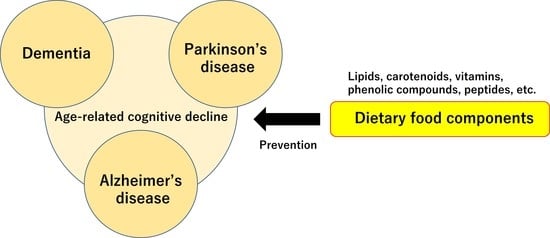
Effects of Dietary Food Components on Cognitive Functions in Older Adults
Abstract
:1. Introduction
2. Method
3. Biological Reactions Associated with Aging
3.1. Reactive Oxygen Species, Reactive Nitrogen Species and Free Radical Theory
3.2. Lipid Peroxidation
4. Role of Functional Food Compounds in Cognitive Performance
4.1. Major Phospholipids
4.2. Plasmalogens
4.3. ω3. Fatty Acids
4.4. Cholesterols
4.5. Carotenoids
4.6. Vitamins
4.7. Phenolic Compounds
4.8. Amino acids, Peptides, and Proteins
4.9. Others
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2017—Highlights (ST/ESA/SER.A/408). Available online: http://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Highlights.pdf (accessed on 28 September 2018).
- Cahill, S. WHO’s global action plan on the public health response to dementia: Some challenges and opportunities. Aging Ment. Health 2020, 24, 197–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blazer, D.G.; Yaffe, K.; Karlawish, J. Cognitive aging: A report from the institute of medicine. J. Am. Med. Assoc. 2015, 313, 2121–2122. [Google Scholar] [CrossRef] [PubMed]
- Luppa, M.; Luck, T.; Weyerer, S.; König, H.-H.; Brähler, E.; Riedel-Heller, S.G. Prediction of institutionalization in the elderly. A systematic review. Age Ageing 2010, 39, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Winblad, B.; Amouyel, P.; Andrieu, S.; Ballard, C.; Brayne, C.; Brodaty, H.; Cedazo-Minguez, A.; Dubois, B.; Edvardsson, D.; Feldman, H.; et al. Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol. 2016, 15, 455–532. [Google Scholar] [CrossRef] [Green Version]
- van de Rest, O.; Berendsen, A.A.M.; Haveman-Nies, A.; CPGM de Groot, L. Dietary patterns, cognitive decline, and dementia: A systematic review. Adv. Nutr. 2015, 6, 154–168. [Google Scholar] [CrossRef]
- Erro, R.; Brigo, F.; Tamburin, S.; Zamboni, M.; Antonini, A.; Tinazzi, M. Nutritional habits, risk, and progression of Parkinson disease. J. Neurol. 2018, 265, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Mumme, K.D.; von Hurst, P.R.; Conlon, C.A.; Jones, B.; Haskell-Ramsay, C.F.; Stonehouse, W.; Heath, A.-L.M.; Coad, J.; Beck, K.L. Study protocol: Associations between dietary patterns, cognitive function and metabolic syndrome in older adults—A cross-sectional study. BMC Public Health 2019, 19, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aridi, Y.S.; Walker, J.L.; Wright, O.R.L. The association between the Mediterranean dietary pattern and cognitive health: A systematic review. Nutrients 2017, 9, 674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, V.H.; MacQueen, G.M. Cognitive dysfunction associated with metabolic syndrome. Obes. Rev. 2007, 8, 409–418. [Google Scholar] [CrossRef]
- Guicciardi, M.; Crisafulli, A.; Doneddu, A.; Fadda, D.; Lecis, R. Effects of metabolic syndrome on cognitive performance of adults during exercise. Front. Physiol. 2019, 10, 1845. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.J.; Yu, B.P. Alterations in mitochondrial membrane fluidity by lipid peroxidation products. Free Radic. Biol. Med. 1994, 17, 411–418. [Google Scholar] [CrossRef]
- Sohal, R.S.; Agarwal, S.; Sohal, B.H. Oxidative stress and aging in the Mongolian gerbil (Meriones unguiculatus). Mech. Ageing Dev. 1995, 81, 15–25. [Google Scholar] [CrossRef]
- Roger, T.D.; Shanlin, F.U.; Roland, S.; Michael, J.D. Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 1997, 324, 1–18. [Google Scholar]
- Mecocci, P.; Fanó, G.; Fulle, S.; MacGarvey, U.; Shinobu, L.; Polidori, M.C.; Cherubini, A.; Vecchiet, J.; Senin, U.; Beal, M.F. Age-dependent increases in oxidative damage to DNA, lipids and proteins in human skeletal muscle. Free Radic. Biol. Med. 1999, 26, 303–308. [Google Scholar] [CrossRef]
- Mecocci, P.; MacGarvey, U.; Kaufman, A.E.; Koontz, D.; Shoffner, J.M.; Wallace, D.C.; Beal, M.F. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann. Neurol. 1993, 34, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-H.; Kao, S.-H.; Lee, H.-C. Simultaneous increase of mitochondrial DNA deletions and lipid peroxidation in human aging. Ann. N. Y. Acad. Sci. 1996, 786, 24–43. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Beckman, K.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef] [Green Version]
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.P.; McAndrew, J.; Sellak, H.; White, C.R.; Jo, H.; Freeman, B.A.; Darley-Usmar, V.M. Biological aspects of reactive nitrogen species. Biochem. Biophys. Acta 1999, 1411, 385–400. [Google Scholar] [CrossRef] [Green Version]
- Drew, B.; Leeuwenburgh, C. Aging and the role of reactive nitrogen species. Ann. N. Y. Acad. Sci. 2002, 959, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, L.; Pannaccione, A.; Cataldi, M.; Secondo, A.; Castaldo, P.; Renzo, G.D.; Taglialatela, M. Modulation of ion channels by reactive oxygen and nitrogen species: A pathophysiological role in brain aging? Neurobiol. Aging 2002, 23, 819–834. [Google Scholar] [CrossRef]
- Miyazawa, T. Lipid hydroperoxides in nutrition, health, and diseases. Proc. Jpn. Acad. Ser. B 2021, 97, 161–196. [Google Scholar] [CrossRef]
- Miyazawa, T.; Yasuda, K.; Fujimoto, K.; Kaneda, T. Presence of phosphatidylcholine hydroperoxide in human plasma. J. Biochem. 1988, 103, 744–746. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Eckley, D.M.; Williamson, J.D.; Launer, L.J.; Rifkind, J.M. Do red blood cell-β-amyloid interactions alter oxygen delivery in Alzheimer’s disease? Adv. Exp. Med. Biol. 2008, 614, 29–35. [Google Scholar] [PubMed]
- Skoumalová, A.; Ivica, J.; Šantorová, P.; Topinková, E.; Wilhelm, J. The lipid peroxidation products as possible markers of Alzheimer’s disease in blood. Exp. Gerontol. 2011, 46, 38–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldeiras, I.; Isabel, S.; Teresa, P.M.; Helena, G.M.; Rui, P.; Ana, R.; Diana, D.; Resende, O.C. Peripheral oxidative damage in mild cognitive impairment and mild Alzheimer’s disease. J. Alzheimer’s Dis. 2008, 15, 117–128. [Google Scholar] [CrossRef]
- Bermejo, P.; Martín-Aragón, S.; Benedí, J.; Susín, C.; Felici, E.; Gil, P.; Ribera, J.M.; Villar, A.M. Peripheral levels of glutathione and protein oxidation as makers in the development of Alzheimer’s disease from mild cognitive impairment. Free Radic. Res. 2008, 42, 162–170. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Liang, H.-J.; Chow, S.-Y.; Chen, S.-M.; Liu, D.-Z. Hemorheological mechanisms in Alzheimer’s disease. Microcirculation 2007, 14, 627–634. [Google Scholar] [CrossRef]
- Kawamoto, E.M.; Munhoz, C.D.; Glezer, I.; Bahia, V.S.; Caramelli, P.; Nitrini, R.; Gorjāo, R.; Curi, R.; Scavone, C.; Marcourakis, T. Oxidative state in platelets and erythrocytes in aging and Alzheimer’s disease. Neurobiol. Aging 2005, 26, 857–864. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kiko, T.; Kuriwada, S.; Miyazawa, T.; Kimura, F.; Miyazawa, T. Amyloid β induces adhesion of erythrocytes to endothelial cells and affects endothelial viability and functionality. Biosci. Biotehonol. Biochem. 2011, 75, 2030–2033. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, S.; Kiko, T.; Fujiwara, H.; Hashimoto, M.; Nakagawa, K.; Kinoshita, M.; Furukawa, K.; Arai, H.; Miyazawa, T. Alterations in the levels of Amyloid-β, phospholipid hydroperoxide, and plasmalogen in the blood of patients with Alzheimer’s disease: Possible interactions between amyloid-β and these lipids. J. Alzheimer’s Dis. 2016, 50, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Suzuki, T.; Yasuda, K.; Fujimoto, K.; Meguro, K.; Sasaki, H. Accumulation of phospholipid hydroperoxides in red blood cell membranes in Alzheimer disease. In Oxygen Radicals; Elsevier Science Publisher: Amsterdam, The Netherlands, 1992; pp. 327–330. [Google Scholar]
- Kiko, T.; Nakagawa, K.; Tsuduki, T.; Suzuki, T.; Arai, H.; Miyazawa, T. Significance of lutein in red blood cells of Alzheimer’s disease patients. J. Alzheimer’s Dis. 2011, 28, 593–600. [Google Scholar] [CrossRef]
- Miyazawa, T. Determination of phospholipid hydroperoxides in human blood plasma by a chemiluminescence-HPLC assay. Free Rad. Biol. Med. 1989, 7, 209–218. [Google Scholar] [CrossRef]
- Miyazawa, T.; Yasuda, K.; Fujimoto, K. Chemiluminescence-high performance liquid chromatography of phosphatidylcholine hydroperoxide. Anal. Lett. 1987, 20, 915–925. [Google Scholar] [CrossRef]
- Seitz, W.R. Chemiluminescence detection of enzymatically generated peroxide. In Bioluminescence and Chemiluminescence; Academic press: New York, NY, USA, 1978; pp. 445–462. [Google Scholar]
- Hoshino, H.; Hinze, W.L. Exploitation of reversed micelles as a medium in analytical chemiluminescence measurements with application to the determination of hydrogen peroxide using luminol. Anal. Chem. 1987, 50, 496–504. [Google Scholar] [CrossRef]
- Miyazawa, T.; Fujimoto, K.; Kaneda, T. Detection of picomole levels in lipid hydroperoxides by a chemiluminescence assay. Agric. Biol. Chem. 1987, 51, 2569–2573. [Google Scholar]
- White, E.H.; Bursey, M.M. Chemiluminescence of luminol and related hydrazides: The light emission step. J. Am. Chem. Soc. 1964, 86, 941–942. [Google Scholar] [CrossRef]
- Yue, L.; Liu, Y.-J. Two conical intersections control luminol chemiluminescence. J. Chem. Theory Comput. 2019, 15, 1798–1805. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Nakagawa, K.; Takekoshi, H.; Higuchi, O.; Kato, S.; Kondo, M.; Kimura, F.; Miyazawa, T. Ingestion of chlorella reduced the oxidation of erythrocyte membrane lipids in senior Japanese subjects. J. Oleo Sci. 2013, 62, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Burdeos, G.C.; Nakagawa, K.; Kimura, F.; Miyazawa, T. Tocotrienol attenuates triglyceride accumulation in HepG2 cells and F344 rats. Lipids 2012, 47, 471–481. [Google Scholar] [CrossRef]
- Vitalakumar, D.; Sharma, A.; Flora, S.J.S. Ferroptosis: A potential therapeutic target for neurodegenerative diseases. J. Biochem. Mol. Toxicol. 2021, e22830. [Google Scholar]
- Langelier, B.; Linard, A.; Bordat, C.; Lavialle, M.; Heberde, C. Long chain-polyunsaturated fatty acids modulate membrane phospholipid composition and protein localization in lipid rafts of neural stem cell cultures. J. Cell Biochem. 2010, 110, 1356–1364. [Google Scholar] [CrossRef]
- Castro-Gómez, P.; Garcia-Serrano, A.; Visioli, F.; Fontecha, J. Relevance of dietary glycerophospholipids and sphingolipids to human health. Prostaglandins Leukot. Essent. Fatty Acids 2015, 101, 41–51. [Google Scholar] [CrossRef]
- Wehrmüller, K. Impact of dietary phospholipids on human health. APL Sci. 2008, 524, 1–15. [Google Scholar]
- Arouri, A.; Mouritsen, O.G. Membrane-perturbing effect of fatty acids and lysolipids. Prog. Lipid Res. 2013, 52, 130–140. [Google Scholar] [CrossRef]
- Chang, H.-C.; Chang, C.-D.; Chen, P.-H.; Chang, C.-J.; Liu, S.-H.; Chen, C.-C. Docosahexaenoic acid and phosphatidylserine improves the antioxidant activities in vitro and in vivo and cognitive functions of the developing brain. Food Chem. 2013, 138, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Meck, W.H.; Williams, C.L. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Dev. Brain Res. 1999, 118, 51–59. [Google Scholar] [CrossRef]
- Cheatham, C.L.; Goldman, B.D.; Fischer, L.M.; da Costa, K.-M.; Reznick, J.S.; Zeisel, S.H. Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2012, 96, 1465–1472. [Google Scholar] [CrossRef]
- Ross, R.G.; Hunter, S.K.; Hoffman, C.; McCarthy, L.; Chambers, B.M.; Law, A.J.; Leonard, S.; Zerbe, G.O.; Freedman, R. Perinatal phosphatidylcholine supplementation and early childhood behavior problems evidence for CHRNA7 moderation. Am. J. Psychiatry 2015, 173, 509–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koval, M.; Pagano, R.E. Intracellular transport and metabolism of sphingomyelin. Biochim. Biophys. Acta 1991, 1082, 113–125. [Google Scholar] [CrossRef]
- Cutler, R.G.; Mattson, M.P. Sphingomyelin and ceramide as regulators of development and lifespan. Mech. Ageing Dev. 2001, 122, 895–908. [Google Scholar] [CrossRef]
- Don, A.S.; Hsiao, J.-H.T.; Bleasel, J.M.; Couttas, T.A.; Halliday, G.M.; Kim, W.S. Altered lipid levels provide evidence for myelin dysfunction in multiple system atrophy. Acta Neuropathol. Commun. 2014, 2, 150. [Google Scholar] [CrossRef]
- Ledesma, M.D.; Brügger, B.; Bünning, C.; Wieland, F.T.; Dotti, C.G. Maturation of the axonal plasma membrane requires upregulation of sphingomyelin synthesis and formation of protein-lipid complexes. EMBO J. 1999, 18, 1761–1771. [Google Scholar] [CrossRef] [Green Version]
- Bienias, K.; Fiedorowicz, A.; Sadowska, A.; Prokopiuk, S.; Car, H. Regulation of sphingomyelin metabolism. Pharmacol. Rep. 2016, 68, 570–581. [Google Scholar] [CrossRef]
- Giusto, N.M.; Roque, M.E.; Ilincheta de Boschero, M.G. Effects of aging on the content, composition and synthesis of sphingomyelin in the central nervous system. Lipids 1992, 27, 835–839. [Google Scholar] [CrossRef]
- Ilincheta de Boschero, M.G.; Roque, M.E.; Giusto, N.M. Alternative pathways for phospholipid synthesis in different brain areas during aging. Exp. Gero. 2000, 35, 653–668. [Google Scholar] [CrossRef]
- Kumar, V.B.; Vyas, K.; Buddhiraju, M.; Alshaher, M.; Flood, J.F.; Morley, J.E. Changes in membrane fatty acids and delta-9 desaturase in senescence accelerated (SAMP8) mouse hippocampus with aging. Life Sci. 1999, 65, 1657–1662. [Google Scholar] [CrossRef]
- Gaiti, A.; Brunetti, M.; Piccinin, G.L.; Woelk, H.; Porcellati, G. The synthesis In Vivo of choline and ethanolamine phosphoglycerides in different brain areas during aging. Lipids 1982, 17, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Gaiti, A.; Sitkievicz, D.; Brunetti, M.; Porcellati, G. Phospholipid metabolism in neuronal and glial cells during aging. Neurochem. Res. 1981, 9, 1549–1558. [Google Scholar] [CrossRef]
- Babenko, N.A.; Semenova, Y.A. Effects of exogenous phosphatidylserine on cognitive functions and phospholipid metabolism in the hippocampus of aged rats. Neurosci. Behav. Physiol. 2011, 41, 97–101. [Google Scholar] [CrossRef]
- Kingsley, M. Effects of phosphatidylserine supplementation on exercising humans. Sports Med. 2006, 36, 657–669. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, M.A.; Maier, S.F.; Einstein, G.O. Brain-specific nutrients: A memory cure? Nutrition 2003, 19, 957–975. [Google Scholar] [CrossRef]
- Pepeu, G.; Pepeu, I.M.; Amaducci, L. A review of phosphatidylserine pharmacological and clinical effects. Is phosphatidylserine a drug for the ageing brain? Pharmacol. Res. 1996, 33, 73–80. [Google Scholar] [CrossRef]
- Nunzi, M.G.; Milan, F.; Guidolin, D.; Toffano, G. Dendritic spine loss in hippocampus of aged rats. Effect of brain phosphatidylserine administration. Neurobiol. Aging 1987, 8, 501–510. [Google Scholar] [CrossRef]
- Wheeler, K.P.; Whittam, R. ATPase activity of the sodium pump needs phosphatidylserine. Nature 1970, 225, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Casamenti, F.; Scali, C.; Pepeu, G. Phosphatidylserine reverses the age-dependent decrease in cortical acetylcholine release: A microdialysis study. Eur. J. Pharmacol. 1991, 194, 11–16. [Google Scholar] [CrossRef]
- Cohen, S.; Müller, W.E. Age-related alterations of NMDA-receptor properties in the mouse forebrain: Partial restoration by chronic phosphatidylserine treatment. Brain Res. 1992, 194, 174–180. [Google Scholar] [CrossRef]
- Suzuki, S.; Yamatoya, H.; Sakai, M.; Kataoka, A.; Furushiro, M.; Kudo, S. Oral administration of soybean lecithin transphosphatidylated phosphatidylserine improves memory impairment in aged rats. J. Nutr. 2001, 131, 2951–2956. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Fleith, M.; Giuffrida, F.; O’Neill, B.V.; Schneider, N. Dietary polar lipids and cognitive development: A narrative review. Adv. Nutr. 2019, 10, 1163–1176. [Google Scholar] [CrossRef]
- Schverer, M.; O’Mahony, S.M.; O’Riordan, K.J.; Donoso, F.; Roy, B.L.; Stanton, C.; Dinan, T.G.; Schellekens, H.; Cryan, J.F. Dietary phospholipids: Role in cognitive processes across the lifespan. Neurosci. Biobehav. Rev. 2020, 111, 183–193. [Google Scholar] [CrossRef]
- Choi, J.; Yin, T.; Shinozaki, K.; Lampe, J.W.; Stevens, J.F.; Becker, L.B.; Kim, J. Comprehensive analysis of phospholipids in the brain, heart, kidney, and liver: Brain phospholipids are least enriched with polyunsaturated fatty acids. Mol. Cell Biochem. 2018, 442, 187–201. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, H.; Guan, W.; Liu, J.; Brennan, C.; Kulasiri, D.; Mohan, M.S. Vesicle properties and health benefits of milk phospholipids: A review. J. Food Bioact. 2019, 5, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Wallace, T.L.; Ballard, T.M.; Glavis-Bloom, C. Animal paradigms to assess cognition with translation to humans. Cogn. Enhanc. 2015, 228, 27–57. [Google Scholar]
- Guan, J.; MacGibbon, A.; Zhang, R.; Elliffe, D.M.; Moon, S.; Liu, D.-X. Supplementation of complex milk lipid concentrate (CMLc) improved the memory of aged rats. Nutr. Neurosci. 2015, 18, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sur, B.-J.; Han, J.-J.; Shim, I.; Her, S.; Lee, H.-J.; Hahm, D.-H. Krill phosphatidylserine improves learning and memory in Morris water maze in aged rats. Prog. Neuropsychophamacol. Biol. Psychiatry 2010, 34, 1085–1093. [Google Scholar] [CrossRef]
- Cenacchi, T.; Bertoldin, T.; Farina, C.; Fiori, M.G.; Crepaldi, G.; Azzini, C.F.; Girardello, R.; Bagozzi, B.; Garuti, R.; Vivaldi, P.; et al. Cognitive decline in the elderly: A double-blind, placebo-controlled multicenter study on efficacy of phosphatidylserine administration. Aging Clin. Exp. Res. 1993, 5, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Engel, R.R.; Satzger, W.; Günther, W.; Kathmann, N.; Bove, D.; Gerke, S.; Münch, U.; Hippius, H. Double-blind cross-over study of phosphatidylserine vs. placebo in patients with early dementia of the Alzheimer type. Eur. Neuropsychopharmacol. 1992, 2, 149–155. [Google Scholar] [CrossRef]
- Kanno, T.; Jin, Y.; Nishizaki, T. DL-/PO-phosphatidylcholine restores restraint stress-induced depression-related behaviors and spatial memory impairment. Behav. Pharmacol. 2014, 25, 575–581. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Neufeld, K.-A.M.; Waworuntu, R.V.; Pusceddu, M.M.; Manurung, S.; Murphy, K.; Strain, C.; Laguna, M.C.; Peterson, V.L.; Stanton, C.; et al. The enduring effects of early-life stress on the microbiota-gut-brain axis are buffered by dietary supplementation with milk fat globule membrane and a prebiotic blend. Eur. J. Neurosci. 2019, 4, 1042–1058. [Google Scholar] [CrossRef]
- Boyle, N.B.; Dye, L.; Arkbåge, K.; Thorell, L.; Frederiksen, P.; Croden, F.; Lawton, C. Effects of milk-based phospholipids on cognitive performance and subjective responses to psychosocial stress: A randomized, double-blind, placebo-controlled trial in high-perfectionist men. Nutrition 2019, 57, 183–193. [Google Scholar] [CrossRef]
- Miller, A.L.; Pitts, F.N., Jr. Brain succinate semialdehyde dehydrogenase.—III. Activities in twenty-four regions of human brain. J. Neurochem. 1967, 14, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.A.; Lake, A.C.; Nagan, N.; Gaposchkin, D.P.; Legner, M.A.; Lieberthal, W. Plasmalogens as endogenous antioxidants: Somatic cell mutants reveal the importance of the vinyl ether. Biochem. J. 1999, 338, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.M.; Yang, K.; Liu, G.; Moon, S.H.; Dilthey, B.G.; Gross, R.W. Cytochrome c is an oxidative stress-activated plasmalogenase that cleaves plasmenylcholine and plasmenylethanolamine at the sn-1 vinyl ether linkage. J. Biol. Chem. 2018, 293, 8693–8709. [Google Scholar] [CrossRef] [Green Version]
- Ginsberg, L.; Rafique, S.; Xuereb, J.H.; Rapoport, S.I.; Gershfeld, N.L. Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer’s disease brain. Brain Res. 1995, 698, 223–226. [Google Scholar] [CrossRef]
- Wells, K.; Farooqui, A.A.; Liss, L.; Horrocks, L.A. Neural membrane phospholipids in Alzheimer disease. Neurochem. Res. 1999, 20, 1329–1333. [Google Scholar] [CrossRef]
- Guan, Z.; Wang, Y.; Cairns, N.J.; Lantos, P.L.; Dallner, G.; Sindelar, P.J. Decrease and structural modifications of phosphatidylethanolamine plasmalogen in the brain with Alzheimer disease. J. Neuropathol. Exp. Neurol. 1999, 58, 740–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, S.; Honjo, A.; Aruga, M.; Nakagawa, K.; Miyazawa, T. Preparation of marine plasmalogen and selective identification of molecular species by LC-MS/MS. J. Oleo Sci. 2014, 63, 423–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, S.; Abe, A.; Nakagawa, K.; Kinoshita, M.; Miyazawa, T. Separation and detection of plasmalogen in marine invertebrates by high-performance liquid chromatography with evaporative light-scattering detection. Lipids 2014, 49, 1261–1273. [Google Scholar] [CrossRef]
- Yamashita, S.; Kanno, S.; Honjo, A.; Otoki, Y.; Nakagawa, K.; Kinoshita, M.; Miyazawa, T. Analysis of plasmalogen species in foodstuffs. Lipids 2016, 51, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Hossain, M.S.; Mawatari, S. Therapeutic efficacy of plasmalogens for Alzheimer’s disease, mild cognitive impairment, and Parkinson’s disease in conjunction with a new hypothesis for the etiology of Alzheimer’s disease. Adv. Exp. Med. Biol. 2020, 1299, 195–212. [Google Scholar]
- Hara, H.; Wakisaka, T.; Aoyama, Y. Lymphatic absorption of plasmalogen in rats. Br. J. Nutr. 2003, 90, 29–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimukai, M.; Wakisaka, T.; Hara, H. Ingestion of plasmalogen markedly increased plasmalogen levels of blood plasma in rats. Lipids 2003, 38, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C.; Michael-Titus, A.T. Neurological benefits of omega-3 fatty acids. Neuromolecular Med. 2008, 10, 219–235. [Google Scholar] [CrossRef]
- Ryan, A.S.; Astwood, J.D.; Gautier, S.; Kuratko, C.N.; Nelson, E.B.; Salem, N., Jr. Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: A review of human studies. Prostaglandins Leukot. Essent. Fatty Acids 2010, 82, 305–314. [Google Scholar] [CrossRef]
- Nilsson, A.; Radeborg, K.; Salo, I.; Björck, I. Effect of supplementation with n-3 polyunsaturated fatty acids on cognitive performance and cardiometabolic risk markers in healthy 51 to 72 years old subjects: A randomized controlled cross-over study. Nutr. J. 2012, 11, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conquer, J.A.; Tierney, M.C.; Zecevic, J.; Bettger, W.J.; Fisher, R.H. Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia and cognitive impairment. Lipids 2000, 35, 1305–1312. [Google Scholar] [CrossRef]
- Kalmijn, S.; van Boxtel, P.J.; Ocké, M.; Verschuren, W.M.M.; Kromhout, D.; Launer, L.J. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology 2004, 62, 275–280. [Google Scholar] [CrossRef]
- Heude, B.; Ducimetiére, P.; Berr, C. Cognitive decline and fatty acid composition of erythrocyte membranes—The EVA study. Am. J. Clin. Nutr. 2003, 77, 803–808. [Google Scholar] [CrossRef] [Green Version]
- Kalmijn, S.; Feskens, E.J.M.; Launer, L.J.; Kromhout, D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am. J. Epidemiol. 1997, 145, 33–41. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Kaufman, J.S.; Satia, J.A.; Rosamond, W.; Folsom, A.R. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: The atherosclerosis risk in communities study. Am. J. Clin. Nutr. 2007, 85, 1103–1111. [Google Scholar] [CrossRef] [Green Version]
- Dullemeijer, C.; Durga, J.; Brouwer, I.A.; van de Rest, O.; Kok, F.J.; Brummer, R.-J.M.; van Boxtel, M.P.J.; Verhoef, P. n-3 fatty acid proportions in plasma and cognitive performance in older adults. Am. J. Clin. Nutr. 2007, 86, 1479–1485. [Google Scholar] [CrossRef] [Green Version]
- Dangour, A.D.; Allen, E.; Elbourne, D.; Fletcher, A.; Richards, M.; Uauy, R. Fish consumption and cognitive function among older people in the UK: Baseline data from the OPAL study. J. Nutr. Health Aging 2009, 13, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S. Fish consumption and cognitive decline with age in a large community study. Arch. Neurol. 2005, 62, 1849–1853. [Google Scholar] [CrossRef] [Green Version]
- van Gelder, B.M.; Tijhuis, M.; Kalmijn, S.; Kromhout, D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: The Zutphen Elderly Study. Am. J. Clin. Nutr. 2007, 85, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, M.H.; Ngandu, T.; Helkala, E.-L.; Tuomilehto, J.; Nissinen, A.; Soininen, H.; Kivipelto, M. Fat intake at midlife and cognitive impairment later in life: A population-based CAIDE study. Int. J. Geriatr. Psychiatry 2008, 23, 741–747. [Google Scholar] [CrossRef]
- Nurk, E.; Drevon, C.A.; Refsum, H.; Solvoll, K.; Vollset, S.E.; Nygård, O.; Nygaard, H.A.; Engedal, K.; Tell, G.S.; Smith, A.D. Cognitive performance among the elderly and dietary fish intake: The Hordaland health study. Am. J. Clin. Nutr. 2007, 86, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease, Control, and Prevention. National Health and Nutrition Examination Survey. Survey Methods and Analytic Guidelines. Available online: https://www.cdc.gov/nchs/nhanes/index (accessed on 15 January 2020).
- Centers for Disease, Control, and Prevention. National Health and Nutrition Examination Survey. Questionnaires, Datasets, and Related Documentation. Available online: https://www.cdc.gov/nchs (accessed on 15 January 2020).
- Dong, X.; Li, S.; Chen, J.; Li, Y.; Wu, Y.; Zhang, D. Association of dietary ω-3 and ω-6 fatty acids intake with cognitive performance in older adults: National health and nutrition examination survey (NHANES) 2011–2014. Nutr. J. 2020, 19, 25. [Google Scholar] [CrossRef] [Green Version]
- Kivipelto, M.; Helkala, E.-L.; Laakso, M.P.; Hänninen, T.; Hallikainen, M.; Alhainen, K.; Iivonen, S.; Mannermaa, A.; Tuomilehto, J.; Nissinen, A.; et al. Apolipoprotein E ε4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann. Intern. Med. 2002, 137, 149–155. [Google Scholar] [CrossRef]
- Notkola, I.L.; Sulkava, R.; Pekkanen, J.; Erkinjuntti, T.; Ehnholm, C.; Kivinen, P.; Tuomilehto, J.; Nissinen, A. Serum total cholesterol, apolipoprotein E ε4 allele, and Alzheimer’s disease. Neuroepidemiology 1998, 17, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Kivipelto, M.; Wolozin, B.; Whitmer, R.A. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement. Geriatr. Cogn. Disord. 2009, 28, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitmer, R.A.; Sidney, S.; Selby, J.; Johnston, S.C.; Yaffe, K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005, 64, 277–281. [Google Scholar] [CrossRef]
- Mielke, M.M.; Zandi, P.P.; Shao, H.; Waern, M.; Östling, S.; Guo, X.; Björkelund, C.; Lissner, L.; Skoog, I.; Gustafson, D.R. The 32-year relationship between cholesterol and dementia from midlife to late life. Neurology 2010, 75, 1888–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalmijn, S.; Foley, D.; White, L.; Burchfiel, C.M.; Curb, J.D.; Petrovitch, H.; Ross, G.W.; Havlik, R.J.; Launer, L.J. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2255–2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, R.; White, L.R.; Xue, Q.-L.; Launer, L.J. Twenty-six-year change in total cholesterol levels and incident dementia: The Honolulu-Asia aging study. Arch. Neurol. 2007, 64, 103–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Z.S.; Seshadri, S.; Beiser, A. Plasma total cholesterol level as a risk factor for Alzheimer disease: The framingham study. Arch. Intern. Med. 2003, 163, 1053–1057. [Google Scholar] [CrossRef] [Green Version]
- Anstey, K.J.; Lipnicki, D.M.; Low, L.-F. Cholesterol as a risk factor for dementia and cognitive decline: A systematic review of prospective studies with meta-analysis. Am. J. Geriatr. Psychiatry 2008, 16, 343–354. [Google Scholar] [CrossRef]
- Mielke, M.M.; Zandi, P.P.; Sjögren, M.; Gustafson, D.; Östling, S.; Steen, B.; Skoog, I. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology 2005, 64, 1689–1695. [Google Scholar] [CrossRef]
- Reitz, C.; Tang, M.-X.; Luchsinger, J.; Mayeux, R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch. Neurol. 2004, 61, 705–714. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Jin, Y.; Unverzagt, F.W.; Su, L.; Yang, L.; Ma, F.; Hake, A.M.; Kettler, C.; Chen, C.; Liu, J.; et al. The relationship between cholesterol and cognitive function is homocysteine-dependent. Clin. Interv. Aging 2014, 9, 1823–1829. [Google Scholar]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Fukushima, W.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Dietary fat intake and risk of Parkinson’s disease: A case-control study in Japan. J. Neurol. Sci. 2010, 288, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Kamanna, V.S.; Kashyap, M.L. Mechanism of action of niacin. Am. J. Cardiol. 2008, 101, S20–S26. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Lee, C.-w.; Nam, M.-J.; Kim, H.; Kwon, D.-Y.; Yoo, J.-W.; Lee, K.N.; Han, K.; Jung, J.-H.; Park, Y.-G.; et al. Association of high-density lipoprotein cholesterol variability and the risk of developing Parkinson disease. Neurology 2021, 96. [Google Scholar]
- Cutler, R.G. Carotenoids and retinol: Their possible importance in determining longevity of primate species. Proc. Natl. Acad. Sci. USA 1984, 81, 7627–7631. [Google Scholar] [CrossRef] [Green Version]
- Akbaraly, N.T.; Faure, H.; Gourlet, V.; Favier, A.; Berr, C. Plasma carotenoid levels and cognitive performance in an elderly population: Results of the EVA study. J. Gerontol. 2007, 62A, 308–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, W.; Sies, H. Lycopene: A biologically important carotenoid for humans? Arch. Biochem. Biophys. 1996, 336, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Paiva, S.A.; Russell, R.M. β-carotene and other carotenoids as antioxidants. J. Am. Coll. Nutr. 1999, 18, 426–433. [Google Scholar] [CrossRef]
- Berr, C.; Balansard, B.; Arnaud, J.; Roussel, A.-M.; Alpérovitch, A. Cognitive decline is associated with systemic oxidative stress: The EVA study. Etude du Vieillissement Arteriel. J. Am. Geriatr. Soc. 2000, 48, 1285–1291. [Google Scholar] [CrossRef]
- Gray, S.L.; Hanlon, J.T.; Landerman, L.R.; Artz, M.; Schmader, K.E.; Fillenbaum, G.G. Is antioxidant use protective of cognitive function in the community-dwelling elderly? Am. J. Geriatr. Pharmacother. 2003, 1, 3–10. [Google Scholar] [CrossRef]
- Jama, J.W.; Launer, L.J.; Witteman, J.C.M.; den Breeijen, J.H.; Breteler, M.M.B.; Grobbee, D.E.; Hofman, A. Dietary antioxidants and cognitive function in a population-based sample of older persons. The Rotterdam study. Am. J. Epidemiol. 1996, 144, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Wilson, R.S. Vitamin E and cognitive decline in older persons. Arch. Neurol. 2002, 59, 1125–1132. [Google Scholar] [CrossRef]
- Perrig, W.J.; Perrig, P.; Stähelin, H.B. The relation between antioxidants and memory performance in the old and very old. J. Am. Geriatr. Soc. 1997, 45, 718–724. [Google Scholar] [CrossRef]
- Smith, A.; Clark, R.; Nutt, D.; Haller, J.; Hayward, S.; Perry, K. Anti-oxidant vitamins and mental performance of the elderly. Hum. Psychopharmacol. 1999, 14, 459–471. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group Impact of antioxidants, zinc, and copper on cognition in the elderly: A randomized, controlled trial. Neurology 2004, 63, 1705–1707. [CrossRef] [PubMed] [Green Version]
- Stahl, W.; Junghans, A.; de Boer, B.; Drionina, E.S.; Briviba, K.; Sies, H. Carotenoid mixtures protect multilamellar liposomes against oxidative damage: Synergistic effects of lycopene and lutein. FEBS Lett. 1998, 427, 305–308. [Google Scholar] [CrossRef] [Green Version]
- Woodall, A.A.; Britton, G.; Jackson, M.J. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: Relationship between carotenoid structure and protective ability. Biochim. Biophys. Acta. 1997, 1336, 575–586. [Google Scholar] [CrossRef]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef]
- Johnson, E.J. A possible role for lutein and zeaxanthin in cognitive function in the elderly. Am. J. Clin. Nutr. 2012, 96, 1161S–1165S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Sookwong, P.; Tsuduki, T.; Satoh, A.; Miyazawa, T. Amyloid β-induced erythrocytic damage and its attenuation by carotenoids. FEBS Lett. 2011, 585, 1249–1254. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Burdeos, G.C.; Kimura, F.; Satoh, A.; Miyazawa, T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011, 105, 1563–1571. [Google Scholar] [CrossRef] [Green Version]
- Sevilla, J.M.; Fernández, F.G.; Grima, E.M. Biotechnological production of lutein and its application. Appl. Microbiol. Biotech. 2010, 86, 27–40. [Google Scholar] [CrossRef]
- Shi, X.-M.; Zhang, X.W.; Chen, F. Heterotrophic production of biomass and lutein by chlorella protothecoides on various nitrogen sources. Enzym. Microb. Technol. 2000, 27, 312–318. [Google Scholar] [CrossRef]
- Miyazawa, T.; Nakagawa, K.; Kumura, F.; Nakashima, Y.; Maruyama, I.; Higuchi, O.; Miyazawa, T. Chlorella is an effective dietary source of lutein for human erythrocytes. J. Oleo Sci. 2013, 62, 773–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mecocci, P.; Polidori, M.C.; Troiano, L.; Cherubini, A.; Cecchetti, R.; Pini, G.; Straatman, M.; Monti, D.; Stahl, W.; Sies, H.; et al. Plasma antioxidants and longevity: A study on healthy centenarians. Free Radic. Biol. Med. 2000, 28, 1243–1248. [Google Scholar] [CrossRef]
- Travica, N.; Ried, K.; Sali, A.; Scholey, A.; Hudson, I.; Pipingas, A. Vitamin C status and cognitive function: A systematic review. Nutrients 2017, 9, 960. [Google Scholar] [CrossRef]
- Rinaldi, P.; Polidori, M.C.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef]
- Rivière, S.; Birlouez-Aragon, I.; Nourhashémi, F.; Vellas, B. Low plasma vitamin C in Alzheimer patients despite an adequate diet. Int. J. Geriat. Psychiatry 1998, 13, 749–754. [Google Scholar] [CrossRef]
- Polidori, M.C.; Patrizia, M. Plasma susceptibility to free radical-induced antioxidant consumption and lipid peroxidation is increased in very old subjects with Alzheimer disease. J. Alzheimer’s Dis. 2002, 4, 517–522. [Google Scholar] [CrossRef]
- Burton, G.W.; Joyce, A.; Ingold, K.U. First proof that vitamin E is major lipid-soluble, chain-breaking antioxidant in human blood plasma. Lancet 1982, 2, 327. [Google Scholar] [CrossRef]
- Burton, G.W.; Joyce, A.; Ingold, K.U. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch. Biochem. Biophys. 1983, 221, 281–290. [Google Scholar] [CrossRef]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory redox interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef]
- Joshi, Y.B.; Pratico, D. Vitamin E in aging, dementia, and Alzheimer’s disease. BioFactors 2012, 38, 90–97. [Google Scholar] [CrossRef]
- Olson, C.R.; Mello, C.V. Significance of vitamin A to brain function, behavior and learning. Mol. Nutr. Food Res. 2010, 54, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Harrison, P.J.; Weinberger, D.R. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol. Phychiatry 2005, 10, 40–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [Green Version]
- Pallet, V.; Touyarot, K. Vitamin A and cognitive processes. Nutr. Healthy Aging 2015, 3, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Polidori, M.C.; Mariani, E.; Baggio, G.; Deiana, L.; Carru, C.; Pes, G.M.; Cecchetti, R.; Franceschi, C.; Senin, U.; Mecocci, P. Different antioxidant profiles in Italian centenarians: The Sardinian peculiarity. Eur. J. Clin. Nutr. 2007, 61, 922–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, L.; Li, J.; Yap, K.-B.; Kua, E.-H.; Ng, T.-P. Vitamin B-12, apolipoprotein E genotype, and cognitive performance in community-living older adults: Evidence of a gene-micronutrient interaction. Am. J. Clin. Nutr. 2009, 89, 1263–1268. [Google Scholar] [CrossRef] [Green Version]
- Riggs, K.M.; Spiro, A., 3rd; Tucker, K.; Rush, D. Relation of vitamin B-12, vitamin B-6, folate and homocysteine to cognitive performance in the normative aging study. Am. J. Clin. Nutr. 1996, 63, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Meehan, M.; Penckofer, S. The role of vitamin D in the aging adult. J. Aging Gerontol. 2014, 2, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Goodwill, A.M.; Szoeke, C.A. systematic review and meta-analysis of the effect of low vitamin D on cognition. J. Am. Geriatr. Soc. 2017, 65, 2161–2168. [Google Scholar] [CrossRef]
- Presse, N.; Belleville, S.; Gaudreau, P.; Greenwood, C.E.; Kergoat, M.-J.; Morais, J.A.; Payette, H.; Shatenstein, B.; Ferland, G. Vitamin K status and cognitive function in healthy older adults. Neurobiol. Aging 2013, 34, 2777–2783. [Google Scholar] [CrossRef]
- Fullard, M.E.; Duda, J.E. A review of the relationship between vitamin D and Parkinson disease symptoms. Front. Neurol. 2020, 11, 454. [Google Scholar] [CrossRef]
- Vauzour, D.; Camprubi-Robles, M.; Miquel-Kergoat, S.; Andres-Lacueva, C.; Bánáti, D.; Barberger-Gateau, P.; Bowman, G.L.; Caberlotto, L.; Clarke, R.; Hogervorst, E.; et al. Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res. Rev. 2017, 35, 222–240. [Google Scholar] [CrossRef] [Green Version]
- Vauzour, D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxid. Med. Cell. Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, A.; Trabelsi, K.; Müller, P.; Bouaziz, B.; Boukhris, O.; Glenn, J.M.; Bott, N.; Driss, T.; Chtourou, H.; Müller, N.; et al. The effect of (poly)phenol-rich interventions on cognitive functions and neuroprotective measures in healthy aging adults: A systematic review and meta-analysis. J. Clin. Med. 2020, 9, 835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamport, D.J.; Dye, L.; Wightman, J.D.; Lawton, C.L. The effects of flavonoid and other polyphenol consumption on cognitive performance: A systematic research review of human experimental and epidemiological studies. Nutr. Aging 2012, 1, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozinoe, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The cocoa, cognition, and aging (cocoa) study-a randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef]
- Whyte, A.R.; Cheng, N.; Fromentin, E.; Williams, C.M. A randomized, double-blinded, placebo-controlled study to compare the safety and efficacy of low dose enhanced wild blueberry powder and wild blueberry extract (thinkblueTM) in maintenance of episodic and working memory in older adults. Nutrients 2018, 10, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wightman, E.L.; Jackson, P.A.; Khan, J.; Forster, J.; Heiner, F.; Feistel, B.; Suarez, C.G.; Pischel, I.; Kennedy, D.O. The acute and chronic cognitive and cerebral blood flow effects of a sidertisscardica (Greek mountain tea) extract: A double blind, randomized, placebo controlled, parallel groups study in healthy humans. Nutrients 2018, 10, 955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witte, A.V.; Kerti, L.; Margulies, D.S.; Flöel, A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef] [Green Version]
- Lamport, D.J.; Pal, D.; Moutsiana, C.; Field, D.T.; Williams, C.M.; Spencer, J.P.E.; Butler, L.T. The effect of flavanol-rich cocoa on cerebral perfusion in healthy older adults during conscious resting state: A placebo controlled, crossover, acute trial. Psychopharmacology 2015, 232, 3227–3234. [Google Scholar] [CrossRef] [Green Version]
- Bowtell, J.L.; Aboo-Bakkar, Z.; Conway, M.E.; Adlam, A.-L.R.; Fulford, J. Enhanced task-related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Appl. Physiol. Nutr. Metab. 2017, 42, 773–779. [Google Scholar] [CrossRef]
- Ho, K.K.; Ferruzzi, M.G.; Wightman, J.D. Potential health benefits of (poly) phenols derived from fruit and 100% fruit juice. Nutr. Rev. 2020, 78, 145–174. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Birru, R.L.; Snitz, B.E.; Ihara, M.; Kakuta, C.; Lopresti, B.J.; Aizenstein, H.J.; Lopez, O.L.; Mathis, C.A.; Miyamoto, Y.; et al. Effects of soy isoflavones on cognitive function: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2020, 78, 134–144. [Google Scholar] [CrossRef]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Leurgans, S.E.; Bennett, D.A.; Booth, S.L.; Morris, M.C. Dietary flavonols and risk of Alzheimer dementia. Neurology 2020, 94. [Google Scholar] [CrossRef]
- Gleason, C.E.; Carlsson, C.M.; Barnet, J.H.; Meade, S.A.; Setchell, K.D.; Atwood, C.S.; Johnson, S.C.; Ries, M.L.; Asthana, S. A preliminary study of the safety, feasibility and cognitive efficacy of soy isoflavone supplements in older men and women. Age Ageing 2009, 38, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Howes, J.B.; Bray, K.; Lorenz, L.; Smerdely, P.; Howes, L.G. The effects of dietary supplementation with isoflavones from red clover on cognitive function in postmenopausal women. Climacteric 2004, 7, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Marsh, C.E.; Carter, H.H.; Guelfi, K.J.; Smith, K.J.; Pike, K.E.; Naylor, L.H.; Green, D.J. Brachial and cerebrovascular functions are enhanced in postmenopausal women after ingestion of chocolate with a high concentration of cocoa. J. Nutr. 2017, 147, 1686–1692. [Google Scholar] [CrossRef]
- Sarker, M.R.; Franks, S.F. Efficacy of curcumin for age-associated cognitive decline: A narrative review of preclinical and clinical studies. GeroScience 2018, 40, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Voulgaropoulou, S.D.; van Amelsvoort, T.A.M.J.; Prickaerts, J.; Vingerhoets, C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: A systematic review of pre-clinical and clinical studies. Brain Res. 2019, 1725, 146476. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.; Lam, C.W.K.; Cheung, S.K.-K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, K.H.M.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015, 29, 642–651. [Google Scholar] [CrossRef]
- Rainey-Smith, S.R.; Brown, B.M.; Sohrabi, H.R.; Shah, T.; Goozee, K.G.; Gupta, V.B.; Martins, R.N. Curcumin and cognition: A randomized, placebo-controlled, double-blind study of community-dwelling older adults. Br. J. Nutr. 2016, 115, 2106–2113. [Google Scholar] [CrossRef]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.-P.; Liu, J.; Merrill, D.A.; et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: A double-blind, placebo-controlled 18-month trial. Am. J. Geriatr. Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef]
- Miyazawa, T.; Nakagawa, K.; Kim, S.H.; Thomas, M.J.; Paul, L.; Zingg, J.-M.; Dolnikowski, G.G.; Roberts, S.B.; Kimura, F.; Miyazawa, T.; et al. Curcumin and piperine supplementation of obese mice under caloric restriction modulates body fat and interleukin-1β. Nutr. Metab. 2018, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Harigae, T.; Nakagawa, K.; Miyazawa, T.; Inoue, N.; Kimura, F.; Ikeda, I.; Miyazawa, T. Metabolic fate of poly-(lactic-co-glycolic acid)-based curcumin nanoparticles following oral administration. Int. J. Nanomed. 2016, 11, 3009–3022. [Google Scholar]
- Shoji, M.; Nakagawa, K.; Watanabe, A.; Tsuduki, T.; Yamada, T.; Kuwahara, S.; Kimura, F.; Miyazawa, T. Comparison of the effects of curcumin and curcumin glucuronide in human hepatocellular carcinoma HepG2 cells. Food Chem. 2014, 151, 126–132. [Google Scholar] [CrossRef]
- Choudhury, A.K.; Raja, S.; Mahapatra, S.; Nagabhushanam, K.; Majeed, M. Synthesis and evaluation of the anti-oxidant capacity of curcumin glucuronides, the major curcumin metabolites. Antioxidants 2015, 4, 750–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Findeis, M.A. The role of amyloid β peptide 42 in Alzheimer’s disease. Pharmacol. Ther. 2007, 116, 266–286. [Google Scholar] [CrossRef]
- Akazawa, N.; Hamasaki, A.; Tanahashi, K.; Kosaki, K.; Yoshikawa, T.; Myoenzono, K.; Maeda, S. Lactotripeptide ingestion increases cerebral blood flow velocity in middle-aged and older adults. Nutr. Res. 2018, 53, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Min, L.-J.; Kobayashi, Y.; Mogi, M.; Tsukuda, K.; Yamada, A.; Yamauchi, K.; Abe, F.; Iwanami, J.; Xiao, J.-Z.; Horiuchi, M. Administration of bovine casein-derived peptide prevents cognitive decline in Alzheimer disease model mice. PLoS ONE 2017, 12, e0171515. [Google Scholar] [CrossRef] [Green Version]
- Yuda, N.; Tanaka, M.; Yamauchi, K.; Abe, F.; Kakiuchi, I.; Kiyosawa, K.; Miyasaka, M.; Sakane, N.; Nakamura, M. Effect of the casein-derived peptide Met-Lys-Pro on cognitive function in community-dwelling adults without dementia: A randomized double-blind, placebo-controlled trial. Clin. Interv. Aging 2020, 15, 743–754. [Google Scholar] [CrossRef]
- Wade, A.M.; Tucker, H.N. Antioxidant characteristics of L-histidine. J. Nutr. Biochem. 1998, 9, 308–315. [Google Scholar] [CrossRef]
- Masuoka, N.; Lei, C.; Li, H.; Hisatsune, T. Influence of imidazole-dipeptides on cognitive status and preservation in elders: A narrative review. Nutrients 2021, 13, 397. [Google Scholar] [CrossRef] [PubMed]
- Fonteh, A.N.; Harrington, R.J.; Tsai, A.; Liao, P.; Harrington, M.G. Free amino acid and dipeptide changes in the body fluids from Alzheimer’s disease subjects. Amino Acids 2007, 32, 213–224. [Google Scholar] [CrossRef]
- Dringen, R.; Gutterer, J.M.; Hirrlinger, J. Glutathione metabolism in brain. Eur. J. Biochem. 2000, 267, 4912–4916. [Google Scholar] [CrossRef]
- Ozawa, H.; Hirayama, A.; Ishikawa, T.; Kudo, R.; Maruyama, M.; Shoji, F.; Doke, T.; Ishimoto, T.; Maruyama, S.; Soga, T.; et al. Comprehensive dipeptide profiling and quantitation by capillary electrophoresis and liquid chromatography coupled with tandem mass spectrometry. Anal. Chem. 2020, 92, 9799–9806. [Google Scholar] [CrossRef]
- Ozawa, H.; Hirayama, A.; Shoji, F.; Maruyama, M.; Suzuki, K.; Yamanaka-Okumura, H.; Tatano, H.; Morine, Y.; Soga, T.; Shimada, M.; et al. Comprehensive dipeptide analysis revealed cancer-specific profile in the liver of patients with hepatocellular carcinoma and hepatitis. Metabolites 2020, 10, 442. [Google Scholar] [CrossRef]
- Glenn, J.M.; Madero, E.N.; Bott, N.T. Dietary protein and amino acid intake: Links to the maintenance of cognitive health. Nutrients 2019, 11, 1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penland, J.G. Dietary boron, brain function, and cognitive performance. Environ. Health Perspect. 1994, 102, 65–72. [Google Scholar] [PubMed] [Green Version]
- Kelly, J.; Fulford, J.; Vanhatalo, A.; Blackwell, J.R.; French, O.; Bailey, S.J.; Gilchrist, M.; Winyard, P.G.; Jones, A.M. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics and muscle and cognitive function in older adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R73–R83. [Google Scholar] [CrossRef] [Green Version]
- Stanaway, L.; Rutherfurd-Markwick, K.; Page, R.; Ali, A. Performance and health benefits of dietary nitrate supplementation in older adults: A systematic review. Nutrients 2017, 9, 1171. [Google Scholar] [CrossRef]
- Nehlig, A. Is caffeine a cognitive enhancer? J. Alzheimer’s Dis. 2010, 20, S85–S94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvis, M.J. Does caffeine intake enhance absolute levels of cognitive performance? Psychopharmacology 1993, 110, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Torga, G.N.; Spers, E.E. Chapter 2—Perspectives of global coffee demand. In Coffee Consumption and Industry Strategies in Brazil; Woodhead Publishing: Sawston, UK, 2020; pp. 21–49. [Google Scholar]




| Samples | PE | PC | PS | PI | SM |
|---|---|---|---|---|---|
| Brain | 55.2% | 31.3% | 8.0% | 5.3% | n.d. |
| Milk | 37.5% | 26% | 7.2% | 6.3% | 23% |
| Egg yolk | 16.6% | 76.9% | n.d. | n.d. | 2.3% |
| Soybean | 26.2% | 44% | n.d. | 14% | n.d. |
| Compounds | Species | Improved Cognitive Functions | References |
|---|---|---|---|
| Complex milk lipids | Rat | Spatial memory | [79] |
| Phosphatidylserine | Human | Learning and verbal memory | [81] |
| Phosphatidylserine | Human | Immediate nonverbal memory | [82] |
| 1,2-dilinoleoyl-sn-glycero-3-phosphocholine | Mouse | Spatial memory | [83] |
| Milk fat globule membrane | Rat | Spatial memory | [84] |
| Bovine milk-derived phospholipid drink | Human | Attention switching | [85] |
| Study Samples | Results | References |
|---|---|---|
| Blood plasma of AD patients, patients of dementia and patients who are cognitively impaired but nondemented | Low levels of ω3 fatty acids in the plasma may be a risk factor for cognitive impairment and/or dementia. | [101] |
| Data from cross-sectional population-based study among subjects aged 45–70 years | Fatty fish and marine ω3 PUFA consumption was associated with a reduced risk and intake of cholesterol and saturated fat with an increased risk of impaired cognitive function in the middle-aged population. | [102] |
| Erythrocyte membrane of men and women (aged 63–74 years) from the Etude du Vieillissement Artériel (EVA) cohort | The inverse association between cognitive decline and the ratio of ω3 to ω6 fatty acid in erythrocyte membranes was confirmed. | [103] |
| Data derived from a cohort of men, aged 69–89 years, who were participants in the Zutphen Elderly Study | High linoleic acid intake was positively associated with cognitive impairment and high fish consumption inversely associated with cognitive impairment. | [104] |
| Plasma of adults aged 50–65 years | Promoting higher intakes of ω3 PUFA in the diet of hypertensive and dyslipidemic people may have substantial benefits in terms of reducing their risk of cognitive decline in the area of verbal fluency. | [105] |
| Plasma of men and women aged 50–70 years | Plasma ω3 PUFA proportions were associated with less decline in the speed-related cognitive domains over 3 years. | [106] |
| Dietaries | Improved Functions | References |
|---|---|---|
| Cocoa with flavanol | Cognitive function, blood pressure control and metabolic profile | [174] |
| Blueberry | Episodic memory performance and cardiovascular disease | [175] |
| Greek mountain tea | Cognitive function | [176] |
| Dietary with resveratrol | Memory performance in association with improved glucose metabolism and increased hippocampal functional conectivity | [177] |
| Cocoa with flavanol | Regional cerebral perfusion | [178] |
| Blueberry | Brain perfusion and activation in brain areas associated with cognitive function | [179] |
| Fruit and 100% fruit juice | Cardiovascular disease, memory/cognition, obesity/diabetes and exercise performance | [180] |
| Soy with isoflavone | Cognitive function | [181] |
| Dietary with flavonol | Developing Alzheimer dementia | [182] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozawa, H.; Miyazawa, T.; Miyazawa, T. Effects of Dietary Food Components on Cognitive Functions in Older Adults. Nutrients 2021, 13, 2804. https://doi.org/10.3390/nu13082804
Ozawa H, Miyazawa T, Miyazawa T. Effects of Dietary Food Components on Cognitive Functions in Older Adults. Nutrients. 2021; 13(8):2804. https://doi.org/10.3390/nu13082804
Chicago/Turabian StyleOzawa, Hitoshi, Taiki Miyazawa, and Teruo Miyazawa. 2021. "Effects of Dietary Food Components on Cognitive Functions in Older Adults" Nutrients 13, no. 8: 2804. https://doi.org/10.3390/nu13082804
APA StyleOzawa, H., Miyazawa, T., & Miyazawa, T. (2021). Effects of Dietary Food Components on Cognitive Functions in Older Adults. Nutrients, 13(8), 2804. https://doi.org/10.3390/nu13082804