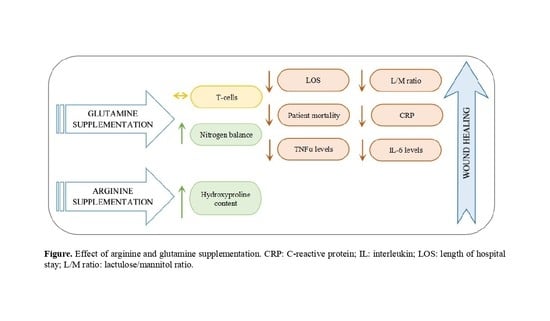
The Effect of Amino Acids on Wound Healing: A Systematic Review and Meta-Analysis on Arginine and Glutamine
Abstract
:1. Introduction
1.1. Nutrition
1.2. Why It Is Important to Do This Review
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.2.1. Population
2.2.2. Intervention
2.2.3. Comparator
2.2.4. Outcomes
2.3. Data Extraction and Management
2.4. Quality Assessment
2.5. Data Analysis
3. Results
3.1. Assessment of Risk of Bias of Included Studies
3.1.1. Risk of Bias of Included Studies on Arginine
3.1.2. Risk of Bias of Included Studies on Glutamine
3.2. Effects of Interventions
3.3. Arginine (Arg)
| Study | Duration | Patient Population | n | Dosage | Control Group | Outcome |
|---|---|---|---|---|---|---|
| Barbul et al. [59] | 14 days | Surgery | 36 | 24.8 g Arg | Not supplemented | ↑ Collagen deposition, ↑ Wound-breaking strength, ↑ Lymphocyte mitogenesis |
| Nussbaum [64] | 14 days | Surgery | 30 | 17 g Arg | Not supplemented | ↑ Collagen synthesis, ↑ T-cell-mediated immune function, ↑ IGF-1 |
| Debats et al. [65] | 5 days | Surgery | 35 | 30 g intravenous Arg | Isonitrogenous solution |  Citrulline, ornithine and NO levels, Citrulline, ornithine and NO levels,  Angiogenesis, Angiogenesis,  Reepithelialisation Reepithelialisation |
| Sigal et al. [66] | 7 days | Abdominal surgery | 30 | 14.7 g intravenous Arg | Isonitrogenous solution |  Lymphocyte proliferation, Lymphocyte proliferation,  NO NO |
| Langkamp-Henken et al. [60] | 4 weeks | Elderly people with pressure ulcers | 33 | 0, 8.5 or 17 g Arg | Not supplemented |  Lymphocyte proliferation, Lymphocyte proliferation,  NO, NO,  IL-2 IL-2 |
 does not increase or decrease.
does not increase or decrease.Collagen Deposition (Hydroxyproline Content)
3.4. Glutamine (Gln)
| Study | Duration | Patient Population | n | Dosage | Control Group | Outcome | |
|---|---|---|---|---|---|---|---|
| Gln (g/kg BW/day) | Gln Dip (g/kg BW/day Ala-Gln) | ||||||
| Oʼriordain et al. [69] | 5 days | Surgery | 22 | 0.18 | 0.27 | Isonitrogenous solution | ↑ T-cell lymphocytes,  IL-2, IL-2,  IL-6, IL-6,  TNFα TNFα |
| De Beaux et al. [70] | 7 days | Critical illness | 14 | 0.22 | 0.33 | Isonitrogenous solution | ↑ Lymphocytes, ↓ IL-8,  IL-6 IL-6 |
| Morlion et al. [71] | 5 days | Surgery | 28 | 0.2 | 0.3 | Isonitrogenous solution | ↑ NO, ↓ LOS and patient mortality, ↑ Mood and general well-being |
| Jacobi et al. [72] | 7 days | Surgery | 34 | 0.27 | 0.4 | Isonitrogenous solution |  IL-10, ↑ Wound healing IL-10, ↑ Wound healing |
| Jiang et al. [73] | 7 days | Abdominal surgery | 60 | 0.34 | 0.5 | Isonitrogenous solution | ↑ NO, ↓ LOS |
| Powell-Tuck et al. [74] | 4–16.5 days | Mixed | 168 | 0.26 | 0.38 | Isonitrogenous solution | ↓ LOS and patient mortality |
| Mertes et al. [75] | 6 days | Abdominal surgery | 37 | 0.34 | 0.5 | Isonitrogenous solution | ↑ NO, ↓ LOS,  IL-6 IL-6 |
| Karwowska et al. [76] | 10 days | Surgery | 30 | 0.2 | 0.3 | Isonitrogenous solution | ↑ IgA and IgG, ↑ T-cell lymphocytes, ↓ LOS |
| Neri et al. [77] | >7 days | Surgery | 33 | 0.2 | 0.3 | Isonitrogenous solution | ↓ LOS |
| Wischmeyer et al. [78] | >7 days | Critical illness | 31 | 0.57 | 0.85 | Isonitrogenous solution | ↓ CRP |
| Goeters et al. [61] | >9 days | Surgery | 144 | 0.2 | 0.3 | Isonitrogenous solution |  NO, ↓ Patient mortality NO, ↓ Patient mortality |
| Lin et al. [79] | 6 days | Abdominal surgery | 48 | 0.28 | 0.417 | Isonitrogenous solution | ↑ NO, ↓ IL-6, ↑ T-cell lymphocytes |
| Ockenga et al. [80] | >7 days | Critical illness | 28 | 0.2 | 0.3 | Isonitrogenous solution | ↑ Lymphocytes, ↓ LOS, ↓ CRP |
| Fuentes-Orozco et al. [81] | 10 days | Surgery | 10 | 0.27 | 0.4 | Isonitrogenous solution | ↑ NO, ↓ Infectious complications, ↑ Lymphocytes CD4 CD8 |
| Xian-Li et al. [62] | 14 days | Critical illness | 69 | 0.27 | 0.4 | Isonitrogenous solution | ↓ LOS and patient mortality |
| Zhou et al. [82] | 12 days | Critical illness | 30 | 0.34 | 0.5 | Isonitrogenous solution | ↑ Wound healing, ↓ Intestinal permeability |
| Quan et al. [83] | 7 days | Abdominal surgery | 20 | 0.53 | 0.78 | Not specified | ↓ Intestinal permeability |
| Kłek et al. [84] | 12 days | Surgery | 105 | 0.27 | 0.4 | Isonitrogenous solution | ↑ Lymphocytes, ↓ LOS |
| Lin et al. [85] | 6 days | Abdominal surgery | 48 | 0.28 | 0.417 | Isonitrogenous solution | ↑ NO, ↓ IL-6 |
| Yao et al. [86] | 5 days | Surgery | 40 | 0.34 | 0.5 | Isonitrogenous solution | ↓ LOS, ↑ CD14 |
| Déchelotte et al. [87] | 5 days | Surgery | 143 | 0.3 | 0.45 | Isonitrogenous solution |  LOS, ↑ NO, ↓ Intestinal permeability LOS, ↑ NO, ↓ Intestinal permeability |
| Şahin et al. [88] | 10.5 ± 3.6 days | Critical illness | 40 | 0.3 | 0.45 | Isonitrogenous solution |  T-cell lymphocytes, ↓ LOS T-cell lymphocytes, ↓ LOS |
| Cai et al. [89] | 14 days | Critical illness | 110 | 0.19 | 0.29 | Isonitrogenous solution | ↑ T-cell lymphocytes, ↓ CRP |
| Duška et al. [90] | 13 days | Critical illness | 30 | 0.2 | 0.3 | Isonitrogenous solution | ↑ NO |
| Estívariz et al. [91] | 7 days | Surgery | 63 | 0.34 | 0.5 | Isonitrogenous solution |  T-cell Lymphocytes, T-cell Lymphocytes,↓ Nosocomial infections |
| Dong et al. [92] | 6 days | Abdominal surgery | 40 | 0.35 | 0.5 | Isonitrogenous solution | ↑ T-cell lymphocytes, ↓ TNFα, ↓ IL-2R |
| Fuentes-Orozco et al. [93] | 10 days | Critical illness | 44 | 0.27 | 0.4 | Isonitrogenous solution | ↑ T-cell lymphocytes, ↑ IgA, ↑ NO, ↓ CRP, ↑ IL-10, ↓ IL-6 |
| Yeh et al. [94] | 7 days | Surgery | 70 | 0.2 | 0.29 | Isonitrogenous solution | ↓ CRP, ↓ LOS,  Patient mortality Patient mortality |
| Asprer et al. [95] | 5 days preoperatively | Abdominal surgery | 34 | 0.2 | 0.3 | Isonitrogenous solution | ↑ Lymphocytes |
| Engel et al. [63] | 3 days | Surgery | 58 | 0.5 | 0.74 | Isonitrogenous solution |  T-cell lymphocytes T-cell lymphocytes |
| Fan et al. [96] | 7 days | Abdominal surgery | 40 | 0.13 | 0.2 | Isonitrogenous solution | ↓ LOS and infectious complications |
| Quan et al. [97] | 4 days | Abdominal surgery | 20 | 0.35 | 0.5 | Normal saline | ↑ NO, ↓ IL-6 |
| Andrews et al. [98] | Up to 7 days | Mixed | 502 | 0.2 | 0.3 | Isonitrogenous solution |  Patient mortality Patient mortality |
| Çekmen et al. [99] | >5 days | Mixed | 30 | 0.35 | 0.5 | Isonitrogenous solution | ↓ CRP, ↓ LOS and patient mortality |
| Grau et al. [100] | 5–9 days | Mixed | 127 | 0.35 | 0.5 | Isonitrogenous solution | ↓ Nosocomial infections |
| Lu et al. [101] | 7 days | Surgery | 50 | 0.3 | 0.45 | Isonitrogenous solution | ↑ NO, ↓ IL-6, ↓ CRP, ↓ Infectious complications |
| Wernerman et al. [102] | 7 days | Mixed | 413 | 0.28 | 0.42 | Normal saline | ↓ LOS and patient mortality |
| Xu et al. [103] | 12 days | Critical illness | 80 | Unknown | Unknown | Isonitrogenous solution | ↓ TNFα |
| Richard et al. [104] | 3 days pre, 4 days postoperatively | Surgery | 22 | 0.53 | 0.78 | Not supplemented | ↓ LOS and patient mortality, ↓ CRP |
 does not increase or decrease.
does not increase or decrease.3.4.1. Nitrogen Balance
3.4.2. Wound Healing Time
3.4.3. Length of Hospital Stay (LOS) and Patient mortality
3.4.4. Lactulose/Mannitol Ratio
3.4.5. C-Reactive Protein
3.4.6. Cytokines
3.4.7. T-Cell Lymphocytes
4. Discussion
4.1. Arginine
4.2. Glutamine
5. Limitations of the Review
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Armstrong, G.D.; Meyr, A.J. Basic Principles of Wound Management. Atlas Small Anim. Wound Manag. Reconstr. Surg. 2018, 4, 33–52. [Google Scholar]
- The Editors of Encyclopaedia Britannica. Encycl. Br. 2019. Available online: https://www.britannica.com/science/wound (accessed on 11 June 2021).
- Gonzalez, A.C.D.O.; Andrade, Z.D.A.; Costa, T.F.; Medrado, A.R.A.P. Wound healing-A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orr, J.W.; Taylor, P.T. Wound healing. In Complications in Gynecological Surgery: Prevention, Recognition, and Management; J. B. Lippincott & Co.: Philadelphia, PA, USA, 2018. [Google Scholar]
- Skover, G.R. Cellular and biochemical dynamics of wound repair. Wound environment in collagen regeneration. Clin. Podiatr. Med. Surg. 1991, 8, 723–756. [Google Scholar] [PubMed]
- Lawrence, W.T. Physiology of the acute wound. Clin. Plast. Surg. 1998, 25, 321–340. [Google Scholar] [CrossRef]
- Hart, J. Inflammation. 1: Its role in the healing of acute wounds. J. Wound Care 2002, 11, 205–209. [Google Scholar] [CrossRef]
- Toy, L.W. Matrix metalloproteinases: Their function in tissue repair. J. Wound Care 2005, 14, 20–22. [Google Scholar] [CrossRef]
- Bacci, S. Cutaneous wound healing: Cellular mechanisms and therapies (an update). Med. Res. Arch. 2019, 7. [Google Scholar] [CrossRef]
- Bunman, S.; Dumavibhat, N.; Chatthanawaree, W.; Ntalapaporn, S.; Thuwachaosuan, T.; Thongchuan, C. Burn Wound Healing: Pathophysiology and Current Management of Burn Injury. Bangkok Med. J. 2017, 13, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Adzick, N.S.; Longaker, M.T. Scarless fetal healing: Therapeutic implications. Ann. Surg. 1992, 215, 3–7. [Google Scholar] [CrossRef]
- Russell, L. Understanding physiology of wound healing and how dressings help. Br. J. Nurs. 2000, 9. [Google Scholar] [CrossRef] [PubMed]
- Zomer, H.D.; Trentin, A.G. Skin wound healing in humans and mice: Challenges in translational research. J. Dermatol. Sci. 2018, 90, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Baum, C.L.; Arpey, C.J. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatol. Surg. 2005, 31, 686. [Google Scholar] [CrossRef]
- Liu, Z.J.; Velazquez, O.C. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid. Redox Signal. 2008, 10, 1869–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masre, S.F.; Yip, G.W.; Sirajudeen, K.N.S.; Ghazali, F.C. Quantitative analysis of sulphated glycosaminoglycans content of Malaysian sea cucumber Stichopus hermanni and Stichopus vastus. Nat. Prod. Res. 2012, 26, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A.F. Cutaneous Wound Healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Leigh, B.; Desneves, K.; Rafferty, J.; Pearce, L.; King, S.; Woodward, M.C.; Brown, D.; Martin, R.; Crowe, T.C. The effect of different doses of an arginine-containing supplement on the healing of pressure ulcers. J. Wound Care 2012, 21, 150–156. [Google Scholar] [CrossRef]
- McNeal, C.J.; Meininger, C.J.; Wilborn, C.D.; Tekwe, C.D.; Wu, G. Safety of dietary supplementation with arginine in adult humans. Amino Acids 2018, 50, 1215–1229. [Google Scholar] [CrossRef]
- Russell, L. Nutritional status in wound healing. Clinical 2001, 10, S42–S49. [Google Scholar]
- Arnold, M.; Barbul, A. Nutrition and wound healing. Plast. Reconstr. Surg. 2006, 117, 42–58. [Google Scholar] [CrossRef]
- Witte, M.B.; Barbul, A. Arginine physiology and its implication for wound healing. Wound Repair Regen. 2003, 11, 419–423. [Google Scholar] [CrossRef]
- Campos, A.C.L.; Groth, A.K.; Branco, A.B. Assessment and nutritional aspects of wound healing. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 281–288. [Google Scholar] [CrossRef]
- Shepherd, A.A. Nutrition for optimum wound healing. Nurs. Stand. 2003, 18, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Tong, B.; Barbul, A. Cellular and Physiological Effects of Arginine. Mini-Reviews Med. Chem. 2004, 4, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Healthc. Mater. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Allan Palmer, T.E.; Griffiths, R.D.; Jones, C. Effect of parenteral l-glutamine on muscle in the very severely Ill. Nutrition 1996, 12, 316–320. [Google Scholar] [CrossRef]
- Labow, B.I.; Souba, W.W. Glutamine. World J. Surg. 2000, 24, 1503–1513. [Google Scholar] [CrossRef]
- Melis, G.C.; Ter Wengel, N.; Boelens, P.G.; Van Leeuwen, P.A.M. Glutamine: Recent developments in research on the clinical significance of glutamine. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 59–70. [Google Scholar] [CrossRef]
- Parry-Billings, M.; Baigrie, R.J.; Lamont, P.M.; Morris, P.J.; Newsholme, E.A. Effects of Major and Minor Surgery on Plasma Glutamine and Cytokine Levels. Arch. Surg. 1992, 127, 1237–1240. [Google Scholar] [CrossRef]
- Field, C.J.; Johnson, I.; Pratt, V.C. Glutamine and arginine: Immunonutrients for improved health. Med. Sci. Sports Exerc. 2000, 32. [Google Scholar] [CrossRef]
- Murphy, C.; Newsholme, P. Importance of glutamine macrophages and human monocytes to L-arginine biosynthesis and rates of nitrite or urea production. Clin. Sci. 1998, 95, 397–407. [Google Scholar] [CrossRef]
- Newsholme, P. Glutamine Metabolism: Nutritional and Clinical Significance Why Is L-Glutamine Metabolism Important to Cells of the Immune System in Health, Postinjury, Surgery or Infection? J. Nutr. 2001, 131, 2515–2522. [Google Scholar] [CrossRef]
- Wu, G.; Brosnan, J.T. Macrophages can convert citrulline into arginine. Biochem. J. 1992, 281, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Coëffier, M.; Claeyssens, S.; Hecketsweiler, B.; Lavoinne, A.; Ducrotté, P.; Déchelotte, P. Enteral glutamine stimulates protein synthesis and decreases ubiquitin mRNA level in human gut mucosa. Am. J. Physiol.-Gastrointest. Liver Physiol. 2003, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Bacquer, O.; Nazih, H.; Hervé Blottiè, R.E.; Meynial-Denis, D.; Laboisse, C.; Darmaun, D. Effects of glutamine deprivation on protein synthesis in a model of human enterocytes in culture. Am. J. Physiol.-Gastrointest. Liver Physiol. 2001, 281. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, S.T.; Smith, R.J.; Hwang, T.L.; Wilmore, D.W. Maintenance of Small Bowel Mucosa with Glutamine-Enriched Parenteral Nutrition. J. Parenter. Enter. Nutr. 1989, 13, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Souba, W.W.; Klimberg, V.S.; Plumley, D.A.; Salloum, R.M.; Flynn, T.C.; Bland, K.I.; Copeland, E.M. The role of glutamine in maintaining a healthy gut and supporting the metabolic response to injury and infection. J. Surg. Res. 1990, 48, 383–391. [Google Scholar] [CrossRef]
- Xue, G.D.; Barekatain, R.; Wu, S.B.; Choct, M.; Swick, R.A. Dietary L-glutamine supplementation improves growth performance, gut morphology, and serum biochemical indices of broiler chickens during necrotic enteritis challenge. Poult. Sci. 2018, 97, 1334–1341. [Google Scholar] [CrossRef]
- De-Souza, D.A.; Greene, L.J. Intestinal permeability and systemic infections in critically ill patients: Effect of glutamine. Crit. Care Med. 2005, 33, 1125–1135. [Google Scholar] [CrossRef] [Green Version]
- Fukatsu, K.; Kudsk, K.A.; Zarzaur, B.L.; Wu, Y.; Hanna, M.K.; DeWitt, R.C. TPN decreases IL-4 and IL-10 mRNA expression in lipopolysaccharide stimulated intestinal lamina propria cells but glutamine supplementation preserves the expression. Shock 2001, 15, 318–322. [Google Scholar] [CrossRef]
- Gianotti, L.; Alexander, J.W.; Gennari, R.; Pyles, T.; Babcock, G.F. Oral glutamine decreases bacterial translocation and improves survival in experimental gut-origin sepsis. J. Parenter. Enter. Nutr. 1995, 19, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.N.; Yeh, S.L.; Lin, M.T.; Shang, H.F.; Yeh, C.L.; Chen, W.J. Glutamine supplementation enhances mucosal immunity in rats with Gut-Derived sepsis. Nutrition 2004, 20, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Van der Hulst, R.R.W.J.; von Meyenfeldt, M.F.; Deutz, N.E.P.; Soeters, P.B.; Brummer, R.J.M.; von Kreel, B.K.; Arends, J.W. Glutamine and the preservation of gut integrity. Lancet 1993, 341, 1363–1365. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Riehm, J.; Singleton, K.D.; Ren, H.; Musch, M.W.; Kahana, M.; Chang, E.B. Glutamine attenuates tumor necrosis factor-α release and enhances heat shock protein 72 in human peripheral blood mononuclear cells. Nutrition 2003, 19, 1–6. [Google Scholar] [CrossRef]
- Zhou, Y.P.; Jiang, Z.M.; Sun, Y.H.; Wang, X.R.; Ma, E.L.; Wilmore, D. The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns: A randomized, double-blind, controlled clinical trial. J. Parenter. Enter. Nutr. 2003, 27, 241–245. [Google Scholar] [CrossRef]
- Ameho, C.K.; Adjei, A.A.; Harrison, E.K.; Takeshita, K.; Morioka, T.; Arakaki, Y.; Ito, E.; Suzuki, I.; Kulkarni, A.D.; Kawajiri, A.; et al. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor α production in trinitrobenzene sulphonic acid induced colitis. Gut 1997, 41, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Spindler-Vesel, A.; Wraber, B.; Vovk, I.; Kompan, L. Intestinal permeability and cytokine inflammatory response in multiply injured patients. J. Interf. Cytokine Res. 2006, 26, 771–776. [Google Scholar] [CrossRef]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Vehe, K.L.; Brown, R.O.; Kuhl, D.A.; Boucher, B.A.; Lutherou, R.W.; Kudsk, K.A. The prognostic inflammatory and nutritional index in traumatized patients receiving enteral nutrition support. J. Am. Coll. Nutr. 1991, 10, 355–363. [Google Scholar] [CrossRef]
- Yeligar, S.M.; Harris, F.L.; Hart, C.M.; Brown, L.A.S. Glutathione attenuates ethanol-induced alveolar macrophage oxidative stress and dysfunction by downregulating NADPH oxidases. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014, 306. [Google Scholar] [CrossRef] [Green Version]
- Bollhalder, L.; Pfeil, A.M.; Tomonaga, Y.; Schwenkglenks, M. A systematic literature review and meta-analysis of randomized clinical trials of parenteral glutamine supplementation. Clin. Nutr. 2013, 32, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, F.; Heyland, D.K.; Avenell, A.; Drover, J.W.; Su, X. Glutamine supplementation in serious illness: A systematic review of the evidence. Crit. Care Med. 2002, 30, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Estarli, M.; Barrera, E.S.A.; et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Rev. Esp. Nutr. Hum. Diet. 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankit Rohatgi WebPlotDigitizer 4.4. Available online: https://automeris.io/WebPlotDigitizer (accessed on 11 June 2021).
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Chichester, UK, 2019; ISBN 9781119536604. [Google Scholar]
- The Nordic Cochrane Centre. Review Manager, Version 5.4; The Cochrane Collaboration: Copenhagen, Denmark, 2020. [Google Scholar]
- Barbul, A.; Lazarou, S.A.; Efron, D.T.; Wasserkrug, H.L.; Efron, G. Arginine enhances wound healing and lymphocyte immune responses in humans. In Proceedings of the Surgery; Mosby Inc.: St. Louis, MO, USA, 1990; Volume 108, pp. 331–337. [Google Scholar]
- Langkamp-Henken, B.; Herrlinger-Garcia, K.A.; Stechmiller, J.K.; Nickerson-Troy, J.A.; Lewis, B.; Moffatt, L. Arginine supplementation is well tolerated but does not enhance mitogen-induced lymphocyte proliferation in elderly nursing home residents with pressure ulcers. J. Parenter. Enter. Nutr. 2000, 24, 280–287. [Google Scholar] [CrossRef]
- Goeters, C.; Wenn, A.; Mertes, N.; Wempe, C.; Van Aken, H.; Stehle, P.; Bone, H.-G. Parenteral L-alanyl-L-glutamine improves 6-month outcome in critically ill patients. Crit. Care Med. 2002, 30, 2032–2037. [Google Scholar] [CrossRef]
- He, X.L.; Ma, Q.J.; Lu, J.G.; Chu, Y.K.; Du, X.L. Effect of total parenteral nutrition (TPN) with and without glutamine dipeptide supplementation on outcome in severe acute pancreatitis (SAP). Clin. Nutr. Suppl. 2004, 1, 43–47. [Google Scholar] [CrossRef]
- Engel, J.M.; Pitz, S.; Mühling, J.; Menges, T.; Martens, F.; Kwapisz, M.; Hempelmann, G. Role of glutamine administration on T-cell derived inflammatory response after cardiopulmonary bypass. Clin. Nutr. 2009, 28, 15–20. [Google Scholar] [CrossRef]
- Nussbaum, M.S. Arginine Stimulates Wound Healing and Immune Function in Elderly Human Beings. J. Parenter. Enter. Nutr. 1994, 18, 194. [Google Scholar] [CrossRef]
- Debats, I.B.J.G.; Koeneman, M.M.; Booi, D.I.; Bekers, O.; Van Der Hulst, R.R.W.J. Intravenous arginine and human skin graft donor site healing: A randomized controlled trial. Burns 2011, 37, 420–426. [Google Scholar] [CrossRef]
- Sigal, R.K.; Shou, J.; Daly, J.M. Parenteral arginine infusion in humans: Nutrient substrate or pharmacologic agent? J. Parenter. Enter. Nutr. 1992, 16, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Gamelli, R.L.; He, L.-K. Incisional Wound Healing: Model and Analysis of Wound Breaking Strength. In Wound Healing; Humana Press: Clifton, NJ, USA, 2003; pp. 037–054. [Google Scholar]
- Fürst, P. Old and new substrates in clinical nutrition. J. Nutr. 1998, 128, 789–796. [Google Scholar] [CrossRef] [PubMed]
- O’Riordain, M.G.; Fearon, K.C.H.; Ross, J.A.; Rogers, P.; Falconer, J.S.; Bartolo, D.C.C.; Garden, O.J.; Carter, D.C. Glutamine-supplemented total parenteral nutrition enhances T-lymphocyte response in surgical patients undergoing colorectal resection. Ann. Surg. 1994, 220, 212–221. [Google Scholar] [CrossRef] [PubMed]
- De Beaux, A.C.; O’Riordain, M.G.; Ross, J.A.; Jodozi, L.; Carter, D.C.; Fearon, K.C.H. Glutamine-supplemented total parenteral nutrition reduces blood mononuclear cell interleukin-8 release in severe acute pancreatitis. Nutrition 1998, 14, 261–265. [Google Scholar] [CrossRef]
- Morlion, B.J.; Stehle, P.; Wachtler, P.; Siedhoff, H.P.; Köller, M.; König, W.; Fürst, P.; Puchstein, C. Total parenteral nutrition with glutamine dipeptide after major abdominal surgery: A randomized, double-blind, controlled study. Ann. Surg. 1998, 227, 302–308. [Google Scholar] [CrossRef]
- Jacobi, C.A.; Ordemann, J.; Zuckermann, H.; Döcke, W.; Volk, H.D.; Müller, J.M. Effect of alanyl-glutamine in postoperative total parenteral nutrition on postoperative immunosuppression and morbidity. Preliminary results of a prospective randomized study. Langenbecks Arch. Chir. Suppl. Kongressbd. 1998, 115, 605–611. [Google Scholar]
- Jiang, Z.M.; Cao, J.D.; Zhu, X.G.; Zhao, W.X.; Yu, J.C.; Ma, E.L.; Wang, X.R.; Zhu, M.W.; Shu, H.; Liu, Y.W. The impact of alanyl-glutamine on clinical safety, nitrogen balance, intestinal permeability, and clinical outcome in postoperative patients: A randomized, double-blind, controlled study of 120 patients. J. Parenter. Enter. Nutr. 1999, 23, S62–S66. [Google Scholar] [CrossRef] [PubMed]
- Powell-Tuck, J.; Jamieson, C.P.; Bettany, G.E.A.; Obeid, O.; Fawcett, H.V.; Archer, C.; Murphy, D.L. A double blind, randomised, controlled trial of glutamine supplementation in parenteral nutrition. Gut 1999, 45, 82–88. [Google Scholar] [CrossRef]
- Mertes, N.; Schulzki, C.; Goeters, C.; Winde, G.; Benzing, S.; Kuhn, K.S.; Van Aken, H.; Stehle, P.; Fürst, P. Cost containment through L-alanyl-L-glutamine supplemented total parenteral nutrition after major abdominal surgery: A prospective randomized double-blind controlled study. Clin. Nutr. 2000, 19, 395–401. [Google Scholar] [CrossRef]
- Karwowska, K.A.; Dworacki, G.; Trybus, M.; Zeromski, J.; Szulc, R. Influence of glutamine-enriched parenteral nutrition on nitrogen balance and immunologic status in patients undergoing elective aortic aneurysm repair. Nutrition 2001, 17, 475–478. [Google Scholar] [CrossRef]
- Neri, A.; Mariani, F.; Piccolomini, A.; Testa, M.; Vuolo, G.; Di Cosmo, L. Glutamine-supplemented total parenteral nutrition in major abdominal surgery. Nutrition 2001, 17, 968–969. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Lynch, J.; Liedel, J.; Wolfson, R.; Riehm, J.; Gottlieb, L.; Kahana, M. Glutamine administration reduces Gram-negative bacteremia in severely burned patients: A prospective, randomized, double-blind trial versus isonitrogenous control. Crit. Care Med. 2001, 29, 2075–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.T.T.; Kung, S.P.P.; Yeh, S.L.L.; Lin, C.; Lin, T.H.H.; Chen, K.H.H.; Liaw, K.Y.Y.; Lee, P.H.H.; Chang, K.J.J.; Chen, W.J.J. The effect of glutamine-supplemented total parenteral nutrition on nitrogen economy depends on severity of diseases in surgical patients. Clin. Nutr. 2002, 21, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Ockenga, J.; Borchert, K.; Rifai, K.; Manns, M.P.; Bischoff, S.C. Effect of glutamine-enriched total parenteral nutrition in patients with acute pancreatitis. Clin. Nutr. 2002, 21, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Orozco, C.; Anaya-Prado, R.; González-Ojeda, A.; Arenas-Márquez, H.; Cabrera-Pivaral, C.; Cervantes-Guevara, G.; Barrera-Zepeda, L.M. L-alanyl-L-glutamine-supplemented parenteral nutrition improves infectious morbidity in secondary peritonitis. Clin. Nutr. 2004, 23, 13–21. [Google Scholar] [CrossRef]
- Zhou, Y.P.; Jiang, Z.M.; Sun, Y.H.; He, G.Z.; Shu, H. The effects of supplemental glutamine dipeptide on gut integrity and clinical outcome after major escharectomy in severe burns: A randomized, double-blind, controlled clinical trial. Clin. Nutr. Suppl. 2004, 1, 55–60. [Google Scholar] [CrossRef]
- Quan, Z.F.; Yang, C.; Li, N.; Li, J.S. Effect of glutamine on change in early postoperative intestinal permeability and its relation to systemic inflammatory response. World J. Gastroenterol. 2004, 10, 1992–1994. [Google Scholar] [CrossRef]
- Kłek, S.; Kulig, J.; Szczepanik, A.M.; Jedrys, J.; Kołodziejczyk, P. The clinical value of parenteral immunonutrition in surgical patients. Acta Chir. Belg. 2005, 105, 175–179. [Google Scholar] [CrossRef]
- Lin, M.T.; Kung, S.P.; Yeh, S.L.; Liaw, K.Y.; Wang, M.Y.; Kuo, M.L.; Lee, P.H.; Chen, W.J. Glutamine-supplemented total parenteral nutrition attenuates plasma interleukin-6 in surgical patients with lower disease severity. World J. Gastroenterol. 2005, 11, 6197–6201. [Google Scholar] [CrossRef]
- Yao, G.X.; Xue, X.B.; Jiang, Z.M.; Yang, N.F.; Wilmore, D.W. Effects of perioperative parenteral glutamine-dipeptide supplementation on plasma endotoxin level, plasma endotoxin inactivation capacity and clinical outcome. Clin. Nutr. 2005, 24, 510–515. [Google Scholar] [CrossRef]
- Déchelotte, P.; Hasselmann, M.; Cynober, L.; Allaouchiche, B.; Coëffier, M.; Hecketsweiler, B.; Merle, V.; Mazerolles, M.; Samba, D.; Guillou, Y.M.; et al. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: The French controlled, randomized, double-blind, multicenter study. Crit. Care Med. 2006, 34, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Şahin, H.; MercanlIgil, S.M.; Inanç, N.; Ok, E. Effects of glutamine-enriched total parenteral nutrition on acute pancreatitis. Eur. J. Clin. Nutr. 2007, 61, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Yan, J.; Zhang, Z.; Yu, Y. Immunomodulatory effects of glutamine-enriched nutritional support in elderly patients with severe sepsis: A prospective, randomized, controlled study. J. Organ Dysfunct. 2008, 4, 31–37. [Google Scholar] [CrossRef]
- Duška, F.; Fric, M.; Waldauf, P.; Pǎout, J.; Anděl, M.; Mokrejš, P.; Tůma, P.; Pachl, J. Frequent intravenous pulses of growth hormone together with glutamine supplementation in prolonged critical illness after multiple trauma: Effects on nitrogen balance, insulin resistance, and substrate oxidation. Crit. Care Med. 2008, 36, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Estívariz, C.F.; Griffith, D.P.; Luo, M.; Szeszycki, E.E.; Bazargan, N.; Dave, N.; Daignault, N.M.; Bergman, G.F.; McNally, T.; Battey, C.H.; et al. Efficacy of Parenteral Nutrition Supplemented With Glutamine Dipeptide to Decrease Hospital Infections in Critically Ill Surgical Patients. J. Parenter. Enter. Nutr. 2008, 32, 389–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, G.L.; Kang, Z.H.; Liu, X.N.; Ji, G.; Wang, C.Y.; Wan, Y.; Liu, D.H.; Wang, W.Z. Effect of alanyl-glutamine on the clinical outcome of patients after total gastrectomy. Chin. J. Clin. Nutr. 2008, 16, 70–73. [Google Scholar]
- Fuentes-Orozco, C.; Cervantes-Guevara, G.; Muciño-Hernández, I.; López-Ortega, A.; Ambriz-González, G.; Gutiérrez-De-La-Rosa, J.L.; Gómez-Herrera, E.; Hermosillo-Sandoval, J.M.; González-Ojeda, A. L-alanyl-L-glutamine-supplemented parenteral nutrition decreases infectious morbidity rate in patients with severe acute pancreatitis. J. Parenter. Enter. Nutr. 2008, 32, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.N.; Lee, H.L.; Liu, Y.Y.; Chiang, K.C.; Hwang, T.L.; Jan, Y.Y.; Chen, M.F. The role of parenteral glutamine supplement for surgical patient perioperatively: Result of a single center, prospective and controlled study. Langenbeck’s Arch. Surg. 2008, 393, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Asprer, J.M.; Llido, L.O.; Sinamban, R.; Schlotzer, E.; Kulkarni, H. Effect on immune indices of preoperative intravenous glutamine dipeptide supplementation in malnourished abdominal surgery patients in the preoperative and postoperative periods. Nutrition 2009, 25, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.P.; Yu, J.C.; Kang, W.M.; Zhang, Q. Effects of glutamine supplementation on patients undergoing abdominal surgery. Chin. Med. Sci. J. 2009, 24, 55–59. [Google Scholar] [CrossRef]
- Quan, Z.; Yuan, Z.; Li, J. Effects of alanyl-glutamine dipeptide administration on postoperative intestinal permeability and systemic inflammatory response. Parenter. Enter. Nutr. 2010, 17, 13–16. [Google Scholar]
- Andrews, P.J.D.; Avenell, A.; Noble, D.W.; Campbell, M.K.; Croal, B.L.; Simpson, W.G.; Vale, L.D.; Battison, C.G.; Jenkinson, D.J.; Cook, J.A.; et al. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ 2011, 342, 695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çekmen, N.; Aydimathn, A.; Erdemli, Ö. The impact of L-alanyl-L-glutamine dipeptide supplemented total parenteral nutrition on clinical outcome in critically patients. e-SPEN 2011, 6, 67–70. [Google Scholar] [CrossRef]
- Grau, T.; Bonet, A.; Miñambres, E.; Piñeiro, L.; Irles, J.A.; Robles, A.; Acosta, J.; Herrero, I.; Palacios, V.; Lopez, J.; et al. The effect of l-alanyl-l-glutamine dipeptide supplemented total parenteral nutrition on infectious morbidity and insulin sensitivity in critically ill patients. Crit. Care Med. 2011, 39, 1263–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.Y.; Shih, Y.L.; Sun, L.C.; Chuang, J.F.; Ma, C.J.; Chen, F.M.; Wu, D.C.; Hsieh, J.S.; Wang, J.Y. The inflammatory modulation effect of glutamine-enriched total parenteral nutrition in postoperative gastrointestinal cancer patients. Am. Surg. 2011, 77, 59–64. [Google Scholar] [CrossRef]
- Wernerman, J.; Kirketeig, T.; Andersson, B.; Berthelson, H.; Ersson, A.; Friberg, H.; Guttormsen, A.B.; Hendrikx, S.; Pettilä, V.; Rossi, P.; et al. Scandinavian glutamine trial: A pragmatic multi-centre randomised clinical trial of intensive care unit patients. Acta Anaesthesiol. Scand. 2011, 55, 812–818. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Y.; Shao, Q.; Hu, J.; Qian, Z.; Zhou, Y.; Ye, Z. Effect of early enteral nutrition supplemented with glutamine on postoperative intestinal mucosal barrier function in patients with gastric carcinoma. Zhonghua Wei Chang Wai Ke Za Zhi 2011, 14, 436–439. [Google Scholar]
- Richard, V.; Dahiya, D.; Kaman, L.; Raj, P.; Behera, A. Effect of perioperative glutamine administration on C-reactive protein and liver function tests in patients undergoing hepatic resection. Polish J. Surg. 2014, 86, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Barchitta, M.; Maugeri, A.; Favara, G.; San Lio, R.M.; Evola, G.; Agodi, A.; Basile, G. Nutrition and wound healing: An overview focusing on the beneficial effects of curcumin. Int. J. Mol. Sci. 2019, 20, 1119. [Google Scholar] [CrossRef] [Green Version]
- Wu, G. Intestinal mucosal amino acid catabolism. J. Nutr. 1998, 128, 1249. [Google Scholar] [CrossRef] [Green Version]
- Castillo, L.; DeRojas, T.C.; Chapman, T.E.; Vogt, J.; Burke, J.F.; Tannenbaum, S.R.; Young, V.R. Splanchnic metabolism of dietary arginine in relation to nitric oxide synthesis in normal adult man. Proc. Natl. Acad. Sci. USA 1993, 90, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Bode-Böger, S.M.; Böger, R.H.; Galland, A.; Tsikas, D.; Frölich, J.C. L-arginine-induced vasodilation in healthy humans: Pharmacokinetic-pharmacodynamic relationship. Br. J. Clin. Pharmacol. 1998, 46, 489–497. [Google Scholar] [CrossRef] [Green Version]
- Heyland, D.K.; Dhaliwal, R.; Drover, J.W.; Gramlich, L.; Dodek, P. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. J. Parenter. Enter. Nutr. 2003, 27, 355–373. [Google Scholar] [CrossRef] [Green Version]
- García-De-Lorenzo, A.; Zarazaga, A.; García-Luna, P.P.; Gonzalez-Huix, F.; López-Martínez, J.; Miján, A.; Quecedo, L.; Casimiro, C.; Usán, L.; Del Llano, J. Clinical evidence for enteral nutritional support with glutamine: A systematic review. Nutrition 2003, 19, 805–811. [Google Scholar] [CrossRef]
- Garlick, P.J. Assessment of the Safety of Glutamine and Other Amino Acids. J. Nutr. 2001, 131, 2556S–2561S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bak, L.K.; Schousboe, A.; Waagepetersen, H.S. The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006, 98, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Sanacora, G.; Zarate, C.A.; Krystal, J.H.; Manji, H.K. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 2008, 7, 426–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, C.; Choi, Y.; Lim, S.M.; Bae, S.; Hong, Y.S.; Kim, J.E.; Lyoo, I.K. Disturbance of the Glutamatergic System in Mood Disorders. Exp. Neurobiol. 2014, 23, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Sanacora, G.; Treccani, G.; Popoli, M. Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 2012, 62, 63–77. [Google Scholar] [CrossRef] [Green Version]















| Patient/Population | Intervention | Outcome | Study Designs | Combining Search Terms |
|---|---|---|---|---|
| Patients | Amino acid | Randomized controlled trial | ||
| Patients undergoing Surgery OR patients with Pressure Ulcers OR Patients with Critical Illness | Amino Acid OR Arginine OR Glutamine OR Nutrition | Inflammation OR Healing OR Wound OR Cytokines OR Interleukin OR Hospital Stay OR C-reactive protein | Clinical trial OR Randomised controlled trial OR controlled clinical trial | Column 1 AND Column 2 AND Column 3 AND Column 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. The Effect of Amino Acids on Wound Healing: A Systematic Review and Meta-Analysis on Arginine and Glutamine. Nutrients 2021, 13, 2498. https://doi.org/10.3390/nu13082498
Arribas-López E, Zand N, Ojo O, Snowden MJ, Kochhar T. The Effect of Amino Acids on Wound Healing: A Systematic Review and Meta-Analysis on Arginine and Glutamine. Nutrients. 2021; 13(8):2498. https://doi.org/10.3390/nu13082498
Chicago/Turabian StyleArribas-López, Elena, Nazanin Zand, Omorogieva Ojo, Martin John Snowden, and Tony Kochhar. 2021. "The Effect of Amino Acids on Wound Healing: A Systematic Review and Meta-Analysis on Arginine and Glutamine" Nutrients 13, no. 8: 2498. https://doi.org/10.3390/nu13082498
APA StyleArribas-López, E., Zand, N., Ojo, O., Snowden, M. J., & Kochhar, T. (2021). The Effect of Amino Acids on Wound Healing: A Systematic Review and Meta-Analysis on Arginine and Glutamine. Nutrients, 13(8), 2498. https://doi.org/10.3390/nu13082498