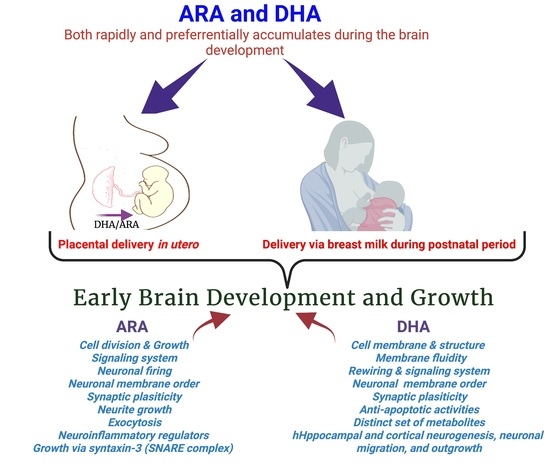
Maternal Supply of Both Arachidonic and Docosahexaenoic Acids Is Required for Optimal Neurodevelopment
Abstract
:1. Introduction
2. Maternal Delivery of DHA and ARA to the Developing Brain
3. The Fatty Acid Uptake System of the Brain
4. Structural and Functional Roles of DHA in the Human Brain
5. Roles of DHA and Its Metabolites in the Brain
6. DHA Deficiency in Utero and Human Brain Function
7. Can DHA Supplementation Improve Brain Function of Infants: Results of Clinical Trials
8. Roles of Arachidonic acid20:4n-6 (ARA) in Brain Development and Function
9. Transport of ARA to the Developing Brain
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Nyaradi, A.; Li, J.; Hickling, S.; Foster, J.; Oddy, W.H. The role of nutrition in children’s neurocognitive development, from pregnancy through childhood. Front. Hum. Neurosci. 2013, 7, 97. [Google Scholar] [CrossRef] [Green Version]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harslof, L.B.; Ciappolino, V.; Agostoni, C. DHA Effects in brain development and function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Martinez, M.; Conde, C.; Ballabriga, A. Some chemical aspects of human brain development. II. Phosphoglyceride fatty acids. Pediatr. Res. 1974, 8, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Clandinin, M.T.; Chappell, J.E.; Leong, S.; Heim, T.; Swyer, P.R.; Chance, G.W. Intrauterine fatty acid accretion rates in human brain: Implications for fatty acid requirements. Early Hum. Dev. 1980, 4, 121–129. [Google Scholar] [CrossRef]
- Agostoni, C.; Trojan, S.; Bellu, R.; Riva, E.; Giovannini, M. Neurodevelopmental quotient of healthy term infants at 4 months and feeding practice: The role of long-chain polyunsaturated fatty acids. Pediatr. Res. 1995, 38, 262–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostoni, C.; Trojan, S.; Bellu, R.; Riva, E.; Bruzzese, M.G.; Giovannini, M. Developmental quotient at 24 months and fatty acid composition of diet in early infancy: A follow up study. Arch. Dis. Child 1997, 76, 421–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helland, I.B.; Smith, L.; Saarem, K.; Saugstad, O.D.; Drevon, C.A. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics 2003, 111, e39–e44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drover, J.R.; Hoffman, D.R.; Castañeda, Y.S.; Morale, S.E.; Garfield, S.; Wheaton, D.H.; Birch, E.E. Cognitive function in 18-month-old term infants of the DIAMOND study: A randomized, controlled clinical trial with multiple dietary levels of docosahexaenoic acid. Early Hum. Dev. 2011, 87, 223–230. [Google Scholar] [CrossRef]
- Innis, S.M. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J. Pediatr. 2003, 143, S1–S8. [Google Scholar] [CrossRef] [Green Version]
- Jensen, C.L.; Voigt, R.G.; Prager, T.C.; Zou, Y.L.; Fraley, J.K.; Rozelle, J.C.; Turcich, M.R.; Llorente, A.M.; Anderson, R.E.; Heird, W.C. Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. Am. J. Clin. Nutr. 2005, 82, 125–132. [Google Scholar] [CrossRef]
- Dutta-Roy, A.K. Transport mechanisms for long-chain polyunsaturated fatty acids in the human placenta. Am. J. Clin. Nutr. 2000, 71, 315S–322S. [Google Scholar] [CrossRef] [Green Version]
- Basak, S.; Mallick, R.; Duttaroy, A.K. Maternal docosahexaenoic acid status during pregnancy and its impact on infant neurodevelopment. Nutrients 2020, 12, 3615. [Google Scholar] [CrossRef]
- Lauritzen, L.; Carlson, S.E. Maternal fatty acid status during pregnancy and lactation and relation to newborn and infant status. Matern. Child Nutr. 2011, 7, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Browning, L.M.; Walker, C.G.; Mander, A.P.; West, A.L.; Madden, J.; Gambell, J.M.; Young, S.; Wang, L.; Jebb, S.A.; Calder, P.C. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 2012, 96, 748–758. [Google Scholar] [CrossRef] [Green Version]
- Innis, S.M.; Friesen, R.W. Essential n-3 fatty acids in pregnant women and early visual acuity maturation in term infants. Am. J. Clin. Nutr. 2008, 87, 548–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavin, E.; Himovichi, E.; Eilam, R. Delayed cell migration in the developing rat brain following maternal omega 3 alpha linolenic acid dietary deficiency. Neuroscience 2009, 162, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Su, K.P. Biological mechanism of antidepressant effect of omega-3 fatty acids: How does fish oil act as a ‘mind-body interface’? Neurosignals 2009, 17, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Su, K.P.; Matsuoka, Y.; Pae, C.U. Omega-3 polyunsaturated fatty acids in prevention of mood and anxiety disorders. Clin. Psychopharmacol. Neurosci. 2015, 13, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunst, K.J.; Enlow, M.B.; Kannan, S.; Carroll, K.N.; Coull, B.A.; Wright, R.J. Effects of prenatal social stress and maternal dietary fatty acid ratio on infant temperament: Does race matter? Epidemiology 2014, 4. [Google Scholar] [CrossRef]
- Ciappolino, V.; DelVecchio, G.; Prunas, C.; Andreella, A.; Finos, L.; Caletti, E.; Siri, F.; Mazzocchi, A.; Botturi, A.; Turolo, S.; et al. The Effect of DHA supplementation on cognition in patients with bipolar disorder: An exploratory randomized control trial. Nutrients 2020, 12, 708. [Google Scholar] [CrossRef] [Green Version]
- Clayton, E.H.; Hanstock, T.L.; Hirneth, S.J.; Kable, C.J.; Garg, M.L.; Hazell, P.L. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids 2008, 43, 1031–1038. [Google Scholar] [CrossRef]
- Hahn-Holbrook, J.; Fish, A.; Glynn, L.M. Human milk omega-3 fatty acid composition is associated with infant temperament. Nutrients 2019, 11, 2964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grey, K.R.; Davis, E.P.; Sandman, C.A.; Glynn, L.M. Human milk cortisol is associated with infant temperament. Psychoneuroendocrinology 2013, 38, 1178–1185. [Google Scholar] [CrossRef] [Green Version]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic acid and cognition throughout the lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef]
- Lin, P.Y.; Su, K.P. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J. Clin. Psychiatry 2007, 68, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, H.S.; Agrawal, R.; Sharma, S.; Huo, Y.X.; Ying, Z.; Gomez-Pinilla, F. Omega-3 fatty acid deficiency during brain maturation reduces neuronal and behavioral plasticity in adulthood. PLoS ONE 2011, 6, e28451. [Google Scholar] [CrossRef] [Green Version]
- Mallick, R.; Basak, S.; Duttaroy, A.K. Docosahexaenoic acid,22:6n-3: Its roles in the structure and function of the brain. Int. J. Develop. Neurosci. Off. J. Int. Soc. Develop. Neurosci. 2019, 79, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras, M.A.; Rapoport, S.I. Recent studies on interactions between n-3 and n-6 polyunsaturated fatty acids in brain and other tissues. Curr. Opin. Lipidol. 2002, 13, 267–272. [Google Scholar] [CrossRef]
- Colombo, J.; Carlson, S.E.; Cheatham, C.L.; Shaddy, D.J.; Kerling, E.H.; Thodosoff, J.M.; Gustafson, K.M.; Brez, C. Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am. J. Clin. Nutr. 2013, 98, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Lepping, R.J.; Honea, R.A.; Martin, L.E.; Liao, K.; Choi, I.Y.; Lee, P.; Papa, V.B.; Brooks, W.M.; Shaddy, D.J.; Carlson, S.E.; et al. Long-chain polyunsaturated fatty acid supplementation in the first year of life affects brain function, structure, and metabolism at age nine years. Dev. Psychobiol. 2019, 61, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNamara, R.K.; Carlson, S.E. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Montes, R.; Chisaguano, A.M.; Castellote, A.I.; Morales, E.; Sunyer, J.; Lopez-Sabater, M.C. Fatty-acid composition of maternal and umbilical cord plasma and early childhood atopic eczema in a Spanish cohort. Eur. J. Clin. Nutr. 2013, 67, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, R.S.; Luxwolda, M.F.; Offringa, P.J.; Boersma, E.R.; Dijck-Brouwer, D.A.; Muskiet, F.A. Fetal intrauterine whole body linoleic, arachidonic and docosahexaenoic acid contents and accretion rates. Prostaglandins Leukot. Essent. Fatty Acids 2012, 86, 13–20. [Google Scholar] [CrossRef]
- Innis, S.M. Essential fatty acids in growth and development. Prog. Lipid Res. 1991, 30, 39–103. [Google Scholar] [CrossRef]
- Martinez, M.; Mougan, I. Fatty acid composition of human brain phospholipids during normal development. J. Neurochem. 1998, 71, 2528–2533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, N., Jr.; Wegher, B.; Mena, P.; Uauy, R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc. Natl. Acad. Sci. USA 1996, 93, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Dijck-Brouwer, D.A.; Hadders-Algra, M.; Bouwstra, H.; Decsi, T.; Boehm, G.; Martini, I.A.; Boersma, E.R.; Muskiet, F.A. Lower fetal status of docosahexaenoic acid, arachidonic acid and essential fatty acids is associated with less favorable neonatal neurological condition. Prostaglandins Leukot. Essent. Fatty Acids 2005, 72, 21–28. [Google Scholar] [CrossRef]
- Sherry, C.L.; Oliver, J.S.; Marriage, B.J. Docosahexaenoic acid supplementation in lactating women increases breast milk and plasma docosahexaenoic acid concentrations and alters infant omega 6:3 fatty acid ratio. Prostaglandins Leukot. Essent. Fatty Acids 2015, 95, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Cetin, I.; Alvino, G.; Cardellicchio, M. Long chain fatty acids and dietary fats in fetal nutrition. J. Physiol. 2009. [Google Scholar] [CrossRef]
- Duttaroy, A.K. Transport of fatty acids across the human placenta: A review. Prog. Lipid Res. 2009, 48, 52–61. [Google Scholar] [CrossRef]
- Innis, S.M. Impact of maternal diet on human milk composition and neurological development of infants. Am. J. Clin. Nutr. 2014, 99, 734S–741S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, A.T.; Brenna, J.T. Dietary docosahexaenoic acid but not arachidonic acid influences central nervous system fatty acid status in baboon neonates. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 105–110. [Google Scholar] [CrossRef]
- Calder, P.C. The DHA content of a cell membrane can have a significant influence on cellular behaviour and responsiveness to signals. Ann. Nutrit. Metab. 2016, 69, 8–21. [Google Scholar] [CrossRef]
- Basak, S.; Das, M.K.; Duttaroy, A.K. Fatty acid-induced angiogenesis in first trimester placental trophoblast cells: Possible roles of cellular fatty acid-binding proteins. Life Sci. 2013, 93, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, G.M.; Basak, S.; Weedon-Fekjaer, M.S.; Staff, A.C.; Duttaroy, A.K. Docosahexaenoic acid stimulates tube formation in first trimester trophoblast cells, HTR8/SVneo. Placenta 2011, 32, 626–632. [Google Scholar] [CrossRef]
- Khong, Y.; Brosens, I. Defective deep placentation. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 301–311. [Google Scholar] [CrossRef]
- Campbell, F.M.; Dutta-Roy, A.K. Plasma membrane fatty acid-binding protein (FABPpm) is exclusively located in the maternal facing membranes of the human placenta. FEBS Lett. 1995, 375, 227–230. [Google Scholar] [CrossRef] [Green Version]
- Bourre, J.M. Dietary omega-3 fatty acids for women. Biomed. Pharmacother. 2007, 61, 105–112. [Google Scholar] [CrossRef]
- Jorgensen, M.H.; Nielsen, P.K.; Michaelsen, K.F.; Lund, P.; Lauritzen, L. The composition of polyunsaturated fatty acids in erythrocytes of lactating mothers and their infants. Matern. Child. Nutr. 2006, 2, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Schmidt, E.; Bremer, H.J.; Haug, M.; Harzer, G. Effects of dietary long-chain polyunsaturated fatty acids on the essential fatty acid status of premature infants. Eur. J. Pediatr. 1989, 148, 669–675. [Google Scholar] [CrossRef]
- Innis, S.M. Chapter 10 Essential fatty acid metabolism during early development. Biol. Grow. Anim. 2005. [Google Scholar] [CrossRef]
- Hibbeln, J.R.; Spiller, P.; Brenna, J.T.; Golding, J.; Holub, B.J.; Harris, W.S.; Kris-Etherton, P.; Lands, B.; Connor, S.L.; Myers, G.; et al. Relationships between seafood consumption during pregnancy and childhood and neurocognitive development: Two systematic reviews. Prostaglandins Leukot. Essent. Fatty Acids 2019, 151, 14–36. [Google Scholar] [CrossRef] [Green Version]
- Oddy, W.H.; Kendall, G.E.; Li, J.; Jacoby, P.; Robinson, M.; de Klerk, N.H.; Silburn, S.R.; Zubrick, S.R.; Landau, L.I.; Stanley, F.J. The long-term effects of breastfeeding on child and adolescent mental health: A pregnancy cohort study followed for 14 years. J. Pediatr. 2010, 156, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Agostoni, C.; Lammardo, A.M.; Giovannini, M.; Galli, C.; Riva, E. Polyunsaturated fatty acid concentrations in human hindmilk are stable throughout 12-months of lactation and provide a sustained intake to the infant during exclusive breastfeeding: An Italian study. Br. J. Nutr. 2000, 84, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Brenna, J.T.; Varamini, B.; Jensen, R.G.; Diersen-Schade, D.A.; Boettcher, J.A.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [CrossRef] [Green Version]
- Makrides, M.; Neumann, M.A.; Gibson, R.A. Effect of maternal docosahexaenoic acid (DHA) supplementation on breast milk composition. Eur. J. Clin. Nutr. 1996, 50, 352–357. [Google Scholar] [PubMed]
- Lattka, E.; Rzehak, P.; Szabo, E.; Jakobik, V.; Weck, M.; Weyermann, M.; Grallert, H.; Rothenbacher, D.; Heinrich, J.; Brenner, H.; et al. Genetic variants in the FADS gene cluster are associated with arachidonic acid concentrations of human breast milk at 1.5 and 6 mo postpartum and influence the course of milk dodecanoic, tetracosenoic, and trans-9-octadecenoic acid concentrations over the duration of lactation. Am. J. Clin. Nutr. 2011, 93, 382–391. [Google Scholar] [CrossRef] [Green Version]
- Van Goor, S.A.; Dijck-Brouwer, D.A.; Hadders-Algra, M.; Doornbos, B.; Erwich, J.J.; Schaafsma, A.; Muskiet, F.A. Human milk arachidonic acid and docosahexaenoic acid contents increase following supplementation during pregnancy and lactation. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.E.; Werkman, S.H.; Peeples, J.M.; Cooke, R.J.; Tolley, E.A. Arachidonic acid status correlates with first year growth in preterm infants. Proc. Natl. Acad. Sci. USA 1993, 90, 1073–1077. [Google Scholar] [CrossRef] [Green Version]
- Uauy-Dagach, R.; Mena, P. Nutritional role of omega-3 fatty acids during the perinatal period. Clin. Perinatol. 1995, 22, 157–175. [Google Scholar] [CrossRef]
- Jimenez, M.J.; Bocos, C.; Panadero, M.; Herrera, E. Fish oil diet in pregnancy and lactation reduces pup weight and modifies newborn hepatic metabolic adaptations in rats. Eur. J. Nutr. 2017, 56, 409–420. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.T.; Bellinger, D.C.; Connor, W.E.; Shaywitz, B.A. A quantitative analysis of prenatal intake of n-3 polyunsaturated fatty acids and cognitive development. Am. J. Prev. Med. 2005, 29, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, J.A.; Simmer, K.; Dixon, G.; Prescott, S.L. Cognitive assessment of children at age 2(1/2) years after maternal fish oil supplementation in pregnancy: A randomised controlled trial. Arch. Dis. Child Fetal. Neonatal. Ed. 2008, 93, F45–F50. [Google Scholar] [CrossRef] [PubMed]
- Oddy, W.H.; Li, J.; Whitehouse, A.J.; Zubrick, S.R.; Malacova, E. Breastfeeding duration and academic achievement at 10 years. Pediatrics 2011, 127, e137–e145. [Google Scholar] [CrossRef] [Green Version]
- Kramer, M.S.; Aboud, F.; Mironova, E.; Vanilovich, I.; Platt, R.W.; Matush, L.; Igumnov, S.; Fombonne, E.; Bogdanovich, N.; Ducruet, T.; et al. Breastfeeding and child cognitive development: New evidence from a large randomized trial. Arch. Gen. Psychiatry 2008, 65, 578–584. [Google Scholar] [CrossRef]
- Caspi, A.; Williams, B.; Kim-Cohen, J.; Craig, I.W.; Milne, B.J.; Poulton, R.; Schalkwyk, L.C.; Taylor, A.; Werts, H.; Moffitt, T.E. Moderation of breastfeeding effects on the IQ by genetic variation in fatty acid metabolism. Proc. Natl. Acad. Sci. USA 2007, 104, 18860–18865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Elswyk, M.E.; Kuratko, C. Achieving adequate DHA in maternal and infant diets. J. Am. Diet Assoc. 2009, 109, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008, 1237, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Dutta-Roy, A.K. Cellular uptake of long-chain fatty acids: Role of membrane-associated fatty-acid-binding/transport proteins. Cell. Mol. Life Sci. 2000, 57, 1360–1372. [Google Scholar] [CrossRef]
- Mitchell, R.W.; On, N.H.; Del Bigio, M.R.; Miller, D.W.; Hatch, G.M. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J. Neurochem. 2011, 117, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Guemez-Gamboa, A.; Nguyen, L.N.; Yang, H.; Zaki, M.S.; Kara, M.; Ben-Omran, T.; Akizu, N.; Rosti, R.O.; Rosti, B.; Scott, E.; et al. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat. Genet. 2015, 47, 809–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, B.H.; Silver, D.L. Mfsd2a: A physiologically important lysolipid transporter in the brain and eye. Adv. Exp. Med. Biol. 2020, 1276, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Campillo, M.; Ruiz-Palacios, M.; Ruiz-Alcaraz, A.J.; Prieto-Sanchez, M.T.; Blanco-Carnero, J.E.; Zornoza, M.; Ruiz-Pastor, M.J.; Demmelmair, H.; Sanchez-Solis, M.; Koletzko, B.; et al. Child head circumference and placental MFSD2a Expression are associated to the level of MFSD2a in maternal blood during pregnancy. Front. Endocrinol. 2020, 11, 38. [Google Scholar] [CrossRef]
- Thies, F.; Delachambre, M.C.; Bentejac, M.; Lagarde, M.; Lecerf, J. Unsaturated fatty acids esterified in 2-acyl-l-lysophosphatidylcholine bound to albumin are more efficiently taken up by the young rat brain than the unesterified form. J. Neurochem. 1992, 59, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Chmurzynska, A. The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism. J. Appl. Genet. 2006, 47, 39–48. [Google Scholar] [CrossRef]
- Owada, Y.; Yoshimoto, T.; Kondo, H. Spatio-temporally differential expression of genes for three members of fatty acid binding proteins in developing and mature rat brains. J. Chem. Neuroanat. 1996, 12, 113–122. [Google Scholar] [CrossRef]
- Cataltepe, O.; Arikan, M.C.; Ghelfi, E.; Karaaslan, C.; Ozsurekci, Y.; Dresser, K.; Li, Y.; Smith, T.W.; Cataltepe, S. Fatty acid binding protein 4 is expressed in distinct endothelial and non-endothelial cell populations in glioblastoma. Neuropathol. Appl. Neurobiol. 2012, 38, 400–410. [Google Scholar] [CrossRef]
- Feng, L.; Hatten, M.E.; Heintz, N. Brain lipid-binding protein (BLBP): A novel signaling system in the developing mammalian CNS. Neuron 1994, 12, 895–908. [Google Scholar] [CrossRef]
- Liang, Y.; Bollen, A.W.; Aldape, K.D.; Gupta, N. Nuclear FABP7 immunoreactivity is preferentially expressed in infiltrative glioma and is associated with poor prognosis in EGFR-overexpressing glioblastoma. BMC Cancer 2006, 6, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaloshi, G.; Mokhtari, K.; Carpentier, C.; Taillibert, S.; Lejeune, J.; Marie, Y.; Delattre, J.Y.; Godbout, R.; Sanson, M. FABP7 expression in glioblastomas: Relation to prognosis, invasion and EGFR status. J. Neurooncol. 2007, 84, 245–248. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, A.; Pellegatta, S.; Rossi, M.; Tunici, P.; Magnoni, L.; Speranza, M.C.; Malusa, F.; Miragliotta, V.; Mori, E.; Finocchiaro, G.; et al. A radial glia gene marker, fatty acid binding protein 7 (FABP7), is involved in proliferation and invasion of glioblastoma cells. PLoS ONE 2012, 7, e52113. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.Z.; Mita, R.; Beaulieu, M.; Gao, Z.; Godbout, R. Fatty acid binding proteins in brain development and disease. Int. J. Dev. Biol. 2010, 54, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Bernlohr, D.A.; Coe, N.R.; Simpson, M.A.; Hertzel, A.V. Regulation of gene expression in adipose cells by polyunsaturated fatty acids. Adv. Exp. Med. Biol. 1997, 422, 145–156. [Google Scholar] [CrossRef]
- Murphy, E.J.; Owada, Y.; Kitanaka, N.; Kondo, H.; Glatz, J.F. Brain arachidonic acid incorporation is decreased in heart fatty acid binding protein gene-ablated mice. Biochemistry 2005, 44, 6350–6360. [Google Scholar] [CrossRef]
- Hanhoff, T.; Lucke, C.; Spener, F. Insights into binding of fatty acids by fatty acid binding proteins. Mol. Cell Biochem. 2002, 239, 45–54. [Google Scholar] [CrossRef]
- Balendiran, G.K.; Schnutgen, F.; Scapin, G.; Borchers, T.; Xhong, N.; Lim, K.; Godbout, R.; Spener, F.; Sacchettini, J.C. Crystal structure and thermodynamic analysis of human brain fatty acid-binding protein. J. Biol. Chem. 2000, 275, 27045–27054. [Google Scholar] [CrossRef]
- Owada, Y. Fatty acid binding protein: Localization and functional significance in the brain. Tohoku J. Exp. Med. 2008, 214, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Maximin, E.; Langelier, B.; Aioun, J.; Al-Gubory, K.H.; Bordat, C.; Lavialle, M.; Heberden, C. Fatty acid binding protein 7 and n-3 poly unsaturated fatty acid supply in early rat brain development. Dev. Neurobiol. 2016, 76, 287–297. [Google Scholar] [CrossRef]
- Kawakita, E.; Hashimoto, M.; Shido, O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience 2006, 139, 991–997. [Google Scholar] [CrossRef]
- Sakayori, N.; Maekawa, M.; Numayama-Tsuruta, K.; Katura, T.; Moriya, T.; Osumi, N. Distinctive effects of arachidonic acid and docosahexaenoic acid on neural stem /progenitor cells. Genes Cells 2011, 16, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Cheon, M.S.; Kim, S.H.; Fountoulakis, M.; Lubec, G. Heart type fatty acid binding protein (H-FABP) is decreased in brains of patients with Down syndrome and Alzheimer's disease. J. Neural. Transm. Suppl. 2003, 225–234. [Google Scholar] [CrossRef]
- Sanchez-Font, M.F.; Bosch-Comas, A.; Gonzalez-Duarte, R.; Marfany, G. Overexpression of FABP7 in Down syndrome fetal brains is associated with PKNOX1 gene-dosage imbalance. Nucleic Acids Res. 2003, 31, 2769–2777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, A.; Toyota, T.; Owada, Y.; Hayashi, T.; Iwayama, Y.; Matsumata, M.; Ishitsuka, Y.; Nakaya, A.; Maekawa, M.; Ohnishi, T.; et al. Fabp7 maps to a quantitative trait locus for a schizophrenia endophenotype. PLoS Biol. 2007, 5, e297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braff, D.L.; Geyer, M.A.; Light, G.A.; Sprock, J.; Perry, W.; Cadenhead, K.S.; Swerdlow, N.R. Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophr. Res. 2001, 49, 171–178. [Google Scholar] [CrossRef]
- Gearhart, D.A.; Toole, P.F.; Warren Beach, J. Identification of brain proteins that interact with 2-methylnorharman. An analog of the parkinsonian-inducing toxin, MPP+. Neurosci. Res. 2002, 44, 255–265. [Google Scholar] [CrossRef]
- Steinacker, P.; Mollenhauer, B.; Bibl, M.; Cepek, L.; Esselmann, H.; Brechlin, P.; Lewczuk, P.; Poser, S.; Kretzschmar, H.A.; Wiltfang, J.; et al. Heart fatty acid binding protein as a potential diagnostic marker for neurodegenerative diseases. Neurosci. Lett. 2004, 370, 36–39. [Google Scholar] [CrossRef]
- Mita, R.; Beaulieu, M.J.; Field, C.; Godbout, R. Brain fatty acid-binding protein and ω-3/ω-6 fatty acids. J. Biol. Chem. 2010, 285, 37005–37015. [Google Scholar] [CrossRef] [Green Version]
- Cunnane, S.C.; Plourde, M.; Pifferi, F.; Begin, M.; Feart, C.; Barberger-Gateau, P. Fish, docosahexaenoic acid and Alzheimer’s disease. Prog. Lipid Res. 2009, 48, 239–256. [Google Scholar] [CrossRef] [Green Version]
- Calderon, F.; Kim, H.Y. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J. Neurochem. 2004, 90, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Xue, R.; Xu, J.; Liu, Z. Effects of docosahexaenoic acid on the survival and neurite outgrowth of rat cortical neurons in primary cultures. J. Nutr. Biochem. 2005, 16, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Insua, M.F.; Garelli, A.; Rotstein, N.P.; German, O.L.; Arias, A.; Politi, L.E. Cell cycle regulation in retinal progenitors by glia-derived neurotrophic factor and docosahexaenoic acid. Investig. Ophthalmol. Visual Sci. 2003, 44, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Aïd, S.; Vancassel, S.; Poumès-Ballihaut, C.; Chalon, S.; Guesnet, P.; Lavialle, M. Effect of a diet-induced n-3 PUFA depletion on cholinergic parameters in the rat hippocampus. J. Lipid Res. 2003, 44, 1545–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalon, S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot. Essential Fatty Acids 2006, 75, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Neuringer, M.; Lin, D.S.; Connor, W.E. Can prenatal N-3 fatty acid deficiency be completely reversed after birth? Effects on retinal and brain biochemistry and visual function in rhesus monkeys. Pediatr. Res. 2005, 58, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Innis, S.M. Dietary (n-3) Fatty Acids and Brain Development. J. Nutr. 2018, 137, 855–859. [Google Scholar] [CrossRef] [Green Version]
- Coti Bertrand, P.; O’Kusky, J.R.; Innis, S.M. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J. Nutr. 2006, 136, 1570–1575. [Google Scholar] [CrossRef] [Green Version]
- Salem, N.; Litman, B.; Kim, H.Y.; Gawrisch, K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 2001, 36, 945–959. [Google Scholar] [CrossRef] [Green Version]
- Lengqvist, J.; Mata de Urquiza, A.; Bergman, A.-C.; Willson, T.M.; Sjövall, J.; Perlmann, T.; Griffiths, W.J. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor α ligand-binding domain. Mol. Cell Proteom. 2004, 3, 692–703. [Google Scholar] [CrossRef] [Green Version]
- Rioux, L.; Arnold, S.E. The expression of retinoic acid receptor alpha is increased in the granule cells of the dentate gyrus in schizophrenia. Psychiatry Res. 2005, 133, 13–21. [Google Scholar] [CrossRef]
- Wada, K.; Nakajima, A.; Katayama, K.; Kudo, C.; Shibuya, A.; Kubota, N.; Terauchi, Y.; Tachibana, M.; Miyoshi, H.; Kamisaki, Y.; et al. Peroxisome proliferator-activated receptor γ-mediated regulation of neural stem cell proliferation and differentiation. J. Biol. Chem. 2006, 281, 12673–12681. [Google Scholar] [CrossRef] [Green Version]
- Cao, D.H.; Xu, J.F.; Xue, R.H.; Zheng, W.F.; Liu, Z.L. Protective effect of chronic ethyl docosahexaenoate administration on brain injury in ischemic gerbils. Pharmacol. Biochem. Behav. 2004, 79, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Green, P.; Glozman, S.; Weiner, L.; Yavin, E. Enhanced free radical scavenging and decreased lipid peroxidation in the rat fetal brain after treatment with ethyl docosahexaenoate. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2001, 1532, 203–212. [Google Scholar] [CrossRef]
- Okada, M.; Amamoto, T.; Tomonaga, M.; Kawachi, A.; Yazawa, K.; Mine, K.; Fujiwara, M. The chronic administration of docosahexaenoic acid reduces the spatial cognitive deficit following transient forebrain ischemia in rats. Neuroscience 1996, 71, 17–25. [Google Scholar] [CrossRef]
- Samson, F.P.; Fabunmi, T.E.; Patrick, A.T.; Jee, D.; Gutsaeva, D.R.; Jahng, W.J. Fatty Acid Composition and Stoichiometry Determine the Angiogenesis Microenvironment. ACS Omega 2021, 6, 5953–5961. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shinto, L.; Connor, W.E.; Quinn, J.F. Nutritional biomarkers in Alzheimer’s disease: The association between carotenoids, n-3 fatty acids, and dementia severity. J. Alzheimers Dis. 2008, 13, 31–38. [Google Scholar] [CrossRef]
- Cao, L.; Tan, L.; Wang, H.F.; Jiang, T.; Zhu, X.C.; Lu, H.; Tan, M.S.; Yu, J.T. Dietary patterns and risk of dementia: A systematic review and meta-analysis of cohort studies. Mol. Neurobiol. 2016, 53, 6144–6154. [Google Scholar] [CrossRef]
- Bousquet, M.; Saint-Pierre, M.; Julien, C.; Salem, N., Jr.; Cicchetti, F.; Calon, F. Beneficial effects of dietary omega-3 polyunsaturated fatty acid on toxin-induced neuronal degeneration in an animal model of Parkinson’s disease. FASEB J. 2008, 22, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Sinn, N.; Milte, C.M.; Street, S.J.; Buckley, J.D.; Coates, A.M.; Petkov, J.; Howe, P.R. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Nutr. 2012, 107, 1682–1693. [Google Scholar] [CrossRef] [Green Version]
- Freund-Levi, Y.; Eriksdotter-Jonhagen, M.; Cederholm, T.; Basun, H.; Faxen-Irving, G.; Garlind, A.; Vedin, I.; Vessby, B.; Wahlund, L.O.; Palmblad, J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: A randomized double-blind trial. Arch. Neurol. 2006, 63, 1402–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R., Jr.; Weiner, M.; et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef]
- Eilander, A.; Hundscheid, D.C.; Osendarp, S.J.; Transler, C.; Zock, P.L. Effects of n-3 long chain polyunsaturated fatty acid supplementation on visual and cognitive development throughout childhood: A review of human studies. Prostaglandins Leukot. Essent. Fatty Acids 2007, 76, 189–203. [Google Scholar] [CrossRef]
- Christie, W.W.; Harwood, J.L. Oxidation of polyunsaturated fatty acids to produce lipid mediators. Essays Biochem. 2020, 64, 401–421. [Google Scholar] [CrossRef]
- Oh, D.Y.; Walenta, E.; Akiyama, T.E.; Lagakos, W.S.; Lackey, D.; Pessentheiner, A.R.; Sasik, R.; Hah, N.; Chi, T.J.; Cox, J.M.; et al. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat. Med. 2014, 20, 942–947. [Google Scholar] [CrossRef] [Green Version]
- Diep, Q.N.; Touyz, R.M.; Schiffrin, E.L. Docosahexaenoic acid, a peroxisome proliferator-activated receptor-alpha ligand, induces apoptosis in vascular smooth muscle cells by stimulation of p38 mitogen-activated protein kinase. Hypertension 2000, 36, 851–855. [Google Scholar] [CrossRef] [Green Version]
- Chitre, N.M.; Wood, B.J.; Ray, A.; Moniri, N.H.; Murnane, K.S. Docosahexaenoic acid protects motor function and increases dopamine synthesis in a rat model of Parkinson’s disease via mechanisms associated with increased protein kinase activity in the striatum. Neuropharmacology 2020, 167, 107976. [Google Scholar] [CrossRef]
- Zúñiga, J.; Cancino, M.; Medina, F.; Varela, P.; Vargas, R.; Tapia, G.; Videla, L.A.; Fernández, V. N-3 PUFA supplementation triggers PPAR-α activation and PPAR-α/NF-κB interaction: Anti-inflammatory implications in liver ischemia-reperfusion injury. PLoS ONE 2011, 6, e28502. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E.; Taylor, D. Stressing the heart of the matter: Re-thinking the mechanisms underlying therapeutic effects of n-3 polyunsaturated fatty acids. F1000 Med. Rep. 2012, 4, 13. [Google Scholar] [CrossRef]
- Robertson, R.; Guihéneuf, F.; Schmid, M.; Stengel, D.; Fitzgerald, G.; Ross, P.; Stanton, C. Algae-Derived Polyunsaturated Fatty Acids: Implications for Human Health; Nova Sciences Publishers, Inc.: New York, NY, USA, 2013; p. 43. [Google Scholar]
- Westphal, C.; Konkel, A.; Schunck, W.H. CYP-eicosanoids--A new link between omega-3 fatty acids and cardiac disease? Prostaglandins Other Lipid Mediat. 2011, 96, 99–108. [Google Scholar] [CrossRef]
- Kuda, O. Bioactive metabolites of docosahexaenoic acid. Biochimie 2017, 136, 12–20. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Bisicchia, E.; Sasso, V.; Catanzaro, G.; Leuti, A.; Besharat, Z.M.; Chiacchiarini, M.; Molinari, M.; Ferretti, E.; Viscomi, M.T.; Chiurchiù, V. Resolvin D1 halts remote neuroinflammation and improves functional recovery after focal brain damage via ALX/FPR2 receptor-regulated microRNAs. Mol. Neurobiol. 2018, 55, 6894–6905. [Google Scholar] [CrossRef] [Green Version]
- Recchiuti, A.; Krishnamoorthy, S.; Fredman, G.; Chiang, N.; Serhan, C.N. MicroRNAs in resolution of acute inflammation: Identification of novel resolvin D1-miRNA circuits. FASEB J. 2010, 25, 544–560. [Google Scholar] [CrossRef] [Green Version]
- Zuo, G.; Zhang, D.; Mu, R.; Shen, H.; Li, X.; Wang, Z.; Li, H.; Chen, G. Resolvin D2 protects against cerebral ischemia/reperfusion injury in rats. Mol. Brain 2018, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Dalli, J.; Winkler, J.W.; Colas, R.A.; Arnardottir, H.; Cheng, C.Y.C.; Chiang, N.; Petasis, N.A.; Serhan, C.N. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem. Biol. 2013, 20, 188–201. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Tjonahen, E.; Morgan, E.L.; Lu, Y.; Serhan, C.N.; Rowley, A.F. Rainbow trout (Oncorhynchus mykiss) brain cells biosynthesize novel docosahexaenoic acid-derived resolvins and protectins-Mediator lipidomic analysis. Prostaglandins Other Lipid Mediat. 2005, 78, 107–116. [Google Scholar] [CrossRef]
- Ariel, A.; Serhan, C.N. New lives given by cell death: Macrophage differentiation following their encounter with apoptotic leukocytes during the resolution of inflammation. Front. Immunol. 2012, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Stables, M.J.; Shah, S.; Camon, E.B.; Lovering, R.C.; Newson, J.; Bystrom, J.; Farrow, S.; Gilroy, D.W. Transcriptomic analyses of murine resolution-phase macrophages. Blood 2011, 118, 192–208. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.J.; Spite, M. Resolvins: Anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Ann. Rev. Nutr. 2012, 32, 203–207. [Google Scholar] [CrossRef]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 2015, 1851, 397–413. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Dalli, J.; Karamnov, S.; Choi, A.; Park, C.-K.; Xu, Z.-Z.; Ji, R.-R.; Zhu, M.; Petasis, N.A. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012, 26, 1755–1765. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2008, 206, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Yanes, O.; Clark, J.; Wong, D.M.; Patti, G.J.; Sánchez-Ruiz, A.; Benton, H.P.; Trauger, S.A.; Desponts, C.; Ding, S.; Siuzdak, G. Metabolic oxidation regulates embryonic stem cell differentiation. Nat. Chem. Biol. 2010, 6, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Xu, G.; Newton, P.T.; Chagin, A.S.; Mkrtchian, S.; Carlström, M.; Zhang, X.M.; Harris, R.A.; Cooter, M.; Berger, M.; et al. Maresin 1 attenuates neuroinflammation in a mouse model of perioperative neurocognitive disorders. Br. J. Anaesth. 2019, 122, 350–360. [Google Scholar] [CrossRef] [Green Version]
- Francos-Quijorna, I.; Santos-Nogueira, E.; Gronert, K.; Sullivan, A.B.; Kopp, M.A.; Brommer, B.; David, S.; Schwab, J.M.; López-Vales, R. Maresin 1 promotes inflammatory resolution, neuroprotection, and functional neurological recovery after spinal cord injury. J. Neurosci. 2017, 37, 11731–11743. [Google Scholar] [CrossRef] [Green Version]
- Chiang, N.; Libreros, S.; Norris, P.C.; de la Rosa, X.; Serhan, C.N. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J. Clin. Investig. 2019, 129, 5294–5311. [Google Scholar] [CrossRef] [Green Version]
- Bazan, N.G.; Molina, M.F.; Gordon, W.C. Docosahexaenoic acid signalolipidomics in nutrition: Significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Ann. Rev. Nutr. 2011, 31, 321–351. [Google Scholar] [CrossRef] [Green Version]
- Balas, L.; Durand, T. Dihydroxylated E, E, Z-docosatrienes. An overview of their synthesis and biological significance. Prog. Lipid Res. 2016, 61, 1–18. [Google Scholar] [CrossRef]
- Bazan, N.G. Neuroprotectin D1 (NPD1): A DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005, 15, 15267–15278. [Google Scholar] [CrossRef]
- Calandria, J.M.; Marcheselli, V.L.; Mukherjee, P.K.; Uddin, J.; Winkler, J.W.; Petasis, N.A.; Bazan, N.G. Selective survival rescue in 15-lipoxygenase-1-deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J. Biol. Chem. 2009, 284, 17877–17882. [Google Scholar] [CrossRef] [Green Version]
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell 2013, 153, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Calon, F.; Julien, C.; Winkler, J.W.; Petasis, N.A.; Lukiw, W.J.; Bazan, N.G. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARγ-mediated mechanisms in Alzheimer’s disease models. PLoS ONE 2011, 6, e15816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Sastre, A. Lessons from lipids in the fight against influenza. Cell 2013, 154, 22–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladine, C.; Laurie, J.-C.; Giulia, C.; Dominique, B.; Corinne, C.; Nathalie, H.; Bart, S.; Giuseppe, Z.; Alessio, P.; Jean-Marie, G.; et al. Neuroprostanes, produced by free-radical mediated peroxidation of DHA, inhibit the inflammatory response of human macrophages. Free Radic. Biol. Med. 2014, 75, S15–S25. [Google Scholar] [CrossRef] [PubMed]
- Groeger, A.L.; Cipollina, C.; Cole, M.P.; Woodcock, S.R.; Bonacci, G.; Rudolph, T.K.; Rudolph, V.; Freeman, B.A.; Schopfer, F.J. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat. Chem. Biol. 2010, 6, 433–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- De Petrocellis, L.; Melck, D.; Bisogno, T.; Milone, A.; Di Marzo, V. Finding of the endocannabinoid signalling system in Hydra, a very primitive organism: Possible role in the feeding response. Neuroscience 1999, 92, 377–387. [Google Scholar] [CrossRef]
- Kim, J.; Carlson, M.E.; Watkins, B.A. Docosahexaenoyl ethanolamide improves glucose uptake and alters endocannabinoid system gene expression in proliferating and differentiating C2C12 myoblasts. Front. Physiol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Soderstrom, K. Endocannabinoids link feeding state and auditory perception-related gene expression. J. Neurosci. 2004, 24, 10013–10024. [Google Scholar] [CrossRef] [Green Version]
- Valenti, M.; Cottone, E.; Martinez, R.; De Pedro, N.; Rubio, M.; Viveros, M.P.; Franzoni, M.F.; Delgado, M.J.; Di Marzo, V. The endocannabinoid system in the brain of Carassius auratus and its possible role in the control of food intake. J. Neurochem. 2005, 95, 662–672. [Google Scholar] [CrossRef]
- Chiang, N.; Takano, T.; Arita, M.; Watanabe, S.; Serhan, C.N. A novel rat lipoxin A4 receptor that is conserved in structure and function. Br. J. Pharmacol. 2003, 139, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Piazza, P.V.; Lafontan, M.; Girard, J. Integrated physiology and pathophysiology of CB1-mediated effects of the endocannabinoid system. Diabetes Metab. 2007, 33, 97–107. [Google Scholar] [CrossRef]
- Masoodi, M.; Kuda, O.; Rossmeisl, M.; Flachs, P.; Kopecky, J. Lipid signaling in adipose tissue: Connecting inflammation & metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 503–518. [Google Scholar]
- D’Addario, C.; Micioni Di Bonaventura, M.V.; Pucci, M.; Romano, A.; Gaetani, S.; Ciccocioppo, R.; Cifani, C.; Maccarrone, M. Endocannabinoid signaling and food addiction. Neurosci. Biobehav. Rev. 2014, 47, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.J.; Alger, B.E. Increased neuronal excitability during depolarization-induced suppression of inhibition in rat hippocampus. J. Physiol. 1996, 495, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.I.; Nicoll, R.A. Neuroscience: Endocannabinoid signaling in the brain. Science 2002, 296, 678–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.C.; MacKie, K. An introduction to the endogenous cannabinoid system. Biol. Psychiatry 2016. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Manku, M.S.; Horrobin, D.F. Long-chain polyunsaturated fatty acids modulate interleukin-1β–induced changes in behavior, monoaminergic neurotransmitters, and brain inflammation in rats. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozzatello, P.; Brignolo, E.; De Grandi, E.; Bellino, S. Supplementation with omega-3 fatty acids in psychiatric disorders: A review of literature data. J. Clin. Med. 2016, 5, 67. [Google Scholar] [CrossRef]
- Hibbeln, J.R. Fish consumption and major depression. Lancet 1998, 351, 1213. [Google Scholar] [CrossRef]
- Kiliaan, A.; Königs, A. Critical appraisal of omega-3 fatty acids in attention-deficit/hyperactivity disorder treatment. Neuropsychiatr. Dis. Treat. 2016, 12, 1869–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukiw, W.J.; Cui, J.G.; Marcheselli, V.L.; Bodker, M.; Botkjaer, A.; Gotlinger, K.; Serhan, C.N.; Bazan, N.G. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Investig. 2005, 115, 2774–2783. [Google Scholar] [CrossRef] [Green Version]
- Boudrault, C.; Bazinet, R.P.; Ma, D.W.L. Experimental models and mechanisms underlying the protective effects of n-3 polyunsaturated fatty acids in Alzheimer’s disease. J. Nutr. Biochem. 2009, 20, 1–10. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hossain, S.; Agdul, H.; Shido, O. Docosahexaenoic acid-induced amelioration on impairment of memory learning in amyloid β-infused rats relates to the decreases of amyloid β and cholesterol levels in detergent-insoluble membrane fractions. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2005, 1738, 91–98. [Google Scholar] [CrossRef]
- Seidl, S.E.; Santiago, J.A.; Bilyk, H.; Potashkin, J.A. The emerging role of nutrition in Parkinson’s disease. Front. Aging Neurosci. 2014, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNamara, R.K. DHA Deficiency and prefrontal cortex neuropathology in recurrent affective disorders. J. Nutr. 2010, 140, 864–868. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Hahn, C.G.; Jandacek, R.; Rider, T.; Tso, P.; Stanford, K.E.; Richtand, N.M. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol. Psychiatry 2007, 62, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Vilasagaram, S.; Duttaroy, A.K. Maternal dietary deficiency of n-3 fatty acids affects metabolic and epigenetic phenotypes of the developing fetus. Prostaglandins Leukot. Essent. Fatty Acids 2020, 158, 102109. [Google Scholar] [CrossRef]
- Srinivas, V.; Molangiri, A.; Mallepogu, A.; Kona, S.R.; Ibrahim, A.; Duttaroy, A.K.; Basak, S. Maternal n-3 PUFA deficiency alters uterine artery remodeling and placental epigenome in the mice. J. Nutr. Biochem. 2021, in press. [Google Scholar] [CrossRef]
- Boucher, O.; Burden, M.J.; Muckle, G.; Saint-Amour, D.; Ayotte, P.; Dewailly, E.; Nelson, C.A.; Jacobson, S.W.; Jacobson, J.L. Neurophysiologic and neurobehavioral evidence of beneficial effects of prenatal omega-3 fatty acid intake on memory function at school age. Am. J. Clin. Nutr. 2011, 93, 1025–1037. [Google Scholar] [CrossRef] [Green Version]
- Mulder, K.A.; King, D.J.; Innis, S.M. Omega-3 fatty acid deficiency in infants before birth identified using a randomized trial of maternal DHA supplementation in pregnancy. PLoS ONE 2014, 9, e83764. [Google Scholar] [CrossRef]
- Bos, D.J.; Oranje, B.; Veerhoek, E.S.; Van Diepen, R.M.; Weusten, J.M.H.; Demmelmair, H.; Koletzko, B.; De Sain-Van Der Velden, M.G.M.; Eilander, A.; Hoeksma, M.; et al. Reduced symptoms of inattention after dietary omega-3 fatty acid supplementation in boys with and without attention deficit/hyperactivity disorder. Neuropsychopharmacology 2015, 40, 2298–2306. [Google Scholar] [CrossRef] [Green Version]
- Parellada, M.; Llorente, C.; Calvo, R.; Gutierrez, S.; Lázaro, L.; Graell, M.; Guisasola, M.; Dorado, M.L.; Boada, L.; Romo, J.; et al. Randomized trial of omega-3 for autism spectrum disorders: Effect on cell membrane composition and behavior. Eur. Neuropsychopharmacol. 2017, 27, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Bauer, I.; Hughes, M.; Rowsell, R.; Cockerell, R.; Pipingas, A.; Crewther, S.; Crewther, D. Omega-3 supplementation improves cognition and modifies brain activation in young adults. Hum. Psychopharmacol. 2014, 29, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Jaremka, L.M.; Derry, H.M.; Bornstein, R.; Prakash, R.S.; Peng, J.; Belury, M.A.; Andridge, R.R.; Malarkey, W.B.; Kiecolt-Glaser, J.K. Omega-3 supplementation and loneliness-related memory problems: Secondary analyses of a randomized controlled trial. Psychosom. Med. 2014, 76, 650–658. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.L.; Rouse, C.A. Docosahexaenoic acid and the preterm infant. Mater. Health Neonatol. Perinatol. 2017, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Albanese, E.; Dangour, A.D.; Uauy, R.; Acosta, D.; Guerra, M.; Guerra, S.S.; Huang, Y.; Jacob, K.S.; de Rodriguez, J.L.; Noriega, L.H.; et al. Dietary fish and meat intake and dementia in Latin America, China, and India: A 10/66 Dementia Research Group population-based study. Am. J. Clin. Nutr. 2009, 90, 392–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milte, C.M.; Sinn, N.; Street, S.J.; Buckley, J.D.; Coates, A.M.; Howe, P.R. Erythrocyte polyunsaturated fatty acid status, memory, cognition and mood in older adults with mild cognitive impairment and healthy controls. Prostaglandins Leukot. Essent. Fatty Acids 2011, 84, 153–161. [Google Scholar] [CrossRef]
- Yin, Y.; Fan, Y.; Lin, F.; Xu, Y.; Zhang, J. Nutrient biomarkers and vascular risk factors in subtypes of mild cognitive impairment: A cross-sectional study. J. Nutr. Health Aging. 2015, 19, 39–47. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Shamim, A.; Mahmood, T.; Ahsan, F.; Kumar, A.; Bagga, P. Lipids: An insight into the neurodegenerative disorders. Clin. Nutr. Exp. 2018, 20, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Lopez, L.B.; Kritz-Silverstein, D.; Barrett Connor, E. High dietary and plasma levels of the omega-3 fatty acid docosahexaenoic acid are associated with decreased dementia risk: The Rancho Bernardo study. J. Nutr. Health Aging. 2011, 15, 25–31. [Google Scholar] [CrossRef]
- Phillips, M.A.; Childs, C.E.; Calder, P.C.; Rogers, P.J. Lower omega-3 fatty acid intake and status are associated with poorer cognitive function in older age: A comparison of individuals with and without cognitive impairment and Alzheimer’s disease. Nutr. Neurosci. 2012, 15, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.J.; Bongard, V.; Beiser, A.S.; Lamon-Fava, S.; Robins, S.J.; Au, R.; Tucker, K.L.; Kyle, D.J.; Wilson, P.W.; Wolf, P.A. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: The Framingham Heart Study. Arch. Neurol. 2006, 63, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Shatenstein, B.; Kergoat, M.J.; Reid, I. Poor nutrient intakes during 1-year follow-up with community-dwelling older adults with early-stage Alzheimer dementia compared to cognitively intact matched controls. J. Am. Diet Assoc. 2007, 107, 2091–2099. [Google Scholar] [CrossRef]
- Kotani, S.; Sakaguchi, E.; Warashina, S.; Matsukawa, N.; Ishikura, Y.; Kiso, Y.; Sakakibara, M.; Yoshimoto, T.; Guo, J.; Yamashima, T. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci. Res. 2006, 56, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, R.J.; Ulus, I.H.; Cansev, M.; Watkins, C.J.; Wang, L.; Marzloff, G. Synaptic proteins and phospholipids are increased in gerbil brain by administering uridine plus docosahexaenoic acid orally. Brain Res. 2006, 1088, 83–92. [Google Scholar] [CrossRef]
- Gomez-Soler, M.; Cordobilla, B.; Morato, X.; Fernandez-Duenas, V.; Domingo, J.C.; Ciruela, F. Triglyceride Form of Docosahexaenoic Acid Mediates Neuroprotection in Experimental Parkinsonism. Front. Neurosci. 2018, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Patrick, R.P.; Ames, B.N. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015, 29, 2207–2222. [Google Scholar] [CrossRef] [Green Version]
- McNamara, R.K.; Jandacek, R.; Rider, T.; Tso, P.; Hahn, C.G.; Richtand, N.M.; Stanford, K.E. Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: Gender differences and partial normalization with antipsychotic medications. Schizophr. Res. 2007, 91, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Sinn, N.; Bryan, J. Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. J. Dev. Behav. Pediatr. 2007, 28, 82–91. [Google Scholar] [CrossRef]
- Sinn, N.; Bryan, J.; Wilson, C. Cognitive effects of polyunsaturated fatty acids in children with attention deficit hyperactivity disorder symptoms: A randomised controlled trial. Prostaglandins Leukot. Essent. Fatty Acids 2008, 78, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Belanger, S.A.; Vanasse, M.; Spahis, S.; Sylvestre, M.P.; Lippe, S.; L'Heureux, F.; Ghadirian, P.; Vanasse, C.M.; Levy, E. Omega-3 fatty acid treatment of children with attention-deficit hyperactivity disorder: A randomized, double-blind, placebo-controlled study. Paediatr. Child Health 2009, 14, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.; Ostlund, S.; Fransson, G.; Kadesjo, B.; Gillberg, C. Omega-3/omega-6 fatty acids for attention deficit hyperactivity disorder: A randomized placebo-controlled trial in children and adolescents. J. Atten. Disord. 2009, 12, 394–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafsson, P.A.; Birberg-Thornberg, U.; Duchen, K.; Landgren, M.; Malmberg, K.; Pelling, H.; Strandvik, B.; Karlsson, T. EPA supplementation improves teacher-rated behaviour and oppositional symptoms in children with ADHD. Acta Paediatr. 2010, 99, 1540–1549. [Google Scholar] [CrossRef]
- Hariri, M.; Djazayery, A.; Djalali, M.; Saedisomeolia, A.; Rahimi, A.; Abdolahian, E. Effect of n-3 supplementation on hyperactivity, oxidative stress and inflammatory mediators in children with attention-deficit-hyperactivity disorder. Malays J. Nutr. 2012, 18, 329–335. [Google Scholar]
- Behdani, F.; Hebrani, P.; Naseraee, A.; Haghighi, M.B.; Akhavanrezayat, A. Does omega-3 supplement enhance the therapeutic results of methylphenidate in attention deficit hyperactivity disorder patients? J. Res. Med. Sci. 2013, 18, 653–658. [Google Scholar] [PubMed]
- Dashti, N.; Hekmat, H.; Soltani, H.R.; Rahimdel, A.; Javaherchian, M. Comparison of therapeutic effects of omega-3 and methylphenidate (ritalin((R))) in treating children with attention deficit hyperactivity disorder. Iran J. Psychiatry Behav. Sci. 2014, 8, 7–11. [Google Scholar]
- Anand, P.; Sachdeva, A. Effect of poly unsaturated fatty acids administration on children with attention deficit hyperactivity disorder: A randomized controlled trial. J. Clin. Diagn. Res. 2016, 10, OC01–OC05. [Google Scholar] [CrossRef] [PubMed]
- Assareh, M.; Davari Ashtiani, R.; Khademi, M.; Jazayeri, S.; Rai, A.; Nikoo, M. Efficacy of Polyunsaturated Fatty Acids (PUFA) in the treatment of attention deficit hyperactivity disorder. J. Atten. Disord. 2017, 21, 78–85. [Google Scholar] [CrossRef]
- Kean, J.D.; Sarris, J.; Scholey, A.; Silberstein, R.; Downey, L.A.; Stough, C. Reduced inattention and hyperactivity and improved cognition after marine oil extract (PCSO-524(R)) supplementation in children and adolescents with clinical and subclinical symptoms of attention-deficit hyperactivity disorder (ADHD): A randomised, double-blind, placebo-controlled trial. Psychopharmacology 2017, 234, 403–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voigt, R.G.; Llorente, A.M.; Jensen, C.L.; Fraley, J.K.; Berretta, M.C.; Heird, W.C. A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. J. Pediatr. 2001, 139, 189–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amminger, G.P.; Schafer, M.R.; Papageorgiou, K.; Klier, C.M.; Cotton, S.M.; Harrigan, S.M.; Mackinnon, A.; McGorry, P.D.; Berger, G.E. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: A randomized, placebo-controlled trial. Arch. Gen. Psychiatry 2010, 67, 146–154. [Google Scholar] [CrossRef]
- Pawelczyk, T.; Grancow-Grabka, M.; Kotlicka-Antczak, M.; Trafalska, E.; Pawelczyk, A. A randomized controlled study of the efficacy of six-month supplementation with concentrated fish oil rich in omega-3 polyunsaturated fatty acids in first episode schizophrenia. J. Psychiatr Res. 2016, 73, 34–44. [Google Scholar] [CrossRef]
- Ammann, E.M.; Pottala, J.V.; Harris, W.S.; Espeland, M.A.; Wallace, R.; Denburg, N.L.; Carnahan, R.M.; Robinson, J.G. omega-3 fatty acids and domain-specific cognitive aging: Secondary analyses of data from WHISCA. Neurology 2013, 81, 1484–1491. [Google Scholar] [CrossRef] [Green Version]
- Maekawa, M.; Watanabe, A.; Iwayama, Y.; Kimura, T.; Hamazaki, K.; Balan, S.; Ohba, H.; Hisano, Y.; Nozaki, Y.; Ohnishi, T.; et al. Polyunsaturated fatty acid deficiency during neurodevelopment in mice models the prodromal state of schizophrenia through epigenetic changes in nuclear receptor genes. Transl. Psychiatry 2017, 7, e1229. [Google Scholar] [CrossRef] [Green Version]
- McNamara, R.K.; Jandacek, R.; Tso, P.; Blom, T.J.; Welge, J.A.; Strawn, J.R.; Adler, C.M.; Strakowski, S.M.; DelBello, M.P. Adolescents with or at ultra-high risk for bipolar disorder exhibit erythrocyte docosahexaenoic acid and eicosapentaenoic acid deficits: A candidate prodromal risk biomarker. Early Interv. Psychiatry 2016, 10, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Stoll, A.L.; Severus, W.E.; Freeman, M.P.; Rueter, S.; Zboyan, H.A.; Diamond, E.; Cress, K.K.; Marangell, L.B. Omega 3 fatty acids in bipolar disorder: A preliminary double-blind, placebo-controlled trial. Arch. Gen. Psychiatry 1999, 56, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Huang, S.Y.; Chen, C.C.; Su, K.P. Omega-3 fatty acids are more beneficial in the depressive phase than in the manic phase in patients with bipolar I disorder. J. Clin. Psychiatry 2005, 66, 1613–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clayton, E.H.; Hanstock, T.L.; Hirneth, S.J.; Kable, C.J.; Garg, M.L.; Hazell, P.L. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur. J. Clin. Nutr. 2009, 63, 1037–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meldrum, S.J.; D'Vaz, N.; Simmer, K.; Dunstan, J.A.; Hird, K.; Prescott, S.L. Effects of high-dose fish oil supplementation during early infancy on neurodevelopment and language: A randomised controlled trial. Br. J. Nutr. 2012, 108, 1443–1454. [Google Scholar] [CrossRef] [Green Version]
- Birch, E.E.; Garfield, S.; Castaneda, Y.; Hughbanks-Wheaton, D.; Uauy, R.; Hoffman, D. Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum. Dev. 2007, 83, 279–284. [Google Scholar] [CrossRef]
- Osendarp, S.J.; Baghurst, K.I.; Bryan, J.; Calvaresi, E.; Hughes, D.; Hussaini, M.; Karyadi, S.J.; van Klinken, B.J.; van der Knaap, H.C.; Lukito, W.; et al. Effect of a 12-mo micronutrient intervention on learning and memory in well-nourished and marginally nourished school-aged children: 2 parallel, randomized, placebo-controlled studies in Australia and Indonesia. Am. J. Clin. Nutr. 2007, 86, 1082–1093. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.J.; Burton, J.R.; Sewell, R.P.; Spreckelsen, T.F.; Montgomery, P. Docosahexaenoic acid for reading, cognition and behavior in children aged 7-9 years: A randomized, controlled trial (the DOLAB Study). PLoS ONE 2012, 7, e43909. [Google Scholar] [CrossRef]
- Dalton, A.; Wolmarans, P.; Witthuhn, R.C.; van Stuijvenberg, M.E.; Swanevelder, S.A.; Smuts, C.M. A randomised control trial in schoolchildren showed improvement in cognitive function after consuming a bread spread, containing fish flour from a marine source. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 143–149. [Google Scholar] [CrossRef]
- Brew, B.K.; Toelle, B.G.; Webb, K.L.; Almqvist, C.; Marks, G.B.; investigators, C. Omega-3 supplementation during the first 5 years of life and later academic performance: A randomised controlled trial. Eur. J. Clin. Nutr. 2015, 69, 419–424. [Google Scholar] [CrossRef]
- Lassek, W.D.; Gaulin, S.J. Sex differences in the relationship of dietary Fatty acids to cognitive measures in american children. Front. Evol. Neurosci. 2011, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Shulkin, M.; Pimpin, L.; Bellinger, D.; Kranz, S.; Fawzi, W.; Duggan, C.; Mozaffarian, D. N-3 fatty acid supplementation in mothers, preterm infants, and term infants and childhood psychomotor and visual development: A systematic review and meta-analysis. J. Nutr. 2018, 149, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.; Shaddy, D.J.; Gustafson, K.; Gajewski, B.J.; Thodosoff, J.M.; Kerling, E.; Carlson, S.E. The Kansas University DHA Outcomes Study (KUDOS) clinical trial: Long-term behavioral follow-up of the effects of prenatal DHA supplementation. Am. J. Clin. Nutr. 2019, 109, 1380–1392. [Google Scholar] [CrossRef]
- Keim, S.A.; Boone, K.M.; Klebanoff, M.A.; Turner, A.N.; Rausch, J.; Nelin, M.A.; Rogers, L.K.; Yeates, K.O.; Nelin, L.; Sheppard, K.W. Effect of docosahexaenoic add supplementation vs placebo on developmental outcomes of toddlers born preterm a randomized clinical. JAMA Pediatr. 2018, 172, 1126–1134. [Google Scholar] [CrossRef] [Green Version]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P.; Doyle, L.W.; Anderson, P.; Else, P.L.; Meyer, B.J.; et al. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA J. Am. Med. Assoc. 2010, 304, 1675–1683. [Google Scholar] [CrossRef] [Green Version]
- Collins, C.T.; Gibson, R.A.; Anderson, P.J.; McPhee, A.J.; Sullivan, T.R.; Gould, J.F.; Ryan, P.; Doyle, L.W.; Davis, P.G.; McMichael, J.E.; et al. Neurodevelopmental outcomes at 7 years' corrected age in preterm infants who were fed high-dose docosahexaenoic acid to term equivalent: A follow-up of a randomised controlled trial. BMJ Open 2015, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smithers, L.G.; Collins, C.T.; Simmonds, L.A.; Gibson, R.A.; McPhee, A.; Makrides, M. Feeding preterm infants milk with a higher dose of docosahexaenoic acid than that used in current practice does not influence language or behavior in early childhood: A follow-up study of a randomized controlled trial. Am. J. Clin. Nutr. 2010, 91, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Duttaroy, A.K. Effects of fatty acids on angiogenic activity in the placental extravillious trophoblast cells. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, F. Arachidonic acid metabolism in brain physiology and pathology: Lessons from genetically altered mouse models. J. Neurochem. 2007, 102, 577–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harauma, A.; Hatanaka, E.; Yasuda, H.; Nakamura, M.T.; Salem, N., Jr.; Moriguchi, T. Effects of arachidonic acid, eicosapentaenoic acid and docosahexaenoic acid on brain development using artificial rearing of delta-6-desaturase knockout mice. Prostaglandins Leukot. Essent. Fatty Acids 2017, 127, 32–39. [Google Scholar] [CrossRef]
- Contreras, M.A.; Greiner, R.S.; Chang, M.C.; Myers, C.S.; Salem, N., Jr.; Rapoport, S.I. Nutritional deprivation of alpha-linolenic acid decreases but does not abolish turnover and availability of unacylated docosahexaenoic acid and docosahexaenoyl-CoA in rat brain. J. Neurochem. 2000, 75, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Coronary heart disease in seven countries. Summary. Circulation 1970, 41, I186–I195.
- Sun, G.Y.; Shelat, P.B.; Jensen, M.B.; He, Y.; Sun, A.Y.; Simonyi, A. Phospholipases A2 and inflammatory responses in the central nervous system. Neuromolecular. Med. 2010, 12, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Bazinet, R.P. Is the brain arachidonic acid cascade a common target of drugs used to manage bipolar disorder? Biochem. Soc. Trans. 2009, 37, 1104–1109. [Google Scholar] [CrossRef]
- Rapoport, S.I. Arachidonic acid and the brain. J. Nutr. 2008, 138, 2515–2520. [Google Scholar] [CrossRef]
- Duncan, R.E.; Bazinet, R.P. Brain arachidonic acid uptake and turnover: Implications for signaling and bipolar disorder. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Fraser, T.; Tayler, H.; Love, S. Fatty acid composition of frontal, temporal and parietal neocortex in the normal human brain and in Alzheimer’s disease. Neurochem. Res. 2010, 35, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Hosono, T.; Mouri, A.; Nishitsuji, K.; Jung, C.G.; Kontani, M.; Tokuda, H.; Kawashima, H.; Shibata, H.; Suzuki, T.; Nabehsima, T.; et al. Arachidonic or docosahexaenoic acid diet prevents memory impairment in Tg2576 mice. J. Alzheimers Dis. 2015, 48, 149–162. [Google Scholar] [CrossRef]
- Hosono, T.; Nishitsuji, K.; Nakamura, T.; Jung, C.G.; Kontani, M.; Tokuda, H.; Kawashima, H.; Kiso, Y.; Suzuki, T.; Michikawa, M. Arachidonic acid diet attenuates brain Abeta deposition in Tg2576 mice. Brain Res. 2015, 1613, 92–99. [Google Scholar] [CrossRef]
- Katsuki, H.; Okuda, S. Arachidonic acid as a neurotoxic and neurotrophic substance. Prog. Neurobiol. 1995, 46, 607–636. [Google Scholar] [CrossRef]
- Sanchez-Mejia, R.O.; Newman, J.W.; Toh, S.; Yu, G.Q.; Zhou, Y.; Halabisky, B.; Cisse, M.; Scearce-Levie, K.; Cheng, I.H.; Gan, L.; et al. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2008, 11, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, S.; Huang, B.; Blumenthal, E.M.; Berg, D.K. Arachidonic acid as a possible negative feedback inhibitor of nicotinic acetylcholine receptors on neurons. J. Neurosci. 1995, 15, 3679–3687. [Google Scholar] [CrossRef]
- Williams, J.H.; Errington, M.L.; Lynch, M.A.; Bliss, T.V. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature 1989, 341, 739–742. [Google Scholar] [CrossRef]
- Fukaya, T.; Gondaira, T.; Kashiyae, Y.; Kotani, S.; Ishikura, Y.; Fujikawa, S.; Kiso, Y.; Sakakibara, M. Arachidonic acid preserves hippocampal neuron membrane fluidity in senescent rats. Neurobiol. Aging. 2007, 28, 1179–1186. [Google Scholar] [CrossRef]
- Wang, Z.J.; Liang, C.L.; Li, G.M.; Yu, C.Y.; Yin, M. Neuroprotective effects of arachidonic acid against oxidative stress on rat hippocampal slices. Chem. Biol. Interact. 2006, 163, 207–217. [Google Scholar] [CrossRef]
- Darios, F.; Davletov, B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature 2006, 440, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Darios, F.; Ruiperez, V.; Lopez, I.; Villanueva, J.; Gutierrez, L.M.; Davletov, B. Alpha-synuclein sequesters arachidonic acid to modulate SNARE-mediated exocytosis. EMBO Rep. 2010, 11, 528–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasojima, K.; Schwab, C.; McGeer, E.G.; McGeer, P.L. Distribution of cyclooxygenase-1 and cyclooxygenase-2 mRNAs and proteins in human brain and peripheral organs. Brain Res. 1999, 830, 226–236. [Google Scholar] [CrossRef]
- McGahon, B.; Clements, M.P.; Lynch, M.A. The ability of aged rats to sustain long-term potentiation is restored when the age-related decrease in membrane arachidonic acid concentration is reversed. Neuroscience 1997, 81, 9–16. [Google Scholar] [CrossRef]
- Angelova, P.R.; Muller, W.S. Arachidonic acid potently inhibits both postsynaptic-type Kv4.2 and presynaptic-type Kv1.4 IA potassium channels. Eur. J. Neurosci. 2009, 29, 1943–1950. [Google Scholar] [CrossRef]
- Connell, E.; Darios, F.; Broersen, K.; Gatsby, N.; Peak-Chew, S.Y.; Rickman, C.; Davletov, B. Mechanism of arachidonic acid action on syntaxin-Munc18. EMBO Rep. 2007, 8, 414–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boneva, N.B.; Kikuchi, M.; Minabe, Y.; Yamashima, T. Neuroprotective and ameliorative actions of polyunsaturated fatty acids against neuronal diseases: Implication of fatty acid-binding proteins (FABP) and G protein-coupled receptor 40 (GPR40) in adult neurogenesis. J. Pharmacol. Sci. 2011, 116, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Scanlon, M.J.; Owada, Y.; Yamamoto, Y.; Porter, C.J.; Nicolazzo, J.A. Fatty acid-binding protein 5 facilitates the blood-brain barrier transport of docosahexaenoic acid. Mol. Pharm. 2015, 12, 4375–4385. [Google Scholar] [CrossRef]
- Marszalek, J.R.; Kitidis, C.; Dirusso, C.C.; Lodish, H.F. Long-chain acyl-CoA synthetase 6 preferentially promotes DHA metabolism. J. Biol. Chem. 2005, 280, 10817–10826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, J.T.; Orr, S.K.; Bazinet, R.P. The emerging role of group VI calcium-independent phospholipase A2 in releasing docosahexaenoic acid from brain phospholipids. J. Lipid Res. 2008, 49, 939–944. [Google Scholar] [CrossRef] [Green Version]
- Martinez, M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 1992, 120, S129–S138. [Google Scholar] [CrossRef]
- Bitsanis, D.; Crawford, M.A.; Moodley, T.; Holmsen, H.; Ghebremeskel, K.; Djahanbakhch, O. Arachidonic acid predominates in the membrane phosphoglycerides of the early and term human placenta. J. Nutr. 2005, 135, 2566–2571. [Google Scholar] [CrossRef] [Green Version]
- Dutta-Roy, A.K.; Sinha, A.K. Purification and properties of prostaglandin E1/prostacyclin receptor of human blood platelets. J. Biol. Chem. 1987, 262, 12685–12691. [Google Scholar] [CrossRef]
- Luo, C.L.; Li, Q.Q.; Chen, X.P.; Zhang, X.M.; Li, L.L.; Li, B.X.; Zhao, Z.Q.; Tao, L.Y. Lipoxin A4 attenuates brain damage and downregulates the production of pro-inflammatory cytokines and phosphorylated mitogen-activated protein kinases in a mouse model of traumatic brain injury. Brain Res. 2013, 1502, 1–10. [Google Scholar] [CrossRef]
- Pamplona, F.A.; Ferreira, J.; Menezes de Lima, O., Jr.; Duarte, F.S.; Bento, A.F.; Forner, S.; Villarinho, J.G.; Bellocchio, L.; Wotjak, C.T.; Lerner, R.; et al. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 21134–21139. [Google Scholar] [CrossRef] [Green Version]
- Qawasmi, A.; Landeros-Weisenberger, A.; Leckman, J.F.; Bloch, M.H. Meta-analysis of long-chain polyunsaturated fatty acid supplementation of formula and infant cognition. Pediatrics 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmer, K.; Patole, S.K.; Rao, S.C. Long-chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Smithers, L.G.; Gibson, R.A.; McPhee, A.; Makrides, M. Effect of long-chain polyunsaturated fatty acid supplementation of preterm infants on disease risk and neurodevelopment: A systematic review of randomized controlled trials. Am. J. Clin. Nutr. 2008, 87, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Lauritzen, L.; Fewtrell, M.; Agostoni, C. Dietary arachidonic acid in perinatal nutrition: A commentary. Pediatr. Res. 2015, 77, 263–269. [Google Scholar] [CrossRef]
- Alshweki, A.; Muñuzuri, A.P.; Baña, A.M.; de Castro, M.J.; Andrade, F.; Aldamiz-Echevarría, L.; de Pipaón, M.S.; Fraga, J.M.; Couce, M.L. Effects of different arachidonic acid supplementation on psychomotor development in very preterm infants; a randomized controlled trial. Nutr. J. 2015, 14, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, M.A.; Costeloe, K.; Ghebremeskel, K.; Phylactos, A.; Skirvin, L.; Stacey, F. Are deficits of arachidonic and docosahexaenoic acids responsible for the neural and vascular complications of preterm babies? Am. J. Clin. Nutr. 1997, 66, 1032S–S1041S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsyth, S.; Calder, P.C.; Zotor, F.; Amuna, P.; Meyer, B.; Holub, B. Dietary Docosahexaenoic Acid and Arachidonic Acid in Early Life: What Is the Best Evidence for Policymakers? Ann. Nutr. Metab. 2018, 72, 210–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Metabolites | Name | Biological Effects |
|---|---|---|
| DHA Metabolites | Maresins | Resolution of inflammation, wound healing, analgesic effects |
| Protectins | Resolution of inflammation, neuroprotection | |
| Resolvins | Resolution of inflammation and wound healing | |
| Electrophilic oxo-derivatives (EFOX) of DHA | Anti-inflammatory, anti-proliferative effects | |
| Epoxides | Anti-hypertensive, analgesic actions | |
| Neuroprostanes | Cardio-protection, wound healing | |
| DHA conjugates | Ethanolamines and glycerol esters | Neural development, immunomodulation, metabolic effects |
| Branched fatty acid esters of hydroxy fatty acids (FAHFA) | Immuno-modulation, resolution of inflammation | |
| N-acyl amides | Metabolic regulation, neuroprotection, neurotransmission | |
| ARA metabolites | Lipoxins A4 | Lowers neuroinflammation by inhibiting microglial activation |
| Lipoxins B4 | Promotes neuroprotection from acute and chronic injuries |
| Study Name | Experimental Setting | Observed Outcome |
|---|---|---|
| The Kansas University DHA outcome study (KUDOS) clinical trial | Cognitive and behavioral development | Improvement of visual attention among infants has been observed to reduce the preterm birth risk [234]. |
| Effect of DHA supplementation vs. placebo on developmental outcomes of toddlers born preterm | Developmental outcomes of toddlers | Daily supplementation of DHA did not improve cognitive function and may adversely affect language development and effortful control in specific subgroups of children [235]. |
| Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children | Neurodevelopmental outcome of children | DHA supplementation during pregnancy did not reduce postpartum depression in mothers, neither did it improve cognitive and language development in their offspring during early childhood [236]. |
| Neurodevelopmental outcomes of preterm infants fed high-amount DHA | Neurodevelopment at 18 months of age | Bayley mental development index scores of preterm infants overall born earlier than 33 weeks were not affected but improved the girls’ Bayley mental development index scores. |
| Neurodevelopmental outcomes at 7 years corrected age in preterm infants who were fed high-dose DHA to term equivalent | Cognitive outcome detected at 18 months age | No evidence of benefit [237]. |
| Feeding preterm infant milk with a higher dose of DHA than that used in current practice | Language or behavior in early childhood | No clinically meaningful change to language development or behavior were observed when assessed in early childhood [238]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basak, S.; Mallick, R.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Maternal Supply of Both Arachidonic and Docosahexaenoic Acids Is Required for Optimal Neurodevelopment. Nutrients 2021, 13, 2061. https://doi.org/10.3390/nu13062061
Basak S, Mallick R, Banerjee A, Pathak S, Duttaroy AK. Maternal Supply of Both Arachidonic and Docosahexaenoic Acids Is Required for Optimal Neurodevelopment. Nutrients. 2021; 13(6):2061. https://doi.org/10.3390/nu13062061
Chicago/Turabian StyleBasak, Sanjay, Rahul Mallick, Antara Banerjee, Surajit Pathak, and Asim K. Duttaroy. 2021. "Maternal Supply of Both Arachidonic and Docosahexaenoic Acids Is Required for Optimal Neurodevelopment" Nutrients 13, no. 6: 2061. https://doi.org/10.3390/nu13062061
APA StyleBasak, S., Mallick, R., Banerjee, A., Pathak, S., & Duttaroy, A. K. (2021). Maternal Supply of Both Arachidonic and Docosahexaenoic Acids Is Required for Optimal Neurodevelopment. Nutrients, 13(6), 2061. https://doi.org/10.3390/nu13062061