) | 5 (7.3) | 7 (14.6) | 9 (17.0) |
| Peanut | 4 (6.1) | 3 (4.4) | 7 (14.6) | 7 (13.2) |
| Apple | 2 (3.0) | 3 (4.4) | 7 (14.6) | 7 (13.2) |
| Carrot | 1 (1.5) | 2 (2.9) | 2 (4.2) | 2 (3.8) |
| Rice | 1 (1.5) | 0 | 1 | 0 |
| Other allergens | ||||
| Mites | 5 (7.6) | 6 (8.8) | 7 (14.6) | 6 (11.3) |
| Grass mix | 6 (9.1) | 5 (7.3) | 6 (12.5) | 5 (9.4) |
| Birch | 5 (7.6) | 5 (7.3) | 8 (16.7) | 5 (9.4) |
| Cat | 4 (6.1) | 4 (5.9) | 6 (12.5) | 5 (9.4) |
| Dog | 4 (6.1) | 3 (4.4) | 6 (12.5) | 5 (9.4) |
| Hourse | 2 (3.0) | 1 (1.5) | 2 (4.2) | 1 (1.9) |
| Alteria alternate | 0 | 1 (1.5) | 2 (4.2) | 2 (3.8) |
| Mugwort | 0 | 1 (1.5) | 0 | 0 |
| Groups | Baseline | after 3-Months Intervention | after 9-Months Follow Up | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Change from Baseline | p-Value Within-Group | p-Value Comparison with Placebo | Mean ± SD | Change from Baseline | p-Value Within-Group | p-Value Comparison with Placebo | |
| All patients with AD | |||||||||
| Probiotic Placebo | 40.4 ± 20.0 35.7 ± 17.8 | 17.6 ± 14.8 18.9 ± 17.5 | −22.8 ± 17.5 −16.7 ± 17.9 | <0.00001 <0.00001 | 0.881 | 12.5 ± 15.4 13.1 ± 13.3 | −28.8 ± 17.1 −23.2 ± 20.9 | <0.00001 <0.00001 | 0.704 |
| Patients with allergen sensitization | |||||||||
| Probiotic Placebo | 45.1 ± 20.1 * 39.6 ± 17.5 # | 17.4 ± 15.9 22.0 ± 18.1 | −27.8 ± 16.8 ** −17.5 ± 18.0 | <0.00001 <0.00001 | 0.186 | 13.2 ± 17.3 14.7 ± 13.4 | −31.2 ± 20.8 −24.5 ± 18.6 | <0.00001 <0.00001 | 0.289 |
| Patients without sensitization | |||||||||
| Probiotic Placebo | 28.0 ± 13.8 * 25.4 ± 14.4 # | 15.3 ± 11.6 11.7 ± 12.9 | −11.7 ± 11.6 ** −13.5 ± 17.9 | 0.004 0.002 | 0.109 | 10.8 ± 9.6 8.0± 12.1 | −17.4 ± 19.7 −16.6 ± 16.7 | 0.013 0.003 | 0.486 |
| Groups | Improvement | Deterioration | No Improvement |
|---|---|---|---|
| All patients with AD | |||
| Probiotic group (n = 66) | 55 (83.3) | 4 (6.1) | 7 (10.6) |
| Placebo group (n = 68) | 45 (66.2) | 9 (13.2) | 14 (20.6) |
| p-value | 0.029 | 0.128 | 0.154 |
| OR (95% CI) | 2.56 (1.13–5.80) | 0.42 (0.12–1.45) | 0.46 (0.17–1.22) |
| p-value for OR | 0.012 | 0.171 | 0.119 |
| Patients with allergen sensitization | |||
| Probiotic group (n = 48) | 44 (91.7) | 1 (2.1) | 3 (6.2) |
| Placebo group (n = 48) | 31 (64.6) | 7 (14.6) | 10 (20.8) |
| p-value | 0.003 | 0.059 | 0.070 |
| OR (95% CI) | 6.03 (1.85–19.67) | 0.12 (0.02–1.06) | 0.25 (0.07–0.99) |
| p-value for OR | 0.001 | 0.028 | 0.023 |
| Patients without sensitization | |||
| Probiotic group (n = 18) | 11 (61.1) | 3 (16.7) | 4 (22.2) |
| Placebo group (n= 20) | 14 (70.0) | 2 (10.0) | 4 (20.0) |
| p-value | 0.503 | 0.209 | 0.101 |
| OR (95% CI) p-value for OR | 0.79 (0.21–2.92) 0.359 | 1.8 (0.27–12.2) 0.547 | 1.14 (0.24–5.44) 0.433 |
| Groups | Improvement | Deterioration | No improvement |
|---|---|---|---|
| All patients with AD | |||
| Probiotic group (n = 48) | 41 (85.4) | 7 (14.6) | 0 |
| Placebo group (n = 53) | 42 (79.2) | 6 (13.3) | 5 (9.4) |
| p-value | 0.448 | 0.769 | 0.058 |
| OR (95% CI) | 1.53 (0.54–4.34) | 1.33 (0.42–4.30) | 0.09 (0.005–1.69) |
| p-value for OR | 0.420 | 0.626 | 0.108 |
| Patients with allergen sensitization | |||
| Probiotic group (n = 34) | 31 (91.2) | 3 (8.8) | 0 |
| Placebo group (n = 37) | 29 (78.4) | 3 (8.1) | 5 (13.5) |
| p-value | 0.675 | 1.0 | 0.055 |
| OR (95% CI) | 2.85 (0.69–11.79) | 1.10 (0.21–5.84) | 0.09 (0.005–1.61) |
| p-value for OR | 0.148 | 0.457 | 0.101 |
| Patients without sensitization | |||
| Probiotic group (n = 14) | 10 (71.4) | 4 (28.6) | 0 |
| Placebo group (n = 16) | 13 (81.3) | 3 (18.7) | 0 |
| p-value | 0.675 | 0.682 | 1.000 |
| OR (95% CI) p-value for OR | 0.58 (0.10–3.19) 0.528 | 1.73 (0.31–9.57) 0.528 | 1.13 (0.002–61.08) 0.949 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cukrowska, B.; Ceregra, A.; Maciorkowska, E.; Surowska, B.; Zegadło-Mylik, M.A.; Konopka, E.; Trojanowska, I.; Zakrzewska, M.; Bierła, J.B.; Zakrzewski, M.; et al. The Effectiveness of Probiotic Lactobacillus rhamnosus and Lactobacillus casei Strains in Children with Atopic Dermatitis and Cow’s Milk Protein Allergy: A Multicenter, Randomized, Double Blind, Placebo Controlled Study. Nutrients 2021, 13, 1169. https://doi.org/10.3390/nu13041169
Cukrowska B, Ceregra A, Maciorkowska E, Surowska B, Zegadło-Mylik MA, Konopka E, Trojanowska I, Zakrzewska M, Bierła JB, Zakrzewski M, et al. The Effectiveness of Probiotic Lactobacillus rhamnosus and Lactobacillus casei Strains in Children with Atopic Dermatitis and Cow’s Milk Protein Allergy: A Multicenter, Randomized, Double Blind, Placebo Controlled Study. Nutrients. 2021; 13(4):1169. https://doi.org/10.3390/nu13041169
Chicago/Turabian StyleCukrowska, Bożena, Aldona Ceregra, Elżbieta Maciorkowska, Barbara Surowska, Maria Agnieszka Zegadło-Mylik, Ewa Konopka, Ilona Trojanowska, Magdalena Zakrzewska, Joanna Beata Bierła, Mateusz Zakrzewski, and et al. 2021. "The Effectiveness of Probiotic Lactobacillus rhamnosus and Lactobacillus casei Strains in Children with Atopic Dermatitis and Cow’s Milk Protein Allergy: A Multicenter, Randomized, Double Blind, Placebo Controlled Study" Nutrients 13, no. 4: 1169. https://doi.org/10.3390/nu13041169
APA StyleCukrowska, B., Ceregra, A., Maciorkowska, E., Surowska, B., Zegadło-Mylik, M. A., Konopka, E., Trojanowska, I., Zakrzewska, M., Bierła, J. B., Zakrzewski, M., Kanarek, E., & Motyl, I. (2021). The Effectiveness of Probiotic Lactobacillus rhamnosus and Lactobacillus casei Strains in Children with Atopic Dermatitis and Cow’s Milk Protein Allergy: A Multicenter, Randomized, Double Blind, Placebo Controlled Study. Nutrients, 13(4), 1169. https://doi.org/10.3390/nu13041169