Joint Effort towards Preventing Nutritional Deficiencies at the Extremes of Life during COVID-19
Abstract
:1. Introduction
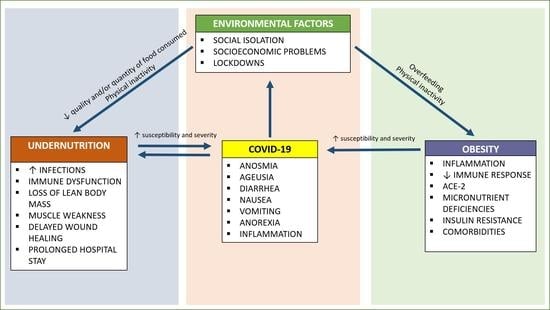
2. Malnutrition at the Two Extremes of Life during the COVID-19
2.1. The Context
2.2. Older Persons
2.3. Infants and Children
3. Management of Nutritional Status at the Two Extremes of Life during COVID-19
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Gandhi, R.T.; Lynch, J.B.; Del Rio, C. Mild or Moderate Covid-19. N. Engl. J. Med. 2020, 383, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Vanacore, N.; Agostoni, C. The two extremes meet: Pediatricians, geriatricians and the life-course approach. Pediatr. Res. 2019, 86, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Bedock, D.; Lassen, P.B.; Mathian, A.; Moreau, P.; Couffignal, J.; Ciangura, C.; Poitou-Bernert, C.; Jeannin, A.-C.; Mosbah, H.; Fadlallah, J.; et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin. Nutr. ESPEN 2020, 40, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Leon-Abarca, J.A. Obesity and immunodeficiencies are the main pre-existing conditions associated with mild to moderate COVID-19 in children. Pediatr. Obes. 2020, 15, e12713. [Google Scholar] [CrossRef] [PubMed]
- Kass, D.A.; Duggal, P.; Cingolani, O. Obesity could shift severe COVID-19 disease to younger ages. Lancet 2020, 395, 1544–1545. [Google Scholar] [CrossRef]
- Zhang, F.; Xiong, Y.; Wei, Y.; Hu, Y.; Wang, F.; Li, G.; Liu, K.; Du, R.; Wang, C.; Zhu, W. Obesity predisposes to the risk of higher mortality in young COVID-19 patients. J. Med. Virol. 2020, 92, 2536–2542. [Google Scholar] [CrossRef]
- Deng, M.; Qi, Y.; Deng, L.; Wang, H.; Xu, Y.; Li, Z.; Meng, Z.; Tang, J.; Dai, Z. Obesity as a Potential Predictor of Disease Severity in Young COVID-19 Patients: A Retrospective Study. Obesity 2020, 28, 1815–1825. [Google Scholar] [CrossRef]
- Elia, M. Defining, Recognizing, and Reporting Malnutrition. Int. J. Low. Extrem. Wounds 2017, 16, 230–237. [Google Scholar] [CrossRef]
- Devine, A.; Lawlis, T. Nutrition and Vulnerable Groups. Nutrients 2019, 11, 1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, L.A.; Gout, B.S.; Crowe, T.C. Hospital Malnutrition: Prevalence, Identification and Impact on Patients and the Healthcare System. Int. J. Environ. Res. Public Health 2011, 8, 514–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Bopp, M.M.; Roberson, P.K.; Sullivan, D.H. Undernutrition and risk of mortality in elderly patients within 1 year of hospital discharge. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M741–M746. [Google Scholar] [CrossRef] [Green Version]
- Bourke, C.D.; Berkley, J.A.; Prendergast, A.J. Immune Dysfunction as a Cause and Consequence of Malnutrition. Trends Immunol. 2016, 37, 386–398. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.K.; Zambruni, M.; Melby, C.L.; Melby, P.C. Impact of Childhood Malnutrition on Host Defense and Infection. Clin. Microbiol. Rev. 2017, 30, 919–971. [Google Scholar] [CrossRef] [Green Version]
- Browne, N.T.; Snethen, J.A.; Greenberg, C.S.; Frenn, M.; Kilanowski, J.F.; Gance-Cleveland, B.; Burke, P.J.; Lewandowski, L. When Pandemics Collide: The Impact of COVID-19 on Childhood Obesity. J. Pediatr. Nurs. Nurs. Care Child. Fam. 2021, 56, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Kuh, D.; New Dynamics of Ageing (NDA) Preparatory Network. A life course approach to healthy aging, frailty, and capability. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 717–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garazzino, S.; Montagnani, C.; Donà, D.; Meini, A.; Felici, E.; Vergine, G.; Bernardi, S.; Giacchero, R.; Vecchio, A.L.; Marchisio, P.; et al. Multicentre Italian study of SARS-CoV-2 infection in children and adolescents, preliminary data as at 10 April 2020. Eurosurveillance 2020, 25, 2000600. [Google Scholar] [CrossRef] [PubMed]
- Giacomet, V.; Barcellini, L.; Stracuzzi, M.; Longoni, E.; Folgori, L.; Leone, A.; Zuccotti, G.V. Gastrointestinal Symptoms in Severe COVID-19 Children. Pediatr. Infect. Dis. J. 2020, 39, e317–e320. [Google Scholar] [CrossRef]
- Akin, H.; Kurt, R.; Tufan, F.; Swi, A.; Ozaras, R.; Tahan, V.; Hammoud, G. Newly Reported Studies on the Increase in Gastrointestinal Symptom Prevalence withCOVID-19 Infection: A Comprehensive Systematic Review and Meta-Analysis. Diseases 2020, 8, 41. [Google Scholar] [CrossRef]
- Nogueira-de-Almeida, C.A.; Del Ciampo, L.A.; Ferraz, I.S.; Del Ciampo, I.R.L.; Contini, A.A.; Ued, F.D.V. COVID-19 and obesity in childhood and adolescence: A clinical review. J. Pediatr. 2020, 96, 546–558. [Google Scholar] [CrossRef]
- Astrup, A.; Bügel, S. Overfed but undernourished: Recognizing nutritional inadequacies/deficiencies in patients with overweight or obesity. Int. J. Obes. 2019, 43, 219–232. [Google Scholar] [CrossRef]
- Visser, M.; Schaap, L.A.; Wijnhoven, H.A.H. Self-Reported Impact of the COVID-19 Pandemic on Nutrition and Physical Activity Behaviour in Dutch Older Adults Living Independently. Nutrients 2020, 12, 3708. [Google Scholar] [CrossRef]
- Butler, M.J.; Barrientos, R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav. Immun. 2020, 87, 53–54. [Google Scholar] [CrossRef]
- Rouget, A.; Vardon-Bounes, F.; Lorber, P.; Vavasseur, A.; Marion, O.; Marcheix, B.; Lairez, O.; Balardy, L.; Fourcade, O.; Conil, J.-M.; et al. Prevalence of malnutrition in coronavirus disease 19: The NUTRICOV study. Br. J. Nutr. 2020, 1–8. [Google Scholar] [CrossRef]
- Parasa, S.; Desai, M.; Chandrasekar, V.T.; Patel, H.K.; Kennedy, K.F.; Roesch, T.; Spadaccini, M.; Colombo, M.; Gabbiadini, R.; Artifon, E.L.A.; et al. Prevalence of Gastrointestinal Symptoms and Fecal Viral Shedding in Patients with Coronavirus Disease 2019. JAMA Netw. Open 2020, 3, e2011335. [Google Scholar] [CrossRef]
- Zhong, P.; Xu, J.; Yang, D.; Shen, Y.; Wang, L.; Feng, Y.; Du, C.; Song, Y.; Wu, C.; Hu, X.; et al. COVID-19-associated gastrointestinal and liver injury: Clinical features and potential mechanisms. Signal Transduct. Target. Ther. 2020, 5, 1–8. [Google Scholar] [CrossRef]
- Morley, J.E.; Kalantar-Zadeh, K.; Anker, S.D. COVID-19: A major cause of cachexia and sarcopenia? J. Cachex Sarcopenia Muscle 2020, 11, 863–865. [Google Scholar] [CrossRef]
- Pan, L.; Mu, M.; Yang, P.; Sun, Y.; Wang, R.; Yan, J.; Li, P.; Hu, B.; Wang, J.; Hu, C.; et al. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020, 115, 766–773. [Google Scholar] [CrossRef]
- Zheng, T.; Yang, C.; Wang, H.-Y.; Chen, X.; Yu, L.; Wu, Z.-L.; Sun, H. Clinical characteristics and outcomes of COVID-19 patients with gastrointestinal symptoms admitted to Jianghan Fangcang Shelter Hospital in Wuhan, China. J. Med. Virol. 2020, 92, 2735–2741. [Google Scholar] [CrossRef]
- Redd, W.D.; Zhou, J.C.; Hathorn, K.E.; Mccarty, T.R.; Bazarbashi, A.N.; Thompson, C.C.; Shen, L.; Chan, W.W. Prevalence and Characteristics of Gastrointestinal Symptoms in Patients with Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology 2020, 159, 765–767. [Google Scholar] [CrossRef]
- Meng, X.; Deng, Y.; Dai, Z.; Meng, Z. COVID-19 and anosmia: A review based on up-to-date knowledge. Am. J. Otolaryngol. 2020, 41, 102581. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Gong, C.; Wang, J.; Liu, B.; Shi, L.; Duan, J. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur. J. Clin. Nutr. 2020, 74, 871–875. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Ye, J.; Chen, M.; Jiang, C.; Lin, W.; Lu, Y.; Ye, H.; Li, Y.; Wang, Y.; Liao, Q.; et al. Erratum to: Malnutrition Prolongs the Hospitalization of Patients with COVID-19 Infection: A Clinical Epidemiological Analysis. J. Nutr. Health Aging 2021, 25, 369–373. [Google Scholar] [CrossRef]
- Allard, L.; Ouedraogo, E.; Molleville, J.; Bihan, H.; Giroux-Leprieur, B.; Sutton, A.; Baudry, C.; Josse, C.; Didier, M.; Deutsch, D.; et al. Malnutrition: Percentage and Association with Prognosis in Patients Hospitalized for Coronavirus Disease 2019. Nutrients 2020, 12, 3679. [Google Scholar] [CrossRef]
- Suleyman, G.; Fadel, R.A.; Malette, K.M.; Hammond, C.; Abdulla, H.; Entz, A.; Demertzis, Z.; Hanna, Z.; Failla, A.; Dagher, C.; et al. Clinical Characteristics and Morbidity Associated With Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit. JAMA Netw. Open 2020, 3, e2012270. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Hajifathalian, K.; Kumar, S.; Newberry, C.; Shah, S.; Fortune, B.; Krisko, T.; Ortiz-Pujols, S.; Zhou, X.K.; Dannenberg, A.J.; Kumar, R.; et al. Obesity is Associated with Worse Outcomes in COVID-19: Analysis of Early Data from New York City. Obesity 2020, 28, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Busetto, L.; Bettini, S.; Fabris, R.; Serra, R.; Dal Pra, C.; Maffei, P.; Rossato, M.; Fioretto, P.; Vettor, R. Obesity and COVID-19: An Italian snapshot. Obesity 2020, 28, 1600–1605. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Patel, U.; Patel, K.; Martin, M.; Shah, C.; Mehta, D.; Malik, F.A.; Sharma, A. Obesity a predictor of outcomes of COVID-19 hospitalized patients-A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 1188–1193. [Google Scholar] [CrossRef]
- Ho, J.S.; Fernando, D.I.; Chan, M.Y.; Sia, C.-H. Obesity in COVID-19: A Systematic Review and Meta-analysis. Ann. Acad. Med. Singap. 2020, 49, 996–1008. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, Y.; Huang, Y.-M.; Wang, M.; Ling, W.; Sui, Y.; Zhao, H.-L. Obesity in patients with COVID-19: A systematic review and meta-analysis. Metabolism 2020, 113, 154378. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Sisto, A.; Marzetti, E. Anorexia of Aging: Risk Factors, Consequences, and Potential Treatments. Nutrients 2016, 8, 69. [Google Scholar] [CrossRef]
- Silverio, R.; Gonçalves, D.C.; Andrade, M.F.; Seelaender, M. Coronavirus Disease 2019 (COVID-19) and Nutritional Status: The Missing Link? Adv. Nutr. 2020. [Google Scholar] [CrossRef]
- Sullivan, D.H.; Sun, S.; Walls, R.C.; Kovacevich, D.S. Protein-Energy undernutrition among elderly hospitalized patients: A Prospective Study. Nutr. Clin. Pract. 1999, 14, 327–328. [Google Scholar] [CrossRef]
- Persson, M.D.; Brismar, K.E.; Katzarski, K.S.; Nordenström, J.; Cederholm, T.E. Nutritional Status Using Mini Nutritional Assessment and Subjective Global Assessment Predict Mortality in Geriatric Patients. J. Am. Geriatr. Soc. 2002, 50, 1996–2002. [Google Scholar] [CrossRef]
- Dupertuis, Y.M.; Kossovsky, M.P.; Kyle, U.G.; Raguso, C.A.; Genton, L.; Pichard, C. Food intake in 1707 hospitalised patients: A prospective comprehensive hospital survey. Clin. Nutr. 2003, 22, 115–123. [Google Scholar] [CrossRef]
- Morley, J.E. COVID-19—The Long Road to Recovery. J. Nutr. Health Aging 2020. [Google Scholar] [CrossRef]
- Orlandoni, P.; Venturini, C.; Peladic, N.J.; Costantini, A.; Di Rosa, M.; Cola, C.; Giorgini, N.; Basile, R.; Fagnani, D.; Sparvoli, D.; et al. Malnutrition upon Hospital Admission in Geriatric Patients: Why Assess It? Front. Nutr. 2017, 4, 50. [Google Scholar] [CrossRef] [Green Version]
- Braunschweig, C.; Gomez, S.; Sheean, P.M. Impact of Declines in Nutritional Status on Outcomes in Adult Patients Hospitalized for More Than 7 days. J. Am. Diet. Assoc. 2000, 100, 1316–1322. [Google Scholar] [CrossRef]
- Stefan, N.; Birkenfeld, A.L.; Schulze, M.B.; Ludwig, D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 2020, 16, 341–342. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Sattar, N.; McInnes, I.B.; McMurray, J.J.V. Obesity Is a Risk Factor for Severe COVID-19 Infection: Multiple Potential Mechanisms. Circulation 2020, 142, 4–6. [Google Scholar] [CrossRef]
- Li, M.-Y.; Li, L.; Zhang, Y.; Wang, X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef]
- Al-Benna, S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes. Med. 2020, 19, 100283. [Google Scholar] [CrossRef]
- Dicker, D.; Bettini, S.; Farpour-Lambert, N.; Frühbeck, G.; Golan, R.; Goossens, G.; Halford, J.; O’Malley, G.; Mullerova, D.; Salas, X.R.; et al. Obesity and COVID-19: The Two Sides of the Coin. Obes. Facts 2020, 13, 430–438. [Google Scholar] [CrossRef]
- Xanthakos, S.A. Nutritional Deficiencies in Obesity and After Bariatric Surgery. Pediatr. Clin. N. Am. 2009, 56, 1105–1121. [Google Scholar] [CrossRef] [Green Version]
- Wells, J.C.; Sawaya, A.L.; Wibaek, R.; Mwangome, M.; Poullas, M.S.; Yajnik, C.S.; Demaio, A. The double burden of malnutrition: Aetiological pathways and consequences for health. Lancet 2020, 395, 75–88. [Google Scholar] [CrossRef]
- Azzolino, D.; Cesari, M. Obesity and COVID-19. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Zamboni, M.; Mazzali, G.; Fantin, F.; Rossi, A.; Di Francesco, V. Sarcopenic obesity: A new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Schrager, M.A.; Metter, E.J.; Simonsick, E.; Ble, A.; Bandinelli, S.; Lauretani, F.; Ferrucci, L. Sarcopenic obesity and inflammation in the InCHIANTI study. J. Appl. Physiol. 2007, 102, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Akseer, N.; Kandru, G.; Keats, E.C.; Bhutta, Z.A. COVID-19 pandemic and mitigation strategies: Implications for maternal and child health and nutrition. Am. J. Clin. Nutr. 2020, 112, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Kluge, H.H.P.; Wickramasinghe, K.; Rippin, H.L.; Mendes, R.; Peters, D.H.; Kontsevaya, A.; Breda, J. Prevention and control of non-communicable diseases in the COVID-19 response. Lancet 2020, 395, 1678–1680. [Google Scholar] [CrossRef]
- Martinez-Ferran, M.; De La Guía-Galipienso, F.; Sanchis-Gomar, F.; Pareja-Galeano, H. Metabolic Impacts of Confinement during the COVID-19 Pandemic Due to Modified Diet and Physical Activity Habits. Nutrients 2020, 12, 1549. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef]
- Sidor, A.; Rzymski, P. Dietary Choices and Habits during COVID-19 Lockdown: Experience from Poland. Nutrients 2020, 12, 1657. [Google Scholar] [CrossRef]
- Mehta, S. Nutritional status and COVID-19: An opportunity for lasting change? Clin. Med. 2020, 20, 270–273. [Google Scholar] [CrossRef]
- Headey, D.; Heidkamp, R.; Osendarp, S.; Ruel, M.; Scott, N.; Black, R.; Shekar, M.; Bouis, H.; Flory, A.; Haddad, L.; et al. Impacts of COVID-19 on childhood malnutrition and nutrition-related mortality. Lancet 2020, 396, 519–521. [Google Scholar] [CrossRef]
- Roberton, T.; Carter, E.D.; Chou, V.B.; Stegmuller, A.R.; Jackson, B.D.; Tam, Y.; Sawadogo-Lewis, T.; Walker, N. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: A modelling study. Lancet Glob. Health 2020, 8, e901–e908. [Google Scholar] [CrossRef]
- Fore, H.H.; Dongyu, Q.; Beasley, D.M.; Ghebreyesus, T.A. Child malnutrition and COVID-19: The time to act is now. Lancet 2020, 396, 517–518. [Google Scholar] [CrossRef]
- Zemrani, B.; Gehri, M.; Masserey, E.; Knob, C.; Pellaton, R. A hidden side of the COVID-19 pandemic in children: The double burden of undernutrition and overnutrition. Int. J. Equity Health 2021, 20, 1–4. [Google Scholar] [CrossRef]
- Storz, M.A. The COVID-19 pandemic: An unprecedented tragedy in the battle against childhood obesity. Clin. Exp. Pediatr. 2020, 63, 477–482. [Google Scholar] [CrossRef]
- An, R. Projecting the impact of the coronavirus disease-2019 pandemic on childhood obesity in the United States: A microsimulation model. J. Sport Heath Sci. 2020, 9, 302–312. [Google Scholar] [CrossRef]
- Finch, A.; Tribble, A.G. The path ahead: From global pandemic to health promotion. Prev. Med. Rep. 2021, 21, 101271. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Diabetologia 1992, 35, 595–601. [Google Scholar] [CrossRef]
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; Van Goudoever, J.B.; De Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. Nutrition During Pregnancy, Lactation and Early Childhood and its Implications for Maternal and Long-Term Child Health: The Early Nutrition Project Recommendations. Ann. Nutr. Metab. 2019, 74, 93–106. [Google Scholar] [CrossRef]
- Ba, J.T.H.; Brown, C.J.; DiMaria-Ghalili, R.A.; Locher, J.L. Undernutrition in Hospitalized Older Adults: Patterns and Correlates, Outcomes, and Opportunities for Intervention with a Focus on Processes of Care. J. Nutr. Elder. 2010, 29, 4–41. [Google Scholar]
- Arlinghaus, K.R.; Truong, C.; Johnston, C.A.; Hernandez, D.C. An Intergenerational Approach to Break the Cycle of Malnutrition. Curr. Nutr. Rep. 2018, 7, 259–267. [Google Scholar] [CrossRef]
- Azzolino, D.; Passarelli, P.C.; D’Addona, A.; Cesari, M. Nutritional strategies for the rehabilitation of COVID-19 patients. Eur. J. Clin. Nutr. 2020, 75, 728–730. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin. Nutr. 2020, 39, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Caccialanza, R.; Laviano, A.; Lobascio, F.; Montagna, E.; Bruno, R.; Ludovisi, S.; Corsico, A.G.; Di Sabatino, A.; Belliato, M.; Calvi, M.; et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): Rationale and feasibility of a shared pragmatic protocol. Nutrition 2020, 74, 110835. [Google Scholar] [CrossRef] [PubMed]
- The WHO Child Growth Standards. Available online: https://www.who.int/tools/child-growth-standards (accessed on 27 January 2021).
- Screening for Malnutrition at Home during Covid-19—Haiti. Available online: https://reliefweb.int/report/haiti/screening-malnutrition-home-during-covid-19 (accessed on 20 March 2021).
- Krznarić, Ž.; Bender, D.V.; Laviano, A.; Cuerda, C.; Landi, F.; Monteiro, R.; Pirlich, M.; Barazzoni, R. A simple remote nutritional screening tool and practical guidance for nutritional care in primary practice during the COVID-19 pandemic. Clin. Nutr. 2020, 39, 1983–1987. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.J.; Bellini, S.G.; Vega, M.W.; Corkins, M.R.; Spear, B.A.; Spoede, E.; Hoy, M.K.; Piemonte, T.A.; Rozga, M. Validity and Reliability of Pediatric Nutrition Screening Tools for Hospital, Outpatient, and Community Settings: A 2018 Evidence Analysis Center Systematic Review. J. Acad. Nutr. Diet. 2020, 120, 288–318. [Google Scholar] [CrossRef]
- Kache, S.; Chisti, M.J.; Gumbo, F.; Mupere, E.; Zhi, X.; Nallasamy, K.; Nakagawa, S.; Lee, J.H.; Di Nardo, M.; De La Oliva, P.; et al. COVID-19 PICU guidelines: For high- and limited-resource settings. Pediatr. Res. 2020, 88, 705–716. [Google Scholar] [CrossRef]
- Mehta, N.M.; Skillman, H.E.; Irving, S.Y.; Coss-Bu, J.A.; Vermilyea, S.; Farrington, E.A.; McKeever, L.; Hall, A.M.; Goday, P.S.; Braunschweig, C. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Pediatric Critically Ill Patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. J. Parenter. Enter. Nutr. 2017, 41, 706–742. [Google Scholar] [CrossRef] [Green Version]
- Tume, L.N.; Valla, F.V.; Joosten, K.; Chaparro, C.J.; Latten, L.; Marino, L.V.; MacLeod, I.; Moullet, C.; Pathan, N.; Rooze, S.; et al. Nutritional support for children during critical illness: European Society of Pediatric and Neonatal Intensive Care (ESPNIC) metabolism, endocrine and nutrition section position statement and clinical recommendations. Intensiv. Care Med. 2020, 46, 411–425. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48. [Google Scholar] [CrossRef]
- Joosten, K.; Embleton, N.; Yan, W.; Senterre, T.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Energy. Clin. Nutr. 2018, 37, 2309–2314. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef] [Green Version]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharadwaj, S.; Ginoya, S.; Tandon, P.; Gohel, T.D.; Guirguis, J.; Vallabh, H.; Jevenn, A.; Hanouneh, I. Malnutrition: Laboratory markers vs nutritional assessment. Gastroenterol. Rep. 2016, 4, 272–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Pereira, S.L.; Luo, M.; Matheson, E.M. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 829. [Google Scholar] [CrossRef] [PubMed]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cereda, E.; Cruz-Jentoft, A.; Goisser, S.; De Groot, L.; Großhauser, F.; Kiesswetter, E.; Norman, K.; et al. Management of Malnutrition in Older Patients—Current Approaches, Evidence and Open Questions. J. Clin. Med. 2019, 8, 974. [Google Scholar] [CrossRef] [Green Version]
- Allen, K.; Hoffman, L. Enteral Nutrition in the Mechanically Ventilated Patient. Nutr. Clin. Pract. 2019, 34, 540–557. [Google Scholar] [CrossRef]
- Pereira, M.; Dantas Damascena, A.; Galvão Azevedo, L.M.; de Almeida Oliveira, T.; da Mota Santana, J. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020, 92, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, K.D.D.S.; Garcia, L.R.S.; Dametto, J.F.D.S.; Assunção, D.G.F.; Maciel, B.L.L. COVID-19 and Nutrition: The Need for Initiatives to Promote Healthy Eating and Prevent Obesity in Childhood. Child Obes. 2020, 16, 235–237. [Google Scholar] [CrossRef]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef]
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Vitamin deficiency as risk factor for SARS-CoV-2 infection: Correlation with susceptibility and prognosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9721–9738. [Google Scholar]
- Cereda, E.; Bogliolo, L.; De Stefano, L.; Caccialanza, R. A brief discussion of the benefit and mechanism of vitamin D supplementation on coronavirus disease 2019. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 102–107. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Sahraian, M.A.; Ebrahimi, M.; Pazoki, M.; Kafan, S.; Tabriz, H.M.; Hadadi, A.; Montazeri, M.; Nasiri, M.; Shirvani, A.; et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS ONE 2020, 15, e0239799. [Google Scholar] [CrossRef]
- Rhodes, J.M.; Subramanian, S.; Laird, E.; Griffin, G.; Kenny, R.A. Perspective: Vitamin D deficiency and COVID-19 severity—Plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J. Intern. Med. 2021, 289, 97–115. [Google Scholar] [CrossRef]
- Nanri, A.; Nakamoto, K.; Sakamoto, N.; Imai, T.; Akter, S.; Nonaka, D.; Mizoue, T. Association of serum 25-hydroxyvitamin D with influenza in case-control study nested in a cohort of Japanese employees. Clin. Nutr. 2017, 36, 1288–1293. [Google Scholar] [CrossRef]
- Lee, M.-D.; Lin, C.-H.; Lei, W.-T.; Chang, H.-Y.; Lee, H.-C.; Yeung, C.-Y.; Chiu, N.-C.; Chi, H.; Liu, J.-M.; Hsu, R.-J.; et al. Does Vitamin D Deficiency Affect the Immunogenic Responses to Influenza Vaccination? A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 409. [Google Scholar] [CrossRef] [Green Version]
- Biesalski, H.K. Obesity, vitamin D deficiency and old age a serious combination with respect to coronavirus disease-2019 severity and outcome. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 18–24. [Google Scholar] [CrossRef]
- Wallace, T.C. Combating COVID-19 and Building Immune Resilience: A Potential Role for Magnesium Nutrition? J. Am. Coll. Nutr. 2020, 39, 685–693. [Google Scholar] [CrossRef]
- Tan, C.W.; Ho, L.P.; Kalimuddin, S.; Cherng, B.P.Z.; Teh, Y.E.; Thien, S.Y.; Wong, H.M.; Tern, P.J.W.; Chandran, M.; Chay, J.W.M.; et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition 2020, 79–80, 111017. [Google Scholar] [CrossRef]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubée, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrition 2020, 12, 3377. [Google Scholar]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a single high dose of Vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: A randomized clinical trial. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef]
- Castillo, M.E.; Costa, L.M.E.; Barrios, J.M.V.; Díaz, J.F.A.; Miranda, J.L.; Bouillon, R.; Gomez, J.M.Q. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Ling, S.F.; Broad, E.; Murphy, R.; Pappachan, J.M.; Pardesi-Newton, S.; Kong, M.-F.; Jude, E.B. High-Dose Cholecalciferol Booster Therapy is Associated with a Reduced Risk of Mortality in Patients with COVID-19: A Cross-Sectional Multi-Centre Observational Study. Nutrition 2020, 12, 3799. [Google Scholar]
- Carlucci, P.M.; Ahuja, T.; Petrilli, C.; Rajagopalan, H.; Jones, S.; Rahimian, J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J. Med. Microbiol. 2020, 69, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.S.; Paguio, J.A.; Dee, E.C.; Tan, H.C.; Moulick, A.; Milazzo, C.; Jurado, J.; Penna, N.D.; Celi, L.A. The Minimal Effect of Zinc on the Survival of Hospitalized Patients with COVID-19: An Observational Study. Chest 2021, 159, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.; Isales, C.M.; Fulzele, S. Low level of Vitamin C and dysregulation of Vitamin C transporter might be involved in the severity of COVID-19 Infection. Aging Dis. 2021, 12, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; Backer, D.D.; Xiang, H.; et al. Pilot Trial of High-dose vitamin C in critically ill COVID-19 patients. Ann. Intensive Care 2020, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, G.; Spano, M.; Grosso, M.; Salvo, A.; Ingallina, C.; Russo, M.; Ritieni, A.; Mannina, L. Food and COVID-19: Preventive/Co-therapeutic Strategies Explored by Current Clinical Trials and in Silico Studies. Foods 2020, 9, 1036. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Rando, H.M.; Covid-Review Consortium; Greene, C.S. Dietary Supplements and Nutraceuticals under Investigation for COVID-19 Prevention and Treatment. arXiv 2021, arXiv:2102.02250v1. [Google Scholar]
| Reference | Study Design and Sample | Aim | Relevant Results |
|---|---|---|---|
| Gastrointestinal symptoms/Anorexia | |||
| Pan et al., 2020 [28] | Cross-sectional study; 204 COVID-19 patients; mean age 52.9 (SD 16) years | Investigate the prevalence and outcomes of COVID-19 patients with digestive symptoms. | 103 patients (50.5%) reported digestive symptoms, including lack of appetite (81 [78.6%] cases), diarrhea (35 [34%] cases), vomiting (4 [3.9%] cases), and abdominal pain (2 [1.9%] cases). |
| Zheng et al., 2020 [29] | Cross-sectional study; 1320 patients; median age 50 (IQR 40–57) years. | Compare clinical characteristics and outcomes between patients with and without GI symptoms. | 192 patients (14.5%) reported gastrointestinal symptoms, including diarrhea (107 [55.7%] cases), abdominal pain (11 [5.7%] cases), anorexia (62 [32.3%] cases), nausea and vomiting (57 [29.7%] cases). |
| Redd et al., 2020 [30] | Multicenter cohort study; 318 patients; mean age 63.4 (SD 16.6) years. | Examine prevalence and features of GI manifestations associated with SARS-CoV-2 infection | 61.3% of patients reported at least 1 gastrointestinal symptom on presentation, most commonly loss of appetite (34.8%), diarrhea (33.7%), and nausea (26.4%). |
| Meng et al., 2020 [31] | Review | Assess the relationship between olfactory dysfunction and COVID-19. | Anosmia ranged from 33.9 to 68% with female dominance. |
| Parasa et al., 2020 [25] | Systematic review and meta-analysis of 23 published and 6 preprint studies; 4805 patients; mean age 52.2 (SD 14.8) years | Examine incidence rates of gastrointestinal symptoms among patients with COVID-19 infection. | 12% of patients with COVID-19 infection reported gastrointestinal symptoms, including diarrhea (7.4%), nausea, and vomiting (4.6%). |
| Undernutrition | |||
| Bedock et al., 2020 [3] | Observational longitudinal study; 114 COVID-19 patients, mean age 59.9 (SD 15.9) years. | Examine the association between malnutrition and disease severity at admission and the impact of malnutrition on clinical outcomes (i.e., ICU transfer or death). | The overall prevalence of malnutrition was 42.1% (moderate: 23.7%, severe: 18.4%). The prevalence of malnutrition reached 66.7% in patients admitted from ICU. |
| Rouget et al., 2020 [24] | Prospective observational cohort study; 80 COVID-19 patients; median age 59.5 (IQR 49.5–68.5). | Evaluate the prevalence of malnutritionin patients hospitalized for COVID-19. | The prevalence of malnutrition was 37.5% with 26% of hospitalized patients who presented severe malnutrition. |
| Li et al., 2020 [32] | Cross-sectional study; 182 COVID-19 older patients; mean age 68.5 (SD 8.8) years. | Investigate the prevalence of malnutrition and its related factors in older patients with COVID-19. | 96 patients (52.7%) were malnourished and 50 patients (27.5%) were at risk of malnutrition |
| Yu et al., 2020 [33] | Retrospective survey study; 139 patients with COVID-19; mean age 61.47 (SD 14.76) years. | Examine the association of malnutrition with duration of hospitalization in patients with COVID-19. | 75 patients had nutritional risk (53.96%). Compared with the patients in the normal nutrition group, the hospitalization time was longer (15.67 [SD 6.26] days versus 27.48 [SD 5.04] days, p = 0.001) |
| Allard et al., 2020 [34] | Retrospective study; 108 COVID-19 patients; mean age 61.8 (SD 15.8). | Determine the percentage of malnutrition and its prognosis in patients admitted for COVID-19. | 42 (38.9%) patients were malnourished. Moderate or severe nutritional risk was found in 83 (84.7%) patients. Malnutrition was not associated with COVID-19 severity, while nutritional risk was associated with severe COVID-19 (p < 0.01). |
| Obesity | |||
| Suleyman et al., 2020 [35] | Case series; 463 patients with COVID-19; mean age 57.5 (SD 16.8) years | Describe the clinical characteristics and outcomes of patients with COVID-19 infection. | Severe obesity (i.e., BMI ≥ 40) was independently associated with intensive care unit admission (OR: 2.0; 95% CI: 1.4–3.6; p = 0.02) |
| Petrilli et al., 2020 [36] | Prospective cohort study; 5279 COVID-19 patients; median age 54 (IQR 38–66) years. | Examine outcomes of people admitted to hospital with COVID-19. | Any increase in BMI (i.e., BMI > 40) was strongly associated with hospital admission (OR: 2.5; CI: 1.8–3.4; average marginal effect: 14%) |
| Simonnet et al., 2020 [37] | Retrospective cohort study; 124 COVID-19 patients admitted in ICU; median age 60 (IQR 51–70) years. | Analyze the relationship between clinical characteristics, including BMI, and the requirement for invasive mechanical ventilation. | Obesity (BMI > 30 kg/m2) and severe obesity (BMI > 35 kg/m2) were present in 47.6% and 28.2% of cases, respectively. The proportion of patients who required IMV increased with BMI categories (p < 0.01, Chi square test for trend) |
| Hajifathalian et al., 2020 [38] | Retrospective review; 770 COVID-19 patients; mean age of 63.5 (SD 17) years | Examine the role of obesity in the clinical course of COVID-19 patients. | Obese patients were more likely to present with fever, cough and shortness of breath. Obesity was also associated with a significantly higher rate of ICU admission or death (RR = 1.58, p = 0.002) |
| Busetto et al., 2020 [39] | Retrospective cohort study; 92 COVID-19 patients; mean age 70.5 (SD 13.3) years | Assess the relationship between the severity of COVID-19 and obesity classes according to BMI. | A higher need for assisted ventilation and a higher admission to intensive or semi-intensive care units were observed in patients with overweight and obesity (p < 0.01 and p < 0.05, respectively) |
| Malik et al., 2021 [40] | Meta-analysis of 14 studies; 10, 233 confirmed COVID-19 patients; | Assess the effect of obesity on outcomes in the COVID-19 hospitalizations. | The overall prevalence of obesity was 33.9% (3473/10,233). COVID-19 patient with obesity had higher odds of poor outcomes (OR: 1.88; 95% CI: 1.25–2.80; p = 0.002). |
| Ho et al., 2020 [41] | Systematic Review and Meta-analysis of 61 studies; 270, 241 patients. | Examine the relationship between COVID-19 and obesity. | The pooled prevalence of obesity was 27.6% (95% CI: 22.0–33.2). Obesity was not significantly associated with increased ICU admission or critical illness (OR: 1.25, 95% CI: 0.99–1.58, p = 0.062) but was significantly associated with more severe disease (OR: 3.13, 95% CI: 1.41–6.92, p = 0.005), mortality (OR: 1.36, 95% CI: 1.09–1.69, p = 0.006) and a positive COVID-19 test (OR: 1.50, 95% CI: 1.25–1.81, p < 0.001). |
| Huang et al., 2020 [42] | Systematic review and meta-analysis of 33 studies (30 studies defined obesity via BMI and 3 studies using VAT adiposity); 45, 650 subjects. | Investigate the effects of obesity with the risk of severe disease among patients with COVID-19. | Higher BMI was associated with severe COVID-19 (OR 1.67, 95% CI: 1.43–1.96; p < 0.001), hospitalization (OR 1.76; 95% CI: 1.21–2.56, p = 0.003), ICU admission (OR 1.67, 95% CI: 1.26–2.21, p < 0.001), IMV requirement (OR: 2.19, 95% CI: 1.56–3.07, p < 0.001), and death (OR 1.37, 95% CI: 1.06–1.75, p = 0.014). Severe COVID-19 cases showed significantly higher VAT (SMD: 0.50, 95% CI: 0.33–0.68, p < 0.001), hospitalization (SMD: 0.49, 95% CI: 0.11–0.87; p = 0.011), ICU admission (SMD: 0.57, 95% CI: 0.33–0.81; p < 0.001) and IMV support (SMD: 0.37, 95% CI: 0.03–0.71; p = 0.035). |
| Reference | Study Design and Sample | Aim | Relevant Results |
|---|---|---|---|
| Gastrointestinal symptoms | |||
| Lu et al., 2020 [16] | Observational study; 171 children with COVID-19; median age 6.7 years (range 1 day–15 years) | Describe the epidemiologic characteristics, clinical features, and radiologic findings of children with COVID-19. | Children had a milder clinical course compared to adults. GI symptoms were not very common in children. 15 patients presented diarrhea (8.8%) and 11 (6.4%) vomiting. |
| Garazzino et al., 2020 [17] | Observational multicentre study; 168 children with COVID-19. | Collect preliminary data on COVID-19 presentation in children | In children, GI symptoms were frequent (18%). |
| Giacomet et al., 2020 [18] | Observational retrospective multicentre study; 127 children with COVID-19 | Explore the presence of GI symptoms in children with COVID-19 and the potential correlation between GI symptoms and severity of illness | GI symptoms were present in 28.3% of the children enrolled. COVID-19 severity was positively correlated with the presence of GI symptoms. |
| Undernutrition | |||
| Akseer et al., 2020 [63] | Review | Identify main risk factors for maternal and child undernutrition during the COVID-19 pandemic and provide guidance to reduce the consequent undernutrition | Children and mothers’ risk of undernutrition may be increase during the pandemic due to food insecurity/poor diet quality, reduced income/limited financial resources, restricted health services, interrupted education, unhealthy household environment. |
| Headey et al., 2020 [69] | Global health projection study | Provide an overview on the impact of COVID-19 on childhood malnutrition and nutrition-related mortality using three different projection models. | Low- and middle-income countries are expected to have an average 7.9% decrease in the gross national income, which might associate to an increase in moderate to severe wasting (chronic malnutrition) in children (up to 14.3%). Together with a projected year average reduction in nutrition and health services coverage of about 25% such event may lead to about 128,605 additional death in children <5 years during 2020. |
| Roberton et al., 2020 [70] | Global health projection study | Estimate the additional child (<5 years) and maternal deaths resulting from potential health systems disruption and decreased access to food. | A reduction by 9.8–51.9% of the coverage of essential maternal and child health interventions might result in increased prevalence of wasting by 10–50% and additional child and maternal death in 2020. |
| Obesity/Overweight | |||
| Nogueira-de-Almeida et al., 2020 [20] | Clinical review | Examine the factors contributing to increased COVID-19 susceptibility and severity in obese children and adolescents. | Obesity related risk factors such as chronic subclinical inflammation, impaired immune response, and association with communicable diseases may explain the increased evidence of higher severity and mortality rate for COVID-19 in the adult as well as in the young population. |
| Storz, 2020 [73] | Review | Present supporting evidence that the COVID-19 pandemic will aggravate the childhood obesity | Through multiple factors (lockdown and movement restrictions, quarantine, home-confinement, and social distancing, school closures, pandemic insecurity and economic hardship) COVID-19 will create an obesogenic environment, increasing childhood obesity |
| Browne et al., 2020 [14] | Report | Address the impact of COVID-19 on children with obesity and propose potential interventions to reduce the negative outcome. | Children with obesity may face biopsychosocial risks during COVID-19, which may lead to stress and consequent impaired inflammation and immune response to COVID-19 Access to timely, comprehensive healthcare is critical during the pandemic. |
| Leon-Abarca, 2020 [4] | Observational study; 21,161 subjects under 18 years old | Identify risk factors and pre-existing conditions associated with COVID-19 illness in childhood. | Obesity (3.1%) was among the most common pre-existing condition in children with COVID-19. Children with obesity had 4.5-fold probability of presenting pneumonia and 2.5-fold probability of being hospitalized. |
| Kass et al., 2020 [5] | Observational study; 265 COVID-19 patients admitted to hospital | Investigate the correlation between BMI and age in COVID-19 patients admitted to the ICU | Significant inverse correlation between age and BMI was observed, suggesting that younger individuals with COVID-19 admitted to hospital and those requiring ICU support are more likely to be obese. |
| Zhang et al., 2020 [6] | Observational retrospective study; 53 young patients (20 to 45 years). | Examine the risk factors of mortality in young patients with COVID-19 with specific attention to the relationships between obesity and COVID-19 mortality. | In young patients, obesity (high BMI) was strongly associated with high risk of mortality for SARS-CoV-2 infection. In addition, aggravated inflammatory response, enhanced cardiac injury and increased coagulation activity were also reported as contributing mechanism to the high mortality, compared to the COVID-19 survivor counterpart. |
| Deng et al., 2020 [7] | Observational retrospective study; 65 COVID-19 hospitalized patients aged 18 to 40 years | Explore the indicators for COVID-19 severity in young patients aged 18 to 40 years. | In young adults, severe COVID-19 cases had higher BMI compared to moderate cases (average 29.23 vs. 22.79 kg/m2, p < 0.01). |
| An R., 2020 [74] | National health projection study | Project the impact of the COVID-19 pandemic on childhood obesity by simulating the BMI z-score trajectory of a representative cohort under a control scenario without COVID-19 or under 4 alternative scenarios with COVID-19. | Relative to the control scenario without COVID-19, scenarios 1, 2, 3, and 4 were associated with an increase in the mean BMI z-score |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spolidoro, G.C.I.; Azzolino, D.; Shamir, R.; Cesari, M.; Agostoni, C. Joint Effort towards Preventing Nutritional Deficiencies at the Extremes of Life during COVID-19. Nutrients 2021, 13, 1616. https://doi.org/10.3390/nu13051616
Spolidoro GCI, Azzolino D, Shamir R, Cesari M, Agostoni C. Joint Effort towards Preventing Nutritional Deficiencies at the Extremes of Life during COVID-19. Nutrients. 2021; 13(5):1616. https://doi.org/10.3390/nu13051616
Chicago/Turabian StyleSpolidoro, Giulia C. I., Domenico Azzolino, Raanan Shamir, Matteo Cesari, and Carlo Agostoni. 2021. "Joint Effort towards Preventing Nutritional Deficiencies at the Extremes of Life during COVID-19" Nutrients 13, no. 5: 1616. https://doi.org/10.3390/nu13051616
APA StyleSpolidoro, G. C. I., Azzolino, D., Shamir, R., Cesari, M., & Agostoni, C. (2021). Joint Effort towards Preventing Nutritional Deficiencies at the Extremes of Life during COVID-19. Nutrients, 13(5), 1616. https://doi.org/10.3390/nu13051616