Endometriosis and Phytoestrogens: Friends or Foes? A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Studies Included
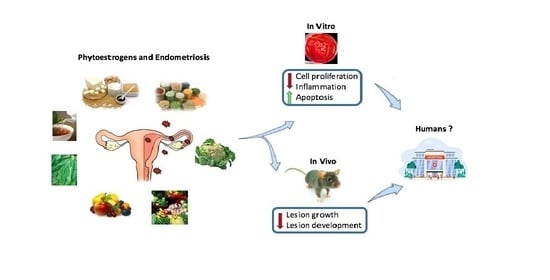
3.1.1. Phytoestrogens and Endometriosis: In Vitro Experimental Human Models
3.1.2. Phytoestrogens and Endometriosis: In Vivo Experimental Animal Models
3.1.3. Phytoestrogen Dietary Intake and the Risk of Endometriosis in Humans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parazzini, F.; Esposito, G.; Tozzi, L.; Noli, S.; Bianchi, S. Epidemiology of endometriosis and its comorbidities. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 3–7. [Google Scholar] [CrossRef]
- Vercellini, P.P.; Vigano, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2013, 10, 261–275. [Google Scholar] [CrossRef]
- Vigano, P.; Candiani, M.; Monno, A.; Giacomini, E.; Vercellini, P.P.; Somigliana, E. Time to redefine endometriosis including its pro-fibrotic nature. Hum. Reprod. 2018, 33, 347–352. [Google Scholar] [CrossRef]
- Vigano, P.; Ottolina, J.; Bartiromo, L.; Bonavina, G.; Schimberni, M.; Villanacci, R.; Candiani, M. Cellular components contributing to fibrosis in endometriosis: A literature review. J. Minim. Invasive Gynecol. 2020, 27, 287–295. [Google Scholar] [CrossRef]
- Huhtinen, K.; Stahle, M.; Perheentupa, A.; Poutanen, M. Estrogen biosynthesis and signaling in endometriosis. Mol. Cell Endocrinol. 2012, 358, 146–154. [Google Scholar] [CrossRef]
- Kyama, C.M.; Debrock, S.; Mwenda, J.M.; D’Hooghe, T.M. Potential involvement of the immune system in the development of endometriosis. Reprod. Biol. Endocrinol. 2003, 1, 123. [Google Scholar] [CrossRef] [Green Version]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat Rev Dis Primers 2018, 19, 4–9. [Google Scholar] [CrossRef]
- Romano, A.; Xanthoulea, S.; Giacomini, E.; Delvoux, B.; Alleva, E.; Vigano, P. Endometriotic cell culture contamination and authenticity: A source of bias in in vitro research? Hum. Reprod. 2020, 35, 364–376. [Google Scholar] [CrossRef] [Green Version]
- Stratton, P.; Berkley, K.J. Chronic pelvic pain and endometriosis: Translational evidence of the relationship and implications. Hum. Reprod. Update 2011, 17, 327–346. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, G.A. p27(kip1) as a key regulator of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 221, 1–4. [Google Scholar] [CrossRef]
- Harris, H.R.; Chavarro, J.E.; Malspeis, S.; Willett, W.C.; Missmer, S.A. Dairy-food, calcium, magnesium and vitamin D intake and endometriosis: A prospective cohort study. Am. J. Epidemiol. 2013, 177, 420–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, H.R.; Eke, A.C.; Chavarro, J.E.; Missmer, S.A. Fruit and vegetable consumption and risk of endometriosis. Hum. Reprod. 2018, 33, 715–727. [Google Scholar] [CrossRef]
- Parazzini, F.; Viganò, P.; Candiani, M.; Fedele, L. Diet and endometriosis risk: A literature review. Reprod. Biomed. Online 2013, 26, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, I.L.; Savić-Gajić, I.M.; Tačić, A.D.; Savić, I.M. Classification and biological activity of phytoestrogens: A review. Adv. Technol. 2017, 6, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Roca, P.; Sastre-Serra, J.; Nadal-Serrano, M.; Pons, D.G.; Blanquer-Rossello, M.D.; Oliver, J. Phytoestrogens and mitochondrial biogenesis in breast cancer. Influence of estrogen receptors ratio. Curr. Pharm. Des. 2014, 20, 5594–5618. [Google Scholar] [CrossRef]
- Kirichenko, T.V.; Myasoedova, V.A.; Orekhova, V.A.; Ravani, A.L.; Nikitina, N.A.; Grechko, A.V.; Sobenin, I.A.; Orekhov, A.N. Phytoestrogen-rich natural preparation for treatment of climacteric syndrome and atherosclerosis prevention in Perimenopausal women. Phytother. Res. 2017, 31, 1209–1214. [Google Scholar] [CrossRef]
- Shukla, V.; Chandra, V.; Sankhwar, P.; Popli, P.; Kaushal, J.B.; Sirohi, V.K.; Dwivedi, A. Phytoestrogen genistein inhibits EGFR/PI3K/NF-kB activation and induces apoptosis in human endometrial hyperplasial cells. RSC Adv. 2015, 5, 56075–56085. [Google Scholar] [CrossRef]
- Nagata, C.; Takatsuka, N.; Kawakami, N.; Shimizu, H. Soy product intake and premenopausal hysterectomy in a follow-up study of Japanese women. Eur. J. Clin. Nutr. 2001, 55, 773–777. [Google Scholar] [CrossRef]
- Cotroneo, M.S.; Lamartiniere, C.A. Pharmacologic, but not dietary, genistein supports endometriosis in a rat model. Toxicol. Sci. 2001, 61, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Edmunds, K.M.; Holloway, A.C.; Crankshaw, D.J.; Agarwal, S.K.; Foster, W.G. The effects of dietary phytoestrogens on aromatase activity in human endometrial stromal cells. Reprod. Nutr. Dev. 2005, 45, 709–720. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, M.; Miura, T.; Hanaoka, T.; Iwasaki, M.; Sasaki, H.; Tanaka, T.; Nakao, H.; Katoh, T.; Ikenoue, T.; Kabuto, M.; et al. Effect of soy isoflavones on endometriosis: Interaction with estrogen receptor 2 gene polymorphism. Epidemiology 2007, 18, 402–408. [Google Scholar] [CrossRef]
- Yavuz, E.; Oktem, M.; Esinler, I.; Toru, S.A.; Zeyneloglu, H.B. Genistein causes regression of endometriotic implants in the rat model. Fertil. Steril. 2007, 88, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Schwender, C.; Scheuer, C.; Vollmar, B.; Menger, M.D. Epigallocatechin-3-gallate inhibits estrogen-induced activation of endometrial cells in vitro and causes regression of endometriotic lesions in vivo. Hum. Reprod. 2008, 23, 2308–2318. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Lui, W.T.; Chu, C.Y.; Ng, P.S.; Wang, C.C.; Rogers, M.S. Anti-angiogenic effects of green tea catechin on an experimental endometriosis mouse model. Hum. Reprod. 2009, 24, 608–618. [Google Scholar] [CrossRef] [Green Version]
- Laschke, M.W.; Schwender, C.; Vollmar, B.; Menger, M.D. Genistein does not affect vascularization and blood perfusion of endometriotic lesions and ovarian follicles in dorsal skinfold chambers of Syrian golden hamsters. Reprod. Sci. 2010, 17, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Bruner-Tran, K.L.; Osteen, K.G.; Taylor, H.S.; Sokalska, A.; Haines, K.; Duleba, A.J. Resveratrol inhibits development of experimental endometriosis in vivo and reduces endometrial stromal cell invasiveness in vitro. Biol. Reprod. 2011, 84, 106–112. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, C.; Shi, S.; Han, J.; Wang, J.; Hu, J.; Liu, Y.; Cai, Z.; Yu, C. Endometriotic implants regress in rat models treated with puerarin by decreasing estradiol level. Reprod. Sci. 2011, 18, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Y.; Han, J.; Zai, D.; Ji, M.; Cheng, W.; Xu, L.; Yang, L.; He, M.; Ni, J.; et al. Puerarin suppresses invasion and vascularization of endometriosis tissue stimulated by 17β-estradiol. PLoS ONE 2011, 6, e25011. [Google Scholar] [CrossRef]
- Xu, H.; Becker, C.M.; Lui, W.T.; Chu, C.Y.; Davis, T.N.; Kung, A.L.; Birsner, A.E.; D’Amato, R.J.; Wai Man, G.C.; Wang, C.C. Green tea epigallocatechin-3-gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C/vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. Fertil. Steril. 2011, 96, 1021–1028. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, L.; Yang, S.; Han, J.; Zhai, D.; Ni, J.; Yu, C.; Cai, Z. Puerarin suppresses proliferation of endometriotic stromal cells partly via the MAPK signaling pathway induced by 17ß-estradiol-BSA. PLoS ONE 2012, 7, e45529. [Google Scholar] [CrossRef] [Green Version]
- Maia, H.; Haddad, C.; Pinheiro, N.; Casoy, J. Advantages of the association of resveratrol with oral contraceptives for management of endometriosis-related pain. Int. J. Womens Health 2012, 4, 543–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudzitis-Auth, J.; Körbel, C.; Scheuer, C.; Menger, M.D.; Laschke, M.W. Xanthohumol inhibits growth and vascularization of developing endometriotic lesions. Hum. Reprod. 2012, 27, 1735–1744. [Google Scholar] [CrossRef] [Green Version]
- Ergenoglu, A.M.; Yeniel, A.O.; Erbas, O.; Aktug, H.; Yildirim, N.; Ulukus, M.; Taskiran, D. Regression of endometrial implants by resveratrol in an experimentally induced endometriosis model in rats. Reprod. Sci. 2013, 20, 1230–1236. [Google Scholar] [CrossRef]
- Ji, M.; Liu, Y.; Yang, S.; Zhai, D.; Zhang, D.; Bai, L.; Wang, Z.; Yu, J.; Yu, C.; Cai, Z. Puerarin suppresses proliferation of endometriotic stromal cells in part via differential recruitment of nuclear receptor coregulators to estrogen receptor-α. J. Steroid Biochem. Mol. Biol. 2013, 138, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.G.; Olivares, C.N.; Bilotas, M.A.; Bastón, J.I.; Singla, J.J.; Meresman, G.F.; Barañao, R.I. Natural therapies assessment for the treatment of endometriosis. Hum. Reprod. 2013, 28, 178–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudzitis-Auth, J.; Menger, M.D.; Laschke, M.W. Resveratrol is a potent inhibitor of vascularization and cell proliferation in experimental endometriosis. Hum. Reprod. 2013, 28, 1339–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.C.; Xu, H.; Man, G.C.; Zhang, T.; Chu, K.O.; Chu, C.Y.; Cheng, J.T.; Li, G.; He, Y.X.; Qin, L.; et al. Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis 2013, 16, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Amaya, S.C.; Savaris, R.F.; Filipovic, C.J.; Wise, J.D.; Hestermann, E.; Young, S.L.; Lessey, B.A. Resveratrol and endometrium: A closer look at an active ingredient of red wine using in vivo and in vitro models. Reprod. Sci. 2014, 21, 1362–1369. [Google Scholar] [CrossRef] [Green Version]
- Demirel, M.A.; Suntar, I.; Ilhan, M.; Keles, H.; Kupeli Akkol, E. Experimental endometriosis remission in rats treated with Achillea biebersteinii Afan: Histopathological evaluation and determination of cytokine levels. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 175, 172–177. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C. Antifibrotic properties of epigallocatechin-3-gallate in endometriosis. Hum. Reprod. 2014, 29, 1677–1687. [Google Scholar] [CrossRef]
- Taguchi, A.; Wada-Hiraike, O.; Kawana, K.; Koga, K.; Yamashita, A.; Shirane, A.; Urata, Y.; Kozuma, S.; Osuga, Y.; Fujii, T. Resveratrol suppresses inflammatory responses in endometrial stromal cells derived from endometriosis: A possible role of the sirtuin 1 pathway. J. Obstet. Gynaecol. Res. 2014, 40, 770–778. [Google Scholar] [CrossRef] [Green Version]
- Yavuz, S.; Aydin, N.E.; Celik, O.; Yilmaz, E.; Ozerol, E.; Tanbek, K. Resveratrol successfully treats experimental endometriosis through modulation of oxidative stress and lipid peroxidation. J. Cancer Res. Ther. 2014, 10, 324–329. [Google Scholar] [CrossRef]
- Bayoglu Tekin, Y.; Guven, S.; Kirbas, A.; Kalkan, Y.; Tumkaya, L.; Guvendag Guven, E.S. Is resveratrol a potential substitute for leuprolide acetate in experimental endometriosis? Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 184, 1–6. [Google Scholar] [CrossRef]
- Cenksoy, O.P.; Oktem, M.; Erdem, O.; Karakaya, C.; Cenksoy, C.; Erdem, A.; Guner, H.; Karabacak, O. A potential novel treatment strategy: Inhibition of angiogenesis and inflammation by resveratrol for regression of endometriosis in an experimental rat model. Gynecol. Endocrinol. 2015, 31, 219–224. [Google Scholar] [CrossRef]
- Singh, A.K.; Chakravarty, B.; Chaudhury, K. Nanoparticle-assisted combinatorial therapy for effective treatment of endometriosis. J. Biomed. Nanotechnol. 2015, 11, 789–804. [Google Scholar] [CrossRef]
- Di Paola, R.; Fusco, R.; Gugliandolo, E.; Crupi, R.; Evangelista, M.; Granese, R.; Cuzzocrea, S. Co-micronized palmitoylethanolamide/polydatin treatment causes endometriotic lesion regression in a rodent model of surgically induced endometriosis. Front. Pharmacol. 2016, 7, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taguchi, A.; Koga, K.; Kawana, K.; Makabe, T.; Sue, F.; Miyashita, M.; Yoshida, M.; Urata, Y.; Izumi, G.; Tkamura, M.; et al. Resveratrol enhances apoptosis in endometriotic stromal cells. Am. J. Reprod. Immunol. 2016, 75, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Woo, J.H.; Kim, H.M.; Oh, M.S.; Jang, D.S.; Choi, J.H. Anti-endometriotic effects of pueraria flower extract in human endometriotic cells and mice. Nutrients 2017, 9, 212. [Google Scholar] [CrossRef] [Green Version]
- Mendes da Silva, D.; Gross, L.A.; Neto, E.P.G.; Lessey, B.A.; Savaris, R.F. The use of resveratrol as an adjuvant treatment of pain in endometriosis: A randomized clinical trial. J. Endocr. Soc. 2017, 1, 359–369. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Naringenin induces mitochondria-mediated apoptosis and endoplasmic reticulum stress by regulating MAPK and AKT signal transduction pathways in endometriosis cells. Mol. Hum. Reprod. 2017, 23, 842–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferella, L.; Bastón, J.I.; Bilotas, M.A.; Singla, J.J.; González, A.M.; Olivares, C.N.; Meresman, G.F. Active compounds present inRosmarinus officinalis leaves andScutellaria baicalensis root evaluated as new therapeutic agents for endometriosis. Reprod. Biomed. Online 2018, 37, 769–782. [Google Scholar] [CrossRef]
- Jouhari, S.; Mohammadzadeh, A.; Soltanghoraee, H.; Mohammadi, Z.; Khazali, S.; Mirzadegan, E.; Lakpour, N.; Fatemi, F.; Zafardoust, S.; Mohazzab, A.; et al. Effects of silymarin, cabergoline and letrozole on rat model of endometriosis. Taiwan J. Obstet. Gynecol. 2018, 57, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Melekoglu, R.; Ciftci, O.; Eraslan, S.; Cetin, A.; Basak, N. The beneficial effects of nerolidol and hesperidin on surgically induced endometriosis in a rat model. Gynecol. Endocrinol. 2018, 34, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Nahari, E.; Razi, M. Silymarin amplifies apoptosis in ectopic endometrial tissue in rats with endometriosis; implication on growth factor GDNF, ERK1/2 and Bcl-6b expression. Acta Histochem. 2018, 120, 757–767. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Apigenin induces ROS-dependent apoptosis and ER stress in human endometriosis cells. J. Cell Physiol. 2018, 233, 3055–3065. [Google Scholar] [CrossRef]
- Signorile, P.G.; Viceconte, R.; Baldi, A. Novel dietary supplement association reduces symptoms in endometriosis patients. J. Cell Physiol. 2018, 233, 5920–5925. [Google Scholar] [CrossRef]
- Takaoka, O.; Mori, T.; Ito, F.; Okimura, H.; Kataoka, H.; Tanaka, Y.; Koshiba, A.; Kusuki, I.; Shigehiro, S.; Amami, T.; et al. Daidzein-rich isoflavone aglycones inhibit cell growth and inflammation in endometriosis. J. Steroid. Biochem. Mol. Biol. 2018, 181, 125–132. [Google Scholar] [CrossRef]
- Wei, X.; Shao, X. Nobiletin alleviates endometriosis via down-regulating NF-κB activity in endometriosis mouse model. Biosci. Rep. 2018, 38, BSR20180470. [Google Scholar] [CrossRef] [Green Version]
- Arablou, T.; Delbandi, A.A.; Khodaverdi, S.; Arefi, S.; Kolahdouz-Mohammadi, R.; Heidari, S.; Mohammadi, T.; Aryaeian, N. Resveratrol reduces the expression of insulin-like growth factor-1 and hepatocyte growth factor in stromal cells of women with endometriosis compared with nonendometriotic women. Phytother. Res. 2019, 33, 1044–1054. [Google Scholar] [CrossRef]
- Ding, D.; Cai, X.; Zheng, H.; Guo, S.W.; Liu, X. Scutellarin suppresses platelet aggregation and stalls lesional progression in mouse with induced endometriosis. Reprod. Sci. 2019, 26, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.; Kim, J.; Bazer, F.W.; Lim, W.; Song, G. Silibinin-induced endoplasmic reticulum stress and mitochondrial dysfunction suppress growth of endometriotic lesions. J. Cell Physiol. 2019, 234, 4327–4341. [Google Scholar] [CrossRef]
- Ilhan, M.; Ali, Z.; Khan, I.A.; Taştan, H.; Küpeli Akkol, E. Bioactivity-guided isolation of flavonoids from Urtica dioica L. and their effect on endometriosis rat model. J. Ethnopharmacol. 2019, 243, 112100. [Google Scholar] [CrossRef]
- Ilhan, M.; Ali, Z.; Khan, I.A.; Tastan, H.; Kupeli Akkol, E. Promising activity of Anthemis austriaca Jacq. on the endometriosis rat model and isolation of its active constituents. Saudi Pharm. J. 2019, 27, 889–899. [Google Scholar] [CrossRef]
- Kapoor, R.; Sirohi, V.K.; Gupta, K.; Dwivedi, A. Naringenin ameliorates progression of endometriosis by modulating Nrf2/Keap1/HO1 axis and inducing apoptosis in rats. J. Nutr. Biochem. 2019, 70, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Kodarahmian, M.; Amidi, F.; Moini, A.; Kashani, L.; Shabani Nashtaei, M.; Pazhohan, A.; Bahramrezai, M.; Berenjian, S.; Sobhani, A. The modulating effects of Resveratrol on the expression of MMP-2 and MMP-9 in endometriosis women: A randomized exploratory trial. Gynecol. Endocrinol. 2019, 35, 719–726. [Google Scholar] [CrossRef]
- Ryu, S.; Bazer, F.W.; Lim, W.; Song, G. Chrysin leads to cell death in endometriosis by regulation of endoplasmic reticulum stress and cytosolic calcium level. J. Cell Physiol. 2019, 234, 2480–2490. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lim, W.; Song, G. Delphinidin induces antiproliferation and apoptosis of endometrial cells by regulating cytosolic calcium levels and mitochondrial membrane potential depolarization. J. Cell Biochem. 2019, 120, 5072–5084. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Whang, K.Y.; Song, G. Quercetin inhibits proliferation of endometriosis regulating cyclin D1 and its target microRNAs in vitro and in vivo. J. Nutr. Biochem. 2019, 63, 87–100. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; You, S.; Song, G. Ameliorative effects of luteolin against endometriosis progression in vitro and in vivo. J. Nutr. Biochem. 2019, 67, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Bina, F.; Daglia, M.; Santarcangelo, C.; Baeeri, M.; Abdollahi, M.; Nabavi, S.M.; Tabarrai, M.; Rahimi, R. Phytochemical profiling and ameliorative effects of Achillea cretica L. on rat model of endometriosis. J. Ethnopharmacol. 2020, 254, 112747. [Google Scholar] [CrossRef]
- Hernandes, C.; de Oliveira, R.N.; de Souza Santos, A.H.; Malvezzi, H.; de Azevedo, B.C.; Gueuvoghlanian-Silva, B.Y.; Pereira, A.M.S.; Podgaec, S. The Effect of rutin and extracts of uncaria guianensis (Aubl.) J. F. gmeland on primary endometriotic cells: A 2D and 3D study. Molecules 2020, 13, 1325. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.W.; Chen, H.Y.; Chiang, Y.F.; Chang, L.C.; Lin, P.H.; Hsia, S.M. The effects of isoliquiritigenin on endometriosis in vivo and in vitro study. Phytomedicine 2020, 77, 153214. [Google Scholar] [CrossRef]
- Ilhan, M.; Ali, Z.; Khan, I.A.; Taştan, H.; Küpeli Akkol, E. The regression of endometriosis with glycosylated flavonoids isolated from Melilotus officinalis (L.) Pall. in an endometriosis rat model. Taiwan J Obstet. Gynecol. 2020, 59, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.R.; Rashidi, Z.; Chobsaz, F.; Niromand, E.; Khazaei, M. Inhibitory effect of resveratrol on the growth and angiogenesis of human endometrial tissue in an In Vitro three-dimensional model of endometriosis. Reprod. Biol. 2020, 20, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Park, M.Y.; Song, G.; Lim, W. 5,7-Dimethoxyflavone induces apoptotic cell death in human endometriosis cell lines by activating the endoplasmic reticulum stress pathway. Phytother. Res. 2020, 34, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Song, G.; Lim, W. Myricetin inhibits endometriosis growth through cyclin E1 down-regulation in vitro and in vivo. J. Nutr. Biochem. 2020, 78, 108328. [Google Scholar] [CrossRef]
- Youseflu, S.; Jahanian Sadatmahalleh, S.H.; Mottaghi, A.; Kazemnejad, A. Dietary phytoestrogen intake and the risk of endometriosis in iranian women: A case-control study. Int. J. Fertil. Steril. 2020, 13, 296–300. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 29, 372. [Google Scholar]
- EFSA Scientific Committee; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A.; et al. The principles and methods behind EFSA’s guidance on uncertainty analysis in scientific assessment. EFSA J. 2018, 16, 5122. [Google Scholar]
- Agarwal, S.K.; Daniels, A.; Drosman, S.R.; Udoff, L.; Foster, W.G.; Pike, M.C.; Spicer, D.V.; Daniels, J.R. Treatment of endometriosis with the GnRHa deslorelin and add-back estradiol and supplementary testosterone. Biomed. Res. Int. 2015, 2015, 934164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu Hashim, H. Potential role of aromatase inhibitors in the treatment of endometriosis. Int. J. Womens Health 2014, 6, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Nawathe, A.; Patwardhan, S.; Yates, D.; Harrison, G.R.; Khan, K.S. Systematic review of the effects of aromatase inhibitors on pain associated with endometriosis. BJOG 2008, 115, 818–822. [Google Scholar] [PubMed]
- Cos, P.; De Bruyne, T.; Apers, S.; Berghe, D.V.; Pieters, L.; Vlietinck, A.J. Phytoestrogens: Recent developments. Planta Med. 2003, 69, 589–599. [Google Scholar]
- Wang, T.-Y.; Li, Q.; Bi, K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavones, flavanones and humanhealth: Epidemiological evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef]
- Chan, K.K.L.; Siu, M.K.Y.; Jiang, Y.-X.; Wang, J.-J.; Leung, T.H.Y.; Ngan, H.Y.S. Estrogen receptor modulators genistein, daidzein and ERB-041 inhibit cell migration, invasion, proliferation and sphere formation via modulation of FAK and PI3K/AKT signaling in ovarian cancer. Cancer Cell Int. 2018, 18, 65. [Google Scholar] [CrossRef] [Green Version]
- Kuiper, G.G.J.M.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.-A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors and β. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Konar, N. Non-isoflavone phytoestrogenic compound contents of various legumes. Eur. Food Res. Technol. 2013, 236, 523–530. [Google Scholar] [CrossRef]
- Onzalez-Mejia, M.E.; Voss, O.H.; Murnan, E.J.; Dose, A.I. Apigenin-induced apoptosis of leukemia cells is mediated by a bimodal and differentially regulated residue-specific phosphorylation of heat-shock protein–27. Cell Death Dis. 2010, 1, e64. [Google Scholar] [CrossRef] [Green Version]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.A.J.; Berghe, D.V. Structure–activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular mechanisms and therapeutic effects of (-) -epicatechin and other polyphenols in cancer, inflammation, diabetes and neurodegeneration. Oxidative Med. Cell. Longev. 2015, 181260, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.S.; Wang, H. Cancer preventive activities of tea catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic potential of naringenin: A Review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Aguzzi, A.; Novellino, E.; Santini, A.; et al. Dietary lignans: Definition, description and research trends in databases development. Molecules 2018, 23, 3251. [Google Scholar] [CrossRef] [Green Version]
- Cotterchio, M.; Boucher, B.; Kreiger, N.; Mills, C.A.; Thompson, L.U. Dietary phytoestrogen intake—Lignans and isoflavones—And breast cancer risk (Canada). Cancer Causes Control 2007, 19, 259–272. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhu, W.; Feng, W.; Lee, S.S.; Leung, A.W.; Shen, J.; Gao, L.; Xu, C. A review of resveratrol as a potent chemoprotective and synergistic agent in cancer chemotherapy. Front. Pharmacol. 2019, 9, 1534. [Google Scholar] [CrossRef]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of natural stilbenes in the prevention of cancer. Oxidative Med. Cell. Longev. 2015, 2016, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic versatility of resveratrol derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef] [Green Version]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinsdale, N.; Nepomnaschy, P.; Crespi, B. The evolutionary biology of endometriosis. Evol. Med. Public Health 2021, 9, 174–191. [Google Scholar] [CrossRef] [PubMed]

| Authors | Date | Substance | Cases | Controls | Results | Adverse Events |
|---|---|---|---|---|---|---|
| Edmunds et al. [20] | 2005 | Genistein, Daidzein, Naringenin or Chrysin (10−4–10−9 M) | EuSC from 11 women with endometriosis | EuSC from 7 women without endometriosis | - PE treatment did not attenuate aromatase activity in EuSC cultures from cases and controls - Genistein (10−9–10−6 M) increased aromatase activity in controls - Naringenin and Chrysin were potent inhibitors of aromatase in FCA - Genistein was inactive in FCA | Genistein consumption in reproductive age may have health risks |
| Wang et al. [28] | 2011 | Puerarin (10−9 M) | EcSC treated with Puerarin | EcSC treated with E2 (10−8 M) Untreated EcSC | - E2 showed a stimulatory effect on EcSC invasion compared with the untreated cells, but the combination of E2 with Puerarin reduced this effect - E2 treatment determined MMP-9 increase and TIMP-1 decrease, but the combination with Puerarin reversed this effect | NR |
| Cheng et al. [30] | 2012 | Puerarin (10−9 M) | EcSC treated with Puerarin +/− E2-BSA | EcSC treated with E2-BSA | - ERK1/2 (MAPK signaling) was highly activated by E2-BSA, which was reversed by Puerarin - E2-BSA induced the proliferation of EcSCs, which was reversed by Puerarin - Puerarin suppressed gene expression of Cyclin D1, COX-2 and cyp19 | NR |
| Ji et al. [34] | 2013 | Puerarin (10−9 M) | EcSC treated with Puerarin +/− E2 | EcSC treated with E2 +/− fulvestrant (anti-E2) | - Puerarin:
| NR |
| Ricci et al. [35] | 2013 | Resveratrol (0, 25, 50 and 100 mM) EGCG (0, 20, 40, 80 and 100 mM). | EuEC from women with endometriosis | EuEC from women without endometriosis | - Both compounds induced reduction in EuEC proliferation and increased apoptosis in both groups - No significant difference in cell proliferation and apoptosis between cases and controls | NR |
| Matsuzaki et al. [40] | 2014 | EGCG (10−9 M) | EcSC and EuSC treated with ECGC (from 45 women with endometriosis) | EuSC and EuSC vehicle-treated or NAC(10 mM) treated (from 45 patients with endometriosis) | - EGCG:
| NR |
| Taguchi et al. [41] | 2014 | Resveratrol (10, 20 or 40 μM) (SIRT-1 activator) Sirtinol at 20 μM (SIRT-1 inhibitor) | EcSC | EuSC from patients without endometriosis | - No difference in the basal expression level of SIRT1 mRNA between EcSC and EuSC - Resveratrol:
| NR |
| Taguchi et al. [47] | 2016 | Resveratrol (40–120 mΜ) TRAIL 100 ng/mL | EcSC treated with resveratrol and TRAIL | EcSC treated with TRAIL | - Resveratrol:
| NR |
| Kim et al. [48] | 2017 | PFE (25, 50, and 100 μg/mL) containing Genistein, Daidzein, Kakkalide, Puerarin, Tectoridin | Human endometriotic (11Z and 12Z) and mesothelial (Met5A) cells treated with PFE | Human endometriotic (11Z and 12Z) and mesothelial (Met5A) cells not treated with PFE | - PFE:
| NR |
| Park et al. [50] | 2017 | Narigenin (100 μM) | VK2/E6E7 and End1/E6E7 cells treated with Narigenin | VK2/E6E7 and End1/E6E7 cells not treated with Narigenin | - Narigenin:
| NR |
| Park et al. [55] | 2018 | Apigenin (20 μM) | VK2/E6E7 and End1/E6E7 cells treated with Apigenin | VK2/E6E7 and End1/E6E7 cells not treated with Apigenin | - Apigenin:
| NR |
| Takaoka et al. [57] | 2018 | DRIAs (0.2, 2, 20 μM) | EcSC from 24 patients with endometriosis | EuSC from 12 patients without endometriosis | - DRIAs:
| NR |
| Arablou et al. [59] | 2019 | Resveratrol (100 μM) | 13 EuSC 8 EcSC from 40 women with endometriosis | 11 EuSC from 15 women without endometriosis | - Basal expression of IGF-1 and HGF gene were significantly higher in EcSC - Resveratrol:
| Resveratrol at 200- and 400-μM concentrations |
| Ham et al. [61] | 2019 | Silibinin (0,2, 5, 10, 25,50 μM) | VK2/E6E7 and End1/E6E7 cells treated with Silibinin | EuSC treated with Silibinin | - Silibinin:
| NR |
| Ryu et al. [66] | 2019 | Chyrisin (0,5,10,20,50, 100 μM | VK2/E6E7 and End1/E6E7 cells treated with Chyrisin | VK2/E6E7 and End1/E6E7 cells not treated with Chyrisin EuC | - Chyrisin:
| NR |
| Park et al. [67] | 2019 | Delphinidin (0,5,10,20,50,100 μM) | VK2/E6E7 and End1/E6E7 cells treated with Delphinidin | VK2/E6E7 and End1/E6E7 cells not treated with Delphinidin | - Delphinidin:
| NR |
| Park et al. [68] | 2019 | Quercetin (0, 2, 5, 10, 20,50 μM) | VK2/E6E7 and End1/E6E7 cells treated with Quercetin | VK2/E6E7 and End1/E6E7 cells not treated with Quercetin | - Quercetin:
- The loss of MMP increased to 2300% in VK2/E6E7 cells and 670% in End1/E6E7 cells at 20 μM | NR |
| Park et al. [69] | 2019 | Luteolin (0, 5, 10, 20, 50 and 100 μM) | VK2/E6E7 and End1/E6E7 cells treated with luteolin | VK2/E6E7 and End1/E6E7 cells not treated with luteolin | - Luteolin:
| NR |
| Hernandes et al. [71] | 2020 | Rutin and extract of Uncaria guianensis | EuSC and EcSC from 4 women with endometriosis | EuSC from 2 women without endometriosis | - Increased ROS levels in EuC from controls treated with ALE, ABE, and ARE and in EuC of patients with endometriosis treated with Rutin, ARE, Rutin + ALE, and Rutin + ARE - Increased ROS levels in EcC treated with ALE - Increased IL-15, IL-17A, IL-4, IL-6, TNF-alfa and VEGF levels in EuC from controls treated with ABE - Increased EGF in EcC treated with ALE | |
| Khazaei et al. [74] | 2020 | Resveratrol (0, 10, 50, 100, 200 μM) | EcSC from 9 patients with endometriosis | EuSC from 9 patients without endometriosis | - Resveratrol (200 μM) completely inhibited growth and angiogenesis in both cells types in a dose-dependent manner - NO level was higher in endometriotic cells. Resveratrol reduced NO level in both endometriotic and endometrial cells - Effect on apoptotic genes (P53, Bax, Bcl2 and caspase 3) and SIRT1 | NR |
| Park et al. [75] | 2020 | DMF (Chyrisin) (0, 20, 50, 100 μM) | VK2/E6E7 and End1/E6E7 cells treated with DMF | VK2/E6E7 and End1/E6E7 cells not treated with DMF | - DMF:
| |
| Park et al. [76] | 2020 | Myricetin (0, 5, 10, 20, 50, 100 μM) | VK2/E6E7 and End1/E6E7 cells treated with Myricetin | VK2/E6E7 and End1/E6E7 cells not treated with Myricetin | - Myricetin
| NR |
| Authors | Date | Model | Substance | Cases (n) | Control (n) | Results |
|---|---|---|---|---|---|---|
| Cotroneo et al. [19] | 2001 | Rats | S.C, genistein:
| 7/8/10 | Vehicle (20) or Estrone (7) | Higher and average dose of Genistein and administration of estrone: - increased uterine/body weight ratios - increased uterine PR expression at all doses - supported growth of the implanted tissue in a dose-responsive manner |
| Laschke et al. [23] | 2008 | Hamsters | I.P. EGCG 65 mg/kg | 7 | Vehicle (10) | - inhibited angiogenesis and blood perfusion of endometriotic lesions |
| Xu et al. [24] | 2009 | Mice | I.P. EGCG 50 mg/kg | 10 | Vitamin E (10) Vehicle (10) | - smaller lesions than control animals - down-regulation of VEGFA mRNA expression - down-regulation of MAPK1 and NFKB mRNA expression |
| Laschke et al. [25] | 2010 | Hamsters | I.P genistein 50/200 mg/kg | Low dose (6) High dose (4) | Vehicle (6) | - blood perfusion and angiogenesis of endometriotic lesions was not affected by Genistein treatment |
| Xu et al. [29] | 2011 | Mice | I.P. EGCG 50 mg/kg | 10 | Vitamin E (10) Vehicle (10) | - decreased lesion size - down-regulation of MMP-9, CXCL3, VEGFC, c-JUN, and IFNγ - suppression of VEGFC mRNA and protein - decreased VEGFC levels in both microvessels and glandular epithelial cells |
| Ergenoglu et al. [33] | 2013 | Rats | I.M. resveratrol 10 mg/kg | 6 | Vehicle (6) | - reduction of implant size - decreased levels of VEGF in peritoneal fluid and plasma - decreased levels of MCP-1 in peritoneal fluid - suppression of VEGF expression in endometriotic tissue |
| Ricci et al. [35] | 2013 | Mice | I.P. resveratrol 10–25 mg/kg; EGCG 20–100 mg/kg by esophageal gavage | Resveratrol (29) EGCG (27) | Vehicle (NR) | - both treatments reduced number and volume of lesions - both diminished proliferation and vascular density of endometriotic lesions - both increased apoptosis |
| Wang et al. [37] | 2013 | Mice | I.P. EGCG 50 mg/kg or pro-EGCG 50 mg/kg | EGCG (8) proEGCG (8) | Vitamin E (8) Vehicle (8) | - decreased lesion size - decreased angiogenesis - increased total apoptopic cell numbers |
| Amaya et al. [38] | 2014 | Mice | S.C. resveratrol 6/30/60 mg/kg | E2 + 6 mg of Resveratrol (4) E2 + 30 mg of Resveratrol (4) E2 + 60 mg of Resveratrol (4) | E2 (4) E2 + P (4) | - reduction in ESR1 and Ki-67 by the highest dose in eutopic endometrial epithelial cells - reduction in Ki-67 expression by the highest dose in endometrial stroma |
| Matsuzaki et al. [40] | 2014 | Mice | I.P. EGCG 50 mg/kg | NR | NR | - lower scores for both Sirius red and Masson trichrome staining |
| Yavuz et al. [42] | 2014 | Rats | I.P resveratrol 1/10 mg/kg | Low dose (8) High dose (8) | Vehicle (8) | - lower implants volume in cases independently from dose - reduced oxidative stress in cases compared to controls in a dose-dependent manner - proliferative scores for glandular tissue and stromal tissue were lower in cases |
| Bayoglu Tekin et al. [43] | 2015 | Mice | I.M. resveratrol 30 mg/kg S.C. 1 mg/kg single dose LA | Resveratrol (NR) LA and resveratrol (NR) | Vehicle (NR) LA (NR) | - reduced implant volumes, histopathological grade and immuno-reactivity to MMP-2, MMP-9 and VEGF - decreased plasma and peritoneal fluid levels of IL-6, IL-8 and TNF-α |
| Singh et al. [45] | 2015 | Mice | I.P. EGCG and doxycycline (NPs) at a dose of 40 mg/kg body weight | 50 | 10 | - decreased ROS and LPO, MMP-2 and MMP-9 activity - decreased angiogenesis and microvessel density |
| Jouhari et al. [52] | 2018 | Rats | S.C. 100 mg/kg silymarin | 8 | Vehicle (8) Letrozole (8) Cabergoline (8) | - smaller volume of implants - lower mean score of the histopathological evaluation of the implants |
| Wei et al. [58] | 2018 | Mice | I.P. nobiletin 10, 20 mg/kg | Low dose Nobiletin (3) High dose Nobiletin (3) | 3 (endometriosis) 3 (sham) | - reduced lesion size - lower PCNA and VEGF immunostaining - higher E-cadherin staining - decreased levels of IL-6, IL-1β, and MMP-3 - reduced levels of TNF-α and MMP-1 - reduced phosphorylation of IKKα, IκBα and p65 factors |
| Ding et al. [60] | 2019 | Mice | I.P. scutellarin
| Low dose (9) High dose (9) | Vehicle (9) | - reduction of lesion weight, improved hyperalgesia, reduced proliferation, angiogenesis, and fibrogenesis of the lesions - reduced the platelet activation rate in peripheral blood |
| Ham et al. [61] | 2019 | Mice | I.P. silibinin 100 μL | 15 | Vehicle (15) | - reduced average size of lesions - decreased expression of TNF-α, IL-1β, and IL-6 mRNA |
| Park et al. [68] | 2019 | Mice | I.P. quercetin 35 mg/kg | 15 | Vehicle (15 | - decreased lesion volume - decreased Ccnd1 mRNA |
| Park et al. [69] | 2019 | Mice | I.P. luteolin 40 mg/kg/day | 6 | Vehicle (6) | - reduced endometriotic lesions growth - decreased mRNA expression of Ccne1, Cdk2 and Cdk4 |
| Park et al. [76] | 2020 | Mice | I.P. myricetin 30 mg/kg | 10 | Vehicle (10) | - decreased lesion size - decreased Ccne1 mRNA expression |
| Authors | Date | Model | Substance | Cases (n) | Control (n) | Results |
|---|---|---|---|---|---|---|
| Cotroneo et al. [19] | 2001 | rats | Genistein 250/1000 mg/kg AIN-76A diet |
12 + 11 (lower/higher dietary intake) | Vehicle (17) |
|
| Yavuz et al. [22] | 2007 | rats |
Genistein 500 mg/kg | 10 | Raloxifene at 10 mg/kg or no vehicle (10 + 13) |
|
| Bruner-Tran et al. [26] | 2011 | mice | Resveratrol 6 mg | 20 | Vehicle (16) |
|
| Chen et al. [27] | 2011 | rats |
Puerarin
| 45 (15 each) |
Danazol at dose of 80 mg/kg or vehicle (15 + 15) |
|
| Rudzitis-Auth et al. [32] | 2012 | mice | Xanthohumol 100 mM | 8 | Vehicle (8) |
|
| Rudzitis-Auth et al. [36] | 2013 | mice | Resveratrol 40 mg/kg | 10 | Vehicle (10) |
|
| Ricci et al. [35] | 2013 | mice | EGCG 20 or 100 mg/kg | 18 (9 each) | Vehicle (9) |
|
| Demirel et al. [39] | 2014 | rats | Extract of Achillea bierbersteinii N-Hexane EtOAc MeOH | 18 | Vehicle or 6 buserelin acetate 20 mg/weekly sc (12) |
|
| Ozcan Censoy et al. [44] | 2015 | rats | Resveratrol 60 mg/kg/day | 7 | Vehicle or leuprolide acetate at 1 mg/kg depot (7 + 8) |
|
| Di Paola et al. [46] | 2016 | rats | mPEA\PLD 10 mg/kg | 5 | Vehicle (5) |
|
| Ferella et al. [51] | 2018 | mice | Wogonin 20 mg/kg/day | 12 | Vehicle (11) |
|
| Nahari et al. [54] | 2018 | rats | Sylimarin (SMN) 50 mg/kg/day | 6 | Vehicle (6) |
|
| Melekoglu et al. [53] | 2018 | rats | Nerolidol 100 mg/kg or Hesperidin 50 mg/kg | 16 (8 each) | (8) |
|
| Takaoka et al. [57] | 2018 | mice | DRIA food at 0.06% | NR | Vehicle (NR) |
|
| Ilhan et al. [62] | 2019 | rats |
Extract of Urtica dioica
| 18 (6 each) | Vehicle or buserelin acetate 20 mg/weekly sc (12) |
|
| Ilhan et al. [63] | 2019 | rats |
Extract of Anthemis austriaca
| 18 (6 each) | Vehicle or buserelin acetate 20 mg/weekly sc (12) |
|
| Kapoor et al. [64] | 2019 | rats |
Narigenin 50 mg/kg/day:
| 12 (6 each) | oral dienogest at dose of 0.3 mg/kg/day for 21 days or nothing (12 endometriosis)(6 sham controls) | Both Narigenin and Dienogest:
|
| Bina et al. [70] | 2020 | rats |
Achillea cretica (A.C.) extract once a day at dose of
| 18 (6 each) | Vehicle or letrozole (12 endometriosis) (6 sham controls) |
|
| Hsu et al. [72] | 2020 | mice |
ISL and estrogens (10 mg/kg/day)
| 12 (6 each) | Vehicle (6) |
|
| Ilhan et al. [73] | 2020 | rats |
Extract of Melilotus officinalis (kaempferol, quercetin, and coumarin derivatives) at 100 mg/kg/day
| 18 (6 each) | Vehicle or buserelin acetate 20 mg/weekly sc (12) | Both MeOH, Fraction C and buserelin acetate:
|
| Authors | Date | Study Design | Substance and Duration | Age (Years, Mean) | Case (n) | Control (n) | Results |
|---|---|---|---|---|---|---|---|
| Nagata et al. [18] | 2001 | prospective cohort study | Genistein, Daidzein in one year | 35–54 42.9 ± 4.4 | 1172 | n.a. | - decreased risk of hysterectomy for pain: RR (95% CI) 0.35 (0.13 ± 0.97) |
| Tsuchiya et al. [21] | 2007 | case-control study | Urinary levels of Genistein/Daidzein, NR | 20–45 Stage I–II: 32.3 ± 3.2 Stage III–IV: 32.6 ± 3.7 | 79 (stage I–II n = 31; stage III–IV n = 48) | 59 | - inversely associated with stage III-IV with aOR 0.21 (95% CI = 0.06–0.76) for Genistein and 0.29 (0.08–1.03) for Daidzein levels - ER-2 RsaI R/r + R/R genotype more frequent than the r/r genotype in advanced stages |
| Maia et al. [31] | 2012 | retrospective study | Resveratrol 30 mg for 2–6 months | 24–40 31 ± 4 | OC+ resveratrol (26) | OC (16) | - reduction in pain scores, with 82% of patients reporting complete resolution of dysmenorrhea and pelvic pain after 2 months - lower COX-2 expression in eutopic endometrium at immunohistochemistry - lower aromatase expression in eutopic endometrium at immunohistochemistry |
| Mendes da Silva et al. [49] | 2017 | randomized clinical trial | Resveratrol 40 mg for 42 days | 20–50 35.4 ± 7.1 | 22 | Placebo (22) | - no difference in pain scores between groups [median difference: 0.75, 95% confidence interval: −1.6 to 2.3] |
| Signorile et al. [56] | 2018 | prospective cohort study | Quercetin 200 mg, titrated Turmeric 20 mg, titrated Parthenium 19.5 mg for three months | 34 ± NR | Group I (30 patients treated with all the ingredients); Group II (30 patients treated with only linseed oil and 5 MTHF calcium salt) | Group III, placebo (30) | - significant reduction of headache (from 14% to 4%), cystitis (from 12% to 2%), muscles ache (from 4% to 1%), irritable colon (from 15% to 6%), dysmenorrhea (from 62% to 18%) and dyspareunia (from 30% to 15%), CPP (from 62% to 18%) - reduction of serum PGE2 level |
| Kodarahmian et al. [65] | 2019 | placebo-controlled, parallel, randomized double-blind exploratory clinical trial | ResveratroL 400 mg for 12–14 weeks | 18–37 30.19 ± 2.4 | 17 | Placebo (17) | - reduced MMP-2 and MMP-9 mRNA and protein levels in eutopic endometrium - reduced level of MMP-2 and MMP-9 in endometrial fluid and serum |
| Youseflu et al. [77] | 2020 | case-control study on dietary data | Isoflavones, lignans, coumestrol, in one year | 15–45 yo 31.01 ± 6.56 | 78 | 78 | - reduced risk of endometriosis for Isoflavones [OR 0.38 (0.33–0.83)], Lignan [OR 0.49 (0.46–0.52)], and Coumestrol [OR 0.38 (0.15–0.96)] assumption |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartiromo, L.; Schimberni, M.; Villanacci, R.; Ottolina, J.; Dolci, C.; Salmeri, N.; Viganò, P.; Candiani, M. Endometriosis and Phytoestrogens: Friends or Foes? A Systematic Review. Nutrients 2021, 13, 2532. https://doi.org/10.3390/nu13082532
Bartiromo L, Schimberni M, Villanacci R, Ottolina J, Dolci C, Salmeri N, Viganò P, Candiani M. Endometriosis and Phytoestrogens: Friends or Foes? A Systematic Review. Nutrients. 2021; 13(8):2532. https://doi.org/10.3390/nu13082532
Chicago/Turabian StyleBartiromo, Ludovica, Matteo Schimberni, Roberta Villanacci, Jessica Ottolina, Carolina Dolci, Noemi Salmeri, Paola Viganò, and Massimo Candiani. 2021. "Endometriosis and Phytoestrogens: Friends or Foes? A Systematic Review" Nutrients 13, no. 8: 2532. https://doi.org/10.3390/nu13082532
APA StyleBartiromo, L., Schimberni, M., Villanacci, R., Ottolina, J., Dolci, C., Salmeri, N., Viganò, P., & Candiani, M. (2021). Endometriosis and Phytoestrogens: Friends or Foes? A Systematic Review. Nutrients, 13(8), 2532. https://doi.org/10.3390/nu13082532