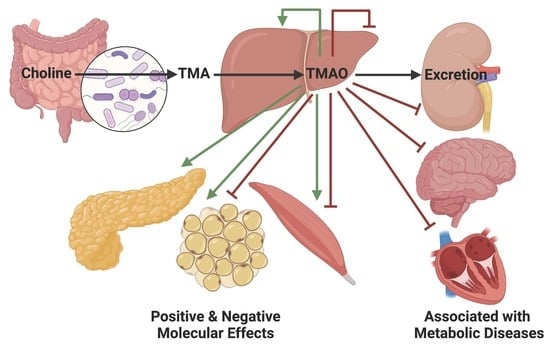
The Accumulation and Molecular Effects of Trimethylamine N-Oxide on Metabolic Tissues: It’s Not All Bad
Abstract
:1. Introduction
2. TMAO Accumulation in Serum
2.1. Intestinal TMA Production
2.2. Hepatic TMAO Production
2.3. Renal TMAO Filtration
3. TMAO Effects on Metabolic Tissues
3.1. TMAO Effects on Liver Function
3.1.1. TMAO and Reverse Cholesterol Transport
3.1.2. TMAO, Oxidative Stress, and Endoplasmic Reticulum Stress
3.2. TMAO Effects on Kidney Function
3.3. TMAO Effects on Brain Function
3.4. TMAO Effects on Adipose Function
3.5. TMAO Effects on Muscle Function
4. TMAO Effects on Blood Glucose Management
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderlini, F. Index of authors’ names. J. Chem. Soc. Abstr. 1894, 66, B493–B539. [Google Scholar] [CrossRef]
- Dunstan, W.R.; Goulding, E. XCVII.—The action of hydrogen peroxide on secondary and tertiary aliphatic amines. Formation of alkylated hydroxylamines and oxamines. J. Chem. Soc. Trans. 1899, 75, 1004–1011. [Google Scholar] [CrossRef] [Green Version]
- Lintzel, W. Trimethylaminoxyd im Menschlichen Harn. Klin. Wochenschr. 1934, 13, 304. [Google Scholar] [CrossRef]
- Baker, J.; Chaykin, S. The biosynthesis of trimethylamine-N-oxide. Biochim. Biophys. Acta 1960, 41, 548–550. [Google Scholar] [CrossRef]
- Baker, J.R.; Struempler, A.; Chaykin, S. A comparative study of trimethylamine-N-oxide biosynthesis. Biochim. Biophys. Acta 1963, 71, 58–64. [Google Scholar] [CrossRef] [PubMed]
- de la Huerga, J.; Popper, H. Urinary excretion of choline metabolites following choline administration in normals and patients with hepatobiliary diseases. J. Clin. Investig. 1951, 30, 463–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarr, H.L.A. The fate of trimethylamine oxide and trimethylamine in man. J. Fish. Res. Board Can. 1941, 5b, 211–216. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Kuffel, A.; Zielkiewicz, J. The hydrogen bond network structure within the hydration shell around simple osmolytes: Urea, tetramethylurea, and trimethylamine-N-oxide, investigated using both a fixed charge and a polarizable water model. J. Chem. Phys. 2010, 133, 035102. [Google Scholar] [CrossRef]
- Bennion, B.J.; Daggett, V. Counteraction of urea-induced protein denaturation by trimethylamine N-oxide: A chemical chaperone at atomic resolution. Proc. Natl. Acad. Sci. USA 2004, 101, 6433–6438. [Google Scholar] [CrossRef] [Green Version]
- Bennion, B.J.; DeMarco, M.L.; Daggett, V. Preventing misfolding of the prion protein by trimethylamine N-oxide. Biochemistry 2004, 43, 12955–12963. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Lynch, G.C.; Kokubo, H.; Pettitt, B.M. Trimethylamine N-oxide influence on the backbone of proteins: An oligoglycine model. Proteins 2010, 78, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulhern, M.L.; Madson, C.J.; Kador, P.F.; Randazzo, J.; Shinohara, T. Cellular osmolytes reduce lens epithelial cell death and alleviate cataract formation in galactosemic rats. Mol. Vis. 2007, 13, 1397–1405. [Google Scholar] [PubMed]
- Arakawa, T.; Timasheff, S.N. The stabilization of proteins by osmolytes. Biophys. J. 1985, 47, 411–414. [Google Scholar] [CrossRef]
- Mashino, T.; Fridovich, I. Effects of urea and trimethylamine-N-oxide on enzyme activity and stability. Arch. Biochem. Biophys. 1987, 258, 356–360. [Google Scholar] [CrossRef]
- Pincus, D.L.; Hyeon, C.; Thirumalai, D. Effects of trimethylamine N-oxide (TMAO) and crowding agents on the stability of RNA hairpins. J. Am. Chem. Soc. 2008, 130, 7364–7372. [Google Scholar] [CrossRef]
- Rezus, Y.L.; Bakker, H.J. Destabilization of the hydrogen-bond structure of water by the osmolyte trimethylamine N-oxide. J. Phys. Chem. B 2009, 113, 4038–4044. [Google Scholar] [CrossRef]
- Chhibber-Goel, J.; Singhal, V.; Parakh, N.; Bhargava, B.; Sharma, A. The metabolite trimethylamine-N-Oxide is an emergent biomarker of human health. Curr. Med. Chem. 2017, 24, 3942–3953. [Google Scholar] [CrossRef]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; DiDonato, J.A.; Sun, Q.; Rimm, E.B.; Hu, F.B.; Rexrode, K.M.; Manson, J.E.; Qi, L. Long-term changes in gut microbial metabolite trimethylamine N-Oxide and coronary heart disease risk. J. Am. Coll. Cardiol. 2020, 75, 763–772. [Google Scholar] [CrossRef]
- Hoyles, L.; Jimenez-Pranteda, M.L.; Chilloux, J.; Brial, F.; Myridakis, A.; Aranias, T.; Magnan, C.; Gibson, G.R.; Sanderson, J.D.; Nicholson, J.K.; et al. Metabolic retroconversion of trimethylamine N-oxide and the gut microbiota. Microbiome 2018, 6, 73. [Google Scholar] [CrossRef] [Green Version]
- Moraes, C.; Fouque, D.; Amaral, A.C.; Mafra, D. Trimethylamine N-Oxide from gut microbiota in chronic kidney disease patients: Focus on diet. J. Ren. Nutr. 2015, 25, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Nagatomo, Y.; Tang, W.H.W. Intersections between microbiome and heart failure: Revisiting the gut hypothesis. J. Card. Fail. 2015, 21, 973–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Organ, C.L.; Otsuka, H.; Bhushan, S.; Wang, Z.; Bradley, J.; Trivedi, R.; Polhemus, D.J.; Tang, W.H.; Wu, Y.; Hazen, S.L.; et al. Choline diet and its gut microbe-derived metabolite, trimethylamine N-Oxide, exacerbate pressure overload-induced heart failure. Circ. Heart Fail. 2016, 9, e002314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servillo, L.; D’Onofrio, N.; Giovane, A.; Casale, R.; Cautela, D.; Castaldo, D.; Iannaccone, F.; Neglia, G.; Campanile, G.; Balestrieri, M.L. Ruminant meat and milk contain delta-valerobetaine, another precursor of trimethylamine N-oxide (TMAO) like gamma-butyrobetaine. Food Chem. 2018, 260, 193–199. [Google Scholar] [CrossRef]
- Tang, W.H.; Hazen, S.L. Microbiome, trimethylamine N-oxide, and cardiometabolic disease. Transl. Res. 2017, 179, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Xu, J.; Jakovlic, I.; Wang, W.M.; Zhao, Y.H. Dietary betaine reduces liver lipid accumulation via improvement of bile acid and trimethylamine-N-oxide metabolism in blunt-snout bream. Food Funct. 2019, 10, 6675–6689. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, Q. Towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Syst. Biol. 2016, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Aldana-Hernandez, P.; Leonard, K.A.; Zhao, Y.Y.; Curtis, J.M.; Field, C.J.; Jacobs, R.L. Dietary choline or trimethylamine N-oxide supplementation does not influence atherosclerosis development in Ldlr-/-and Apoe-/-male mice. J. Nutr. 2020, 150, 249–255. [Google Scholar] [CrossRef]
- Lindskog Jonsson, A.; Caesar, R.; Akrami, R.; Reinhardt, C.; Fak Hallenius, F.; Boren, J.; Backhed, F. Impact of gut microbiota and diet on the development of atherosclerosis in Apoe(-/-) mice. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Boini, K.M.; Hussain, T.; Li, P.L.; Koka, S. Trimethylamine-N-Oxide instigates NLRP3 inflammasome activation and endothelial dysfunction. Cell Physiol. Biochem. 2017, 44, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, L.; Sawicka, A.K.; Szarmach, A.; Winklewski, P.J.; Olek, R.A.; Gabbianelli, R. A pilot study on the effects of l-carnitine and trimethylamine-N-Oxide on platelet mitochondrial DNA methylation and CVD biomarkers in aged women. Int. J. Mol. Sci. 2020, 21, 1047. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.L.; Zhu, X.H.; Ran, L.; Lang, H.D.; Yi, L.; Mi, M.T. Trimethylamine-N-Oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J. Am. Heart Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Weng, Z.; Liu, Q.; Shao, W.; Guo, W.; Chen, C.; Jiao, L.; Wang, Q.; Lu, Q.; Sun, H.; et al. FMO3 and its metabolite TMAO contribute to the formation of gallstones. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2576–2585. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Qiu, X.; Liu, Y.; Yuan, C.; Yang, X. Trimethylamine N-oxide promotes tissue factor expression and activity in vascular endothelial cells: A new link between trimethylamine N-oxide and atherosclerotic thrombosis. Thromb. Res. 2019, 177, 110–116. [Google Scholar] [CrossRef]
- Collins, H.L.; Drazul-Schrader, D.; Sulpizio, A.C.; Koster, P.D.; Williamson, Y.; Adelman, S.J.; Owen, K.; Sanli, T.; Bellamine, A. L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE(-/-) transgenic mice expressing CETP. Atherosclerosis 2016, 244, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croyal, M.; Saulnier, P.J.; Aguesse, A.; Gand, E.; Ragot, S.; Roussel, R.; Halimi, J.M.; Ducrocq, G.; Cariou, B.; Montaigne, D.; et al. Plasma trimethylamine N-Oxide and risk of cardiovascular events in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2020, 105, dgaa188. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chang, M.; Guo, Y.; Zhang, L.; Xue, C.; Yanagita, T.; Zhang, T.; Wang, Y. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism. Lipids Health Dis. 2018, 17, 286. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Zhao, M.; Wang, D.; Hu, H.; Guo, C.; Chen, W.; Li, Q.; Zheng, L.; Chen, B. Coronary plaque characterization assessed by optical coherence tomography and plasma trimethylamine-N-oxide levels in patients with coronary artery disease. Am. J. Cardiol. 2016, 118, 1311–1315. [Google Scholar] [CrossRef]
- Haghikia, A.; Li, X.S.; Liman, T.G.; Bledau, N.; Schmidt, D.; Zimmermann, F.; Krankel, N.; Widera, C.; Sonnenschein, K.; Haghikia, A.; et al. Gut microbiota-dependent Trimethylamine N-Oxide predicts risk of cardiovascular events in patients with stroke and is related to proinflammatory monocytes. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2225–2235. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Zhang, Y.; Zheng, D.; Shi, H.; Zou, C.; Zhang, H.; Lu, Z.; Du, H. Increasing trimethylamine N-oxide levels as a predictor of early neurological deterioration in patients with acute ischemic stroke. Neurol. Res. 2020, 42, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Kanitsoraphan, C.; Rattanawong, P.; Charoensri, S.; Senthong, V. Trimethylamine N-Oxide and risk of cardiovascular disease and mortality. Curr. Nutr. Rep. 2018, 7, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Pan, B.; Chen, Y.; Guo, C.; Zhao, M.; Zheng, L.; Chen, B. Trimethylamine N-oxide in atherogenesis: Impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci. Rep. 2017, 37, BSR20160244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Dai, M. Trimethylamine N-Oxide generated by the gut microbiota is associated with vascular inflammation: New insights into atherosclerosis. Mediat. Inflamm. 2020, 2020, 4634172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mafune, A.; Iwamoto, T.; Tsutsumi, Y.; Nakashima, A.; Yamamoto, I.; Yokoyama, K.; Yokoo, T.; Urashima, M. Associations among serum trimethylamine-N-oxide (TMAO) levels, kidney function and infarcted coronary artery number in patients undergoing cardiovascular surgery: A cross-sectional study. Clin. Exp. Nephrol. 2016, 20, 731–739. [Google Scholar] [CrossRef] [Green Version]
- Miao, J.; Ling, A.V.; Manthena, P.V.; Gearing, M.E.; Graham, M.J.; Crooke, R.M.; Croce, K.J.; Esquejo, R.M.; Clish, C.B.; Vicent, D.; et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat. Commun. 2015, 6, 6498. [Google Scholar] [CrossRef]
- Al-Obaide, M.A.I.; Singh, R.; Datta, P.; Rewers-Felkins, K.A.; Salguero, M.V.; Al-Obaidi, I.; Kottapalli, K.R.; Vasylyeva, T.L. Gut microbiota-dependent Trimethylamine-N-Oxide and serum biomarkers in patients with T2DM and advanced CKD. J. Clin. Med. 2017, 6, 86. [Google Scholar] [CrossRef] [Green Version]
- Argyridou, S.; Davies, M.J.; Biddle, G.J.H.; Bernieh, D.; Suzuki, T.; Dawkins, N.P.; Rowlands, A.V.; Khunti, K.; Smith, A.C.; Yates, T. Evaluation of an 8-week vegan diet on plasma Trimethylamine-N-Oxide and postchallenge glucose in adults with dysglycemia or obesity. J. Nutr. 2021, 151, 1844–1853. [Google Scholar] [CrossRef]
- Canyelles, M.; Tondo, M.; Cedo, L.; Farras, M.; Escola-Gil, J.C.; Blanco-Vaca, F. Trimethylamine N-Oxide: A link among diet, gut microbiota, gene regulation of liver and intestine cholesterol homeostasis and HDL function. Int. J. Mol. Sci. 2018, 19, 3228. [Google Scholar] [CrossRef] [Green Version]
- Dambrova, M.; Latkovskis, G.; Kuka, J.; Strele, I.; Konrade, I.; Grinberga, S.; Hartmane, D.; Pugovics, O.; Erglis, A.; Liepinsh, E. Diabetes is Associated with Higher Trimethylamine N-oxide Plasma Levels. Exp. Clin. Endocrinol. Diabetes 2016, 124, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Liang, Z.; Guo, M.; Hu, S.; Shen, Z.; Hai, X. The association between plasma levels of Trimethylamine N-Oxide and the risk of coronary heart disease in chinese patients with or without type 2 diabetes mellitus. Dis. Markers 2018, 2018, 1578320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, E.; Oste, M.C.J.; Bennett, D.W.; Jeyarajah, E.J.; Shalaurova, I.; Gruppen, E.G.; Hazen, S.L.; Otvos, J.D.; Bakker, S.J.L.; Dullaart, R.P.F.; et al. High betaine, a Trimethylamine N-Oxide related metabolite, is prospectively associated with low future risk of type 2 diabetes mellitus in the PREVEND study. J. Clin. Med. 2019, 8, 1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koppe, L.; Fouque, D.; Soulage, C.O. Metabolic abnormalities in diabetes and kidney disease: Role of uremic toxins. Curr. Diab. Rep. 2018, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Leustean, A.M.; Ciocoiu, M.; Sava, A.; Costea, C.F.; Floria, M.; Tarniceriu, C.C.; Tanase, D.M. Implications of the intestinal microbiota in diagnosing the progression of diabetes and the presence of cardiovascular complications. J. Diabetes Res. 2018, 2018, 5205126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lever, M.; George, P.M.; Slow, S.; Bellamy, D.; Young, J.M.; Ho, M.; McEntyre, C.J.; Elmslie, J.L.; Atkinson, W.; Molyneux, S.L.; et al. Betaine and Trimethylamine-N-Oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: An observational study. PLoS ONE 2014, 9, e114969. [Google Scholar] [CrossRef] [Green Version]
- McEntyre, C.J.; Lever, M.; Chambers, S.T.; George, P.M.; Slow, S.; Elmslie, J.L.; Florkowski, C.M.; Lunt, H.; Krebs, J.D. Variation of betaine, N,N-dimethylglycine, choline, glycerophosphorylcholine, taurine and trimethylamine-N-oxide in the plasma and urine of overweight people with type 2 diabetes over a two-year period. Ann. Clin. Biochem 2015, 52, 352–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mente, A.; Chalcraft, K.; Ak, H.; Davis, A.D.; Lonn, E.; Miller, R.; Potter, M.A.; Yusuf, S.; Anand, S.S.; McQueen, M.J. The relationship between Trimethylamine-N-Oxide and prevalent cardiovascular disease in a multiethnic population living in Canada. Can. J. Cardiol. 2015, 31, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.M.; Allenspach, M.; Othman, A.; Saely, C.H.; Muendlein, A.; Vonbank, A.; Drexel, H.; von Eckardstein, A. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis 2015, 243, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Oellgaard, J.; Winther, S.A.; Hansen, T.S.; Rossing, P.; von Scholten, B.J. Trimethylamine N-oxide (TMAO) as a new potential therapeutic target for insulin resistance and cancer. Curr. Pharm. Des. 2017, 23, 3699–3712. [Google Scholar] [CrossRef] [PubMed]
- Ottiger, M.; Nickler, M.; Steuer, C.; Bernasconi, L.; Huber, A.; Christ-Crain, M.; Henzen, C.; Hoess, C.; Thomann, R.; Zimmerli, W.; et al. Gut, microbiota-dependent trimethylamine-N-oxide is associated with long-term all-cause mortality in patients with exacerbated chronic obstructive pulmonary disease. Nutrition 2018, 45, 135–141.e1. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; Bullo, M.; Zheng, Y.; Ruiz-Canela, M.; Yu, E.; Guasch-Ferre, M.; Toledo, E.; Clish, C.; Corella, D.; Estruch, R.; et al. Plasma trimethylamine-N-oxide and related metabolites are associated with type 2 diabetes risk in the prevencion con dieta mediterranea (PREDIMED) trial. Am. J. Clin. Nutr. 2018, 108, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Konigsrainer, I.; Konigsrainer, A.; Balletshofer, B.; et al. Relationship of serum Trimethylamine N-Oxide (TMAO) levels with early atherosclerosis in humans. Sci. Rep. 2016, 6, 26745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, Z.; Sun, T.; Huang, H.; Chen, S.; Chen, L.; Luo, C.; Yang, W.; Yang, X.; Yao, P.; Cheng, J.; et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am. J. Clin. Nutr. 2017, 106, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.; Wang, Z.; Li, X.S.; Fan, Y.; Li, D.S.; Wu, Y.; Hazen, S.L. Increased Trimethylamine N-Oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin. Chem. 2017, 63, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Winther, S.A.; Ollgaard, J.C.; Tofte, N.; Tarnow, L.; Wang, Z.; Ahluwalia, T.S.; Jorsal, A.; Theilade, S.; Parving, H.H.; Hansen, T.W.; et al. Utility of plasma concentration of Trimethylamine N-Oxide in predicting cardiovascular and renal complications in individuals with type 1 diabetes. Diabetes Care 2019, 42, 1512–1520. [Google Scholar] [CrossRef]
- Zhuang, R.; Ge, X.; Han, L.; Yu, P.; Gong, X.; Meng, Q.; Zhang, Y.; Fan, H.; Zheng, L.; Liu, Z.; et al. Gut microbe-generated metabolite trimethylamine N-oxide and the risk of diabetes: A systematic review and dose-response meta-analysis. Obes. Rev. 2019, 20, 883–894. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The good, the bad and the unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef] [Green Version]
- Angiletta, C.J.; Griffin, L.E.; Steele, C.N.; Baer, D.J.; Novotny, J.A.; Davy, K.P.; Neilson, A.P. Impact of short-term flavanol supplementation on fasting plasma trimethylamine N-oxide concentrations in obese adults. Food Funct. 2018, 9, 5350–5361. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.E.; Caudill, M.A. Trimethylamine-N-Oxide: Friend, foe, or simply caught in the cross-fire? Trends Endocrinol. Metab. 2017, 28, 121–130. [Google Scholar] [CrossRef]
- Nowinski, A.; Ufnal, M. Trimethylamine N-oxide: A harmful, protective or diagnostic marker in lifestyle diseases? Nutrition 2018, 46, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; More, M.; Bellamine, A. Trimethylamine N-Oxide in relation to cardiometabolic health-cause or effect? Nutrients 2020, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- Washburn, R.L.; Cox, J.E.; Muhlestein, J.B.; May, H.T.; Carlquist, J.F.; Le, V.T.; Anderson, J.L.; Horne, B.D. Pilot study of novel intermittent fasting effects on metabolomic and Trimethylamine N-oxide changes during 24-hour water-only fasting in the FEELGOOD trial. Nutrients 2019, 11, 246. [Google Scholar] [CrossRef] [Green Version]
- Baugh, M.E.; Steele, C.N.; Angiletta, C.J.; Mitchell, C.M.; Neilson, A.P.; Davy, B.M.; Hulver, M.W.; Davy, K.P. Inulin supplementation does not reduce plasma Trimethylamine N-Oxide concentrations in individuals at risk for type 2 diabetes. Nutrients 2018, 10, 793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smits, L.P.; Kootte, R.S.; Levin, E.; Prodan, A.; Fuentes, S.; Zoetendal, E.G.; Wang, Z.; Levison, B.S.; Cleophas, M.C.P.; Kemper, E.M.; et al. Effect of vegan fecal microbiota transplantation on carnitine- and choline-derived Trimethylamine-N-Oxide production and vascular inflammation in patients with metabolic syndrome. J. Am. Heart Assoc. 2018, 7, e008342. [Google Scholar] [CrossRef]
- Svingen, G.F.; Schartum-Hansen, H.; Pedersen, E.R.; Ueland, P.M.; Tell, G.S.; Mellgren, G.; Njolstad, P.R.; Seifert, R.; Strand, E.; Karlsson, T.; et al. Prospective associations of systemic and urinary choline metabolites with incident type 2 diabetes. Clin. Chem. 2016, 62, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Roberts, A.B.; Gu, X.; Buffa, J.A.; Hurd, A.G.; Wang, Z.; Zhu, W.; Gupta, N.; Skye, S.M.; Cody, D.B.; Levison, B.S.; et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat. Med. 2018, 24, 1407–1417. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Mitchell, S.C.; Smith, R.L. Dietary precursors of trimethylamine in man: A pilot study. Food Chem. Toxicol. 1999, 37, 515–520. [Google Scholar] [CrossRef]
- Muramatsu, H.; Matsuo, H.; Okada, N.; Ueda, M.; Yamamoto, H.; Kato, S.; Nagata, S. Characterization of ergothionase from Burkholderia sp. HME13 and its application to enzymatic quantification of ergothioneine. Appl. Microbiol. Biotechnol. 2013, 97, 5389–5400. [Google Scholar] [CrossRef]
- Meyer, K.A.; Shea, J.W. Dietary choline and betaine and risk of CVD: A systematic review and meta-analysis of prospective studies. Nutrients 2017, 9, 711. [Google Scholar] [CrossRef] [Green Version]
- Tindall, A.M.; Petersen, K.S.; Kris-Etherton, P.M. Dietary patterns affect the gut microbiome-the link to risk of cardiometabolic diseases. J. Nutr. 2018, 148, 1402–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, J.; van Daalen, K.R.; Thayyil, A.; Cocco, M.; Caputo, D.; Oliver-Williams, C. A Systematic review of the association between vegan diets and risk of cardiovascular disease. J. Nutr. 2021, 151, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Costabile, G.; Vetrani, C.; Bozzetto, L.; Giacco, R.; Bresciani, L.; Del Rio, D.; Vitale, M.; Della Pepa, G.; Brighenti, F.; Riccardi, G.; et al. Plasma TMAO increase after healthy diets: Results from 2 randomized controlled trials with dietary fish, polyphenols, and whole-grain cereals. Am. J. Clin. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Wishnok, J.S.; Blusztajn, J.K. Formation of methylamines from ingested choline and lecithin. J. Pharm. Exp. Ther. 1983, 225, 320–324. [Google Scholar]
- Rebouche, C.J.; Chenard, C.A. Metabolic fate of dietary carnitine in human adults: Identification and quantification of urinary and fecal metabolites. J. Nutr. 1991, 121, 539–546. [Google Scholar] [CrossRef] [Green Version]
- Al-Waiz, M.; Mitchell, S.C.; Idle, J.R.; Smith, R.L. The metabolism of 14C-labelled trimethylamine and its N-oxide in man. Xenobiotica 1987, 17, 551–558. [Google Scholar] [CrossRef]
- Ufnal, M.; Zadlo, A.; Ostaszewski, R. TMAO: A small molecule of great expectations. Nutrition 2015, 31, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.K.; Chen, C.C.; Liu, P.Y.; Panyod, S.; Liao, B.Y.; Chen, P.C.; Kao, H.L.; Kuo, H.C.; Kuo, C.H.; Chiu, T.H.T.; et al. Identification of TMAO-producer phenotype and host-diet-gut dysbiosis by carnitine challenge test in human and germ-free mice. Gut 2019, 68, 1439–1449. [Google Scholar] [CrossRef] [Green Version]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaworska, K.; Konop, M.; Hutsch, T.; Perlejewski, K.; Radkowski, M.; Grochowska, M.; Bielak-Zmijewska, A.; Mosieniak, G.; Sikora, E.; Ufnal, M. Trimethylamine but not Trimethylamine Oxide increases with age in rat plasma and affects smooth muscle cells viability. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1276–1283. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pinky, P.D.; Steinke, I.; Bloemer, J.; Ramesh, S.; Kariharan, T.; Rella, R.T.; Bhattacharya, S.; Dhanasekaran, M.; Suppiramaniam, V.; et al. Gut metabolite TMAO induces synaptic plasticity deficits by promoting endoplasmic reticulum stress. Front. Mol. Neurosci. 2020, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, Y.; Gua, C.; Li, X. Elevated circulating Trimethylamine N-Oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Front. Physiol. 2017, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.K.; Panyod, S.; Liu, P.Y.; Chen, C.C.; Kao, H.L.; Chuang, H.L.; Chen, Y.H.; Zou, H.B.; Kuo, H.C.; Kuo, C.H.; et al. Characterization of TMAO productivity from carnitine challenge facilitates personalized nutrition and microbiome signatures discovery. Microbiome 2020, 8, 162. [Google Scholar] [CrossRef]
- Falony, G.; Vieira-Silva, S.; Raes, J. Microbiology meets big data: The case of gut microbiota-derived trimethylamine. Annu. Rev. Microbiol. 2015, 69, 305–321. [Google Scholar] [CrossRef]
- Martinez-del Campo, A.; Bodea, S.; Hamer, H.A.; Marks, J.A.; Haiser, H.J.; Turnbaugh, P.J.; Balskus, E.P. Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut bacteria. mBio 2015, 6, e00042. [Google Scholar] [CrossRef] [Green Version]
- Kalnins, G.; Kuka, J.; Grinberga, S.; Makrecka-Kuka, M.; Liepinsh, E.; Dambrova, M.; Tars, K. Structure and function of CutC choline lyase from human microbiota bacterium klebsiella pneumoniae. J. Biol. Chem. 2015, 290, 21732–21740. [Google Scholar] [CrossRef] [Green Version]
- Craciun, S.; Marks, J.A.; Balskus, E.P. Characterization of choline trimethylamine-lyase expands the chemistry of glycyl radical enzymes. ACS Chem. Biol. 2014, 9, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Craciun, S.; Balskus, E.P. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc. Natl. Acad. Sci. USA 2012, 109, 21307–21312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalnins, G.; Sevostjanovs, E.; Hartmane, D.; Grinberga, S.; Tars, K. CntA oxygenase substrate profile comparison and oxygen dependency of TMA production in Providencia rettgeri. J. Basic Microbiol. 2018, 58, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Samulak, J.J.; Sawicka, A.K.; Hartmane, D.; Grinberga, S.; Pugovics, O.; Lysiak-Szydlowska, W.; Olek, R.A. L-carnitine supplementation increases Trimethylamine-N-Oxide but not markers of atherosclerosis in healthy aged women. Ann. Nutr. Metab. 2019, 74, 11–17. [Google Scholar] [CrossRef]
- Samulak, J.J.; Sawicka, A.K.; Samborowska, E.; Olek, R.A. Plasma Trimethylamine-N-oxide following cessation of l-carnitine supplementation in healthy aged women. Nutrients 2019, 11, 1322. [Google Scholar] [CrossRef] [Green Version]
- Koeth, R.A.; Levison, B.S.; Culley, M.K.; Buffa, J.A.; Wang, Z.; Gregory, J.C.; Org, E.; Wu, Y.; Li, L.; Smith, J.D.; et al. Gamma-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014, 20, 799–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.; Buffa, J.A.; Roberts, A.B.; Sangwan, N.; Skye, S.M.; Li, L.; Ho, K.J.; Varga, J.; DiDonato, J.A.; Tang, W.H.W.; et al. Targeted inhibition of gut microbial Trimethylamine N-Oxide production reduces renal tubulointerstitial fibrosis and functional impairment in a murine model of chronic kidney disease. Arter. Thromb. Vasc. Biol. 2020, 40, 1239–1255. [Google Scholar] [CrossRef]
- Fortino, M.; Marino, T.; Russo, N.; Sicilia, E. Mechanistic investigation of trimethylamine-N-oxide reduction catalysed by biomimetic molybdenum enzyme models. Phys. Chem. Chem. Phys. 2016, 18, 8428–8436. [Google Scholar] [CrossRef]
- Kruk, M.; Lee, J.S. Inhibition of escherichia coli Trimethylamine-N-oxide reductase by food preservatives (1). J. Food Prot. 1982, 45, 241–243. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.Y.; Li, S.; Koh, Y.C.; Wu, J.C.; Yang, M.J.; Ho, C.T.; Pan, M.H. Oolong tea extract and citrus peel polymethoxyflavones reduce transformation of l-carnitine to Trimethylamine-N-Oxide and decrease vascular inflammation in l-carnitine feeding mice. J. Agric. Food Chem. 2019, 67, 7869–7879. [Google Scholar] [CrossRef]
- He, Z.; Hao, W.; Kwek, E.; Lei, L.; Liu, J.; Zhu, H.; Ma, K.Y.; Zhao, Y.; Ho, H.M.; He, W.S.; et al. Fish oil is more potent than flaxseed oil in modulating gut microbiota and reducing Trimethylamine-N-oxide-exacerbated atherogenesis. J. Agric. Food Chem. 2019, 67, 13635–13647. [Google Scholar] [CrossRef] [PubMed]
- Kuka, J.; Videja, M.; Makrecka-Kuka, M.; Liepins, J.; Grinberga, S.; Sevostjanovs, E.; Vilks, K.; Liepinsh, E.; Dambrova, M. Metformin decreases bacterial trimethylamine production and trimethylamine N-oxide levels in db/db mice. Sci. Rep. 2020, 10, 14555. [Google Scholar] [CrossRef]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, J.L. Bile formation and secretion. Compr. Physiol. 2013, 3, 1035–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, G.B. The lipid composition of human bile. Biochim. Biophys. Acta 1960, 41, 361–363. [Google Scholar] [CrossRef]
- al-Waiz, M.; Mikov, M.; Mitchell, S.C.; Smith, R.L. The exogenous origin of trimethylamine in the mouse. Metabolism 1992, 41, 135–136. [Google Scholar] [CrossRef]
- Brunt, V.E.; Gioscia-Ryan, R.A.; Richey, J.J.; Zigler, M.C.; Cuevas, L.M.; Gonzalez, A.; Vazquez-Baeza, Y.; Battson, M.L.; Smithson, A.T.; Gilley, A.D.; et al. Suppression of the gut microbiome ameliorates age-related arterial dysfunction and oxidative stress in mice. J. Physiol. 2019, 597, 2361–2378. [Google Scholar] [CrossRef] [Green Version]
- Warrier, M.; Shih, D.M.; Burrows, A.C.; Ferguson, D.; Gromovsky, A.D.; Brown, A.L.; Marshall, S.; McDaniel, A.; Schugar, R.C.; Wang, Z.; et al. The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015, 10, 326–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, S.E.; Lopez, C.A.; Baumler, A.J. The dynamics of gut-associated microbial communities during inflammation. EMBO Rep. 2013, 14, 319–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balagam, B.; Richardson, D.E. The mechanism of carbon dioxide catalysis in the hydrogen peroxide N-oxidation of amines. Inorg. Chem. 2008, 47, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Fraser-Andrews, E.A.; Manning, N.J.; Ashton, G.H.; Eldridge, P.; McGrath, J.; Menage Hdu, P. Fish odour syndrome with features of both primary and secondary trimethylaminuria. Clin. Exp. Dermatol 2003, 28, 203–205. [Google Scholar] [CrossRef]
- Al-Waiz, M.; Ayesh, R.; Mitchell, S.C.; Idle, J.R.; Smith, R.L. Trimethylaminuria (‘fish-odour syndrome’): A study of an affected family. Clin. Sci. 1988, 74, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Al-Waiz, M.; Ayesh, R.; Mitchell, S.C.; Idle, J.R.; Smith, R.L. Trimethylaminuria (fish-odour syndrome): An inborn error of oxidative metabolism. Lancet 1987, 1, 634–635. [Google Scholar] [CrossRef]
- D’Angelo, R.; Esposito, T.; Calabro, M.; Rinaldi, C.; Robledo, R.; Varriale, B.; Sidoti, A. FMO3 allelic variants in Sicilian and Sardinian populations: Trimethylaminuria and absence of fish-like body odor. Gene 2013, 515, 410–415. [Google Scholar] [CrossRef]
- Humbert, J.A.; Hammond, K.B.; Hathaway, W.E. Trimethylaminuria: The fish-odour syndrome. Lancet 1970, 2, 770–771. [Google Scholar] [CrossRef]
- Krueger, S.K.; Williams, D.E. Mammalian flavin-containing monooxygenases: Structure/function, genetic polymorphisms and role in drug metabolism. Pharm. Ther. 2005, 106, 357–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherrington, N.J.; Cao, Y.; Cherrington, J.W.; Rose, R.L.; Hodgson, E. Physiological factors affecting protein expression of flavin-containing monooxygenases 1, 3 and 5. Xenobiotica 1998, 28, 673–682. [Google Scholar] [CrossRef]
- Zhang, J.; Cashman, J.R. Quantitative analysis of FMO gene mRNA levels in human tissues. Drug Metab. Dispos. 2006, 34, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernetti, L.; Gough, A.; Baetz, N.; Blutt, S.; Broughman, J.R.; Brown, J.A.; Foulke-Abel, J.; Hasan, N.; In, J.; Kelly, E.; et al. Functional coupling of human microphysiology systems: Intestine, liver, kidney proximal tubule, blood-brain barrier and skeletal muscle. Sci. Rep. 2017, 7, 42296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falls, J.G.; Blake, B.L.; Cao, Y.; Levi, P.E.; Hodgson, E. Gender differences in hepatic expression of flavin-containing monooxygenase isoforms (FMO1, FMO3, and FMO5) in mice. J. Biochem. Toxicol. 1995, 10, 171–177. [Google Scholar] [CrossRef]
- Xu, M.; Bhatt, D.K.; Yeung, C.K.; Claw, K.G.; Chaudhry, A.S.; Gaedigk, A.; Pearce, R.E.; Broeckel, U.; Gaedigk, R.; Nickerson, D.A.; et al. Genetic and nongenetic factors associated with protein abundance of flavin-containing monooxygenase 3 in human liver. J. Pharm. Exp. 2017, 363, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Schugar, R.C.; Willard, B.; Wang, Z.; Brown, J.M. Postprandial gut microbiota-driven choline metabolism links dietary cues to adipose tissue dysfunction. Adipocyte 2018, 7, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Miyake, T.; Mizuno, T.; Mochizuki, T.; Kimura, M.; Matsuki, S.; Irie, S.; Ieiri, I.; Maeda, K.; Kusuhara, H. Involvement of organic cation transporters in the kinetics of Trimethylamine N-oxide. J. Pharm. Sci. 2017, 106, 2542–2550. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Liu, Y.; Long, J.; Chen, S.; Liao, G.; Wu, S.; Li, C.; Wang, L.; Ling, W.; Zhu, H. Trimethylamine N-Oxide aggravates liver steatosis through modulation of bile acid metabolism and inhibition of farnesoid x receptor signaling in nonalcoholic fatty liver disease. Mol. Nutr. Food Res. 2019, 63, e1900257. [Google Scholar] [CrossRef]
- Liao, B.M.; McManus, S.A.; Hughes, W.E.; Schmitz-Peiffer, C. Flavin-containing monooxygenase 3 reduces endoplasmic reticulum stress in lipid-treated hepatocytes. Mol. Endocrinol. 2016, 30, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Henderson, A.; Petriello, M.C.; Romano, K.A.; Gearing, M.; Miao, J.; Schell, M.; Sandoval-Espinola, W.J.; Tao, J.; Sha, B.; et al. Trimethylamine N-Oxide binds and activates PERK to promote metabolic dysfunction. Cell Metab. 2019, 30, 1141–1151 e1145. [Google Scholar] [CrossRef] [PubMed]
- Borbas, T.; Benko, B.; Dalmadi, B.; Szabo, I.; Tihanyi, K. Insulin in flavin-containing monooxygenase regulation. Flavin-containing monooxygenase and cytochrome P450 activities in experimental diabetes. Eur. J. Pharm. Sci. 2006, 28, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, Z.; Tang, W.H.W.; Hazen, S.L. Gut microbe-generated Trimethylamine N-Oxide from dietary choline is prothrombotic in subjects. Circulation 2017, 135, 1671–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gessner, A.; Konig, J.; Fromm, M.F. Contribution of multidrug and toxin extrusion protein 1 (MATE1) to renal secretion of trimethylamine-N-oxide (TMAO). Sci. Rep. 2018, 8, 6659. [Google Scholar] [CrossRef]
- Teft, W.A.; Morse, B.L.; Leake, B.F.; Wilson, A.; Mansell, S.E.; Hegele, R.A.; Ho, R.H.; Kim, R.B. Identification and characterization of Trimethylamine-N-oxide uptake and efflux transporters. Mol. Pharm. 2017, 14, 310–318. [Google Scholar] [CrossRef]
- Krause, R.J.; Lash, L.H.; Elfarra, A.A. Human kidney flavin-containing monooxygenases and their potential roles in cysteine s-conjugate metabolism and nephrotoxicity. J. Pharm. Exp. Ther. 2003, 304, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Ripp, S.L.; Itagaki, K.; Philpot, R.M.; Elfarra, A.A. Species and sex differences in expression of flavin-containing monooxygenase form 3 in liver and kidney microsomes. Drug Metab. Dispos. 1999, 27, 46–52. [Google Scholar]
- Mitchell, S.M.; Milan, A.M.; Mitchell, C.J.; Gillies, N.A.; D’Souza, R.F.; Zeng, N.; Ramzan, F.; Sharma, P.; Knowles, S.O.; Roy, N.C.; et al. Protein intake at twice the RDA in older men increases circulatory concentrations of the microbiome metabolite Trimethylamine-N-Oxide (TMAO). Nutrients 2019, 11, 2207. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.M.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent Trimethylamine N-Oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prokopienko, A.J.; West, R.E., 3rd; Schrum, D.P.; Stubbs, J.R.; Leblond, F.A.; Pichette, V.; Nolin, T.D. Metabolic activation of flavin monooxygenase-mediated Trimethylamine-N-Oxide formation in experimental kidney disease. Sci. Rep. 2019, 9, 15901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, C.; Prokopienko, A.J.; West, R.E., 3rd; Nolin, T.D.; Stubbs, J.R. Decreased kidney function is associated with enhanced hepatic flavin monooxygenase activity and increased circulating Trimethylamine N-Oxide concentrations in mice. Drug Metab. Dispos. 2018, 46, 1304–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, K.Y.; Xia, G.H.; Lu, J.Q.; Chen, M.X.; Zhen, X.; Wang, S.; You, C.; Nie, J.; Zhou, H.W.; Yin, J. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci. Rep. 2017, 7, 1445. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, J.R.; House, J.A.; Ocque, A.J.; Zhang, S.; Johnson, C.; Kimber, C.; Schmidt, K.; Gupta, A.; Wetmore, J.B.; Nolin, T.D.; et al. Serum Trimethylamine-N-Oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J. Am. Soc. Nephrol. 2016, 27, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.B.; Morse, B.L.; Djurdjev, O.; Tang, M.; Muirhead, N.; Barrett, B.; Holmes, D.T.; Madore, F.; Clase, C.M.; Rigatto, C.; et al. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016, 89, 1144–1152. [Google Scholar] [CrossRef]
- Organ, C.L.; Li, Z.; Sharp, T.E., 3rd; Polhemus, D.J.; Gupta, N.; Goodchild, T.T.; Tang, W.H.W.; Hazen, S.L.; Lefer, D.J. Nonlethal inhibition of gut microbial Trimethylamine N-oxide production improves cardiac function and remodeling in a murine model of heart failure. J. Am. Heart Assoc. 2020, 9, e016223. [Google Scholar] [CrossRef]
- Bordoni, L.; Samulak, J.J.; Sawicka, A.K.; Pelikant-Malecka, I.; Radulska, A.; Lewicki, L.; Kalinowski, L.; Gabbianelli, R.; Olek, R.A. Trimethylamine N-oxide and the reverse cholesterol transport in cardiovascular disease: A cross-sectional study. Sci. Rep. 2020, 10, 18675. [Google Scholar] [CrossRef]
- Bordoni, L.; Petracci, I.; Pelikant-Malecka, I.; Radulska, A.; Piangerelli, M.; Samulak, J.J.; Lewicki, L.; Kalinowski, L.; Gabbianelli, R.; Olek, R.A. Mitochondrial DNA copy number and trimethylamine levels in the blood: New insights on cardiovascular disease biomarkers. FASEB J. 2021, 35, e21694. [Google Scholar] [CrossRef] [PubMed]
- Latkovskis, G.; Makarova, E.; Mazule, M.; Bondare, L.; Hartmane, D.; Cirule, H.; Grinberga, S.; Erglis, A.; Liepinsh, E.; Dambrova, M. Loop diuretics decrease the renal elimination rate and increase the plasma levels of trimethylamine-N-oxide. Br. J. Clin. Pharm. 2018, 84, 2634–2644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missailidis, C.; Hallqvist, J.; Qureshi, A.R.; Barany, P.; Heimburger, O.; Lindholm, B.; Stenvinkel, P.; Bergman, P. Serum Trimethylamine-N-Oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS ONE 2016, 11, e0141738. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, T.; Rohrmann, S.; Sookthai, D.; Johnson, T.; Katzke, V.; Kaaks, R.; von Eckardstein, A.; Muller, D. Intra-individual variation of plasma trimethylamine-N-oxide (TMAO), betaine and choline over 1 year. Clin. Chem. Lab. Med. 2017, 55, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Dumas, M.E.; Barton, R.H.; Toye, A.; Cloarec, O.; Blancher, C.; Rothwell, A.; Fearnside, J.; Tatoud, R.; Blanc, V.; Lindon, J.C.; et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc. Natl. Acad. Sci. USA 2006, 103, 12511–12516. [Google Scholar] [CrossRef] [Green Version]
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; Gromovsky, A.D.; Heine, M.; Chatterjee, A.; et al. The TMAO-producing enzyme flavin-containing monooxygenase 3 regulates obesity and the beiging of white adipose tissue. Cell Rep. 2017, 19, 2451–2461. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, Y.; Liu, J.; Yang, G.; Zhao, J.; Liao, G.; Shi, M.; Yuan, Y.; He, S.; Lu, Y.; et al. Serum metabolic variables associated with impaired glucose tolerance induced by high-fat-high-cholesterol diet in Macaca mulatta. Exp. Biol. Med. 2012, 237, 1310–1321. [Google Scholar] [CrossRef]
- Chen, Y.M.; Liu, Y.; Zhou, R.F.; Chen, X.L.; Wang, C.; Tan, X.Y.; Wang, L.J.; Zheng, R.D.; Zhang, H.W.; Ling, W.H.; et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci. Rep. 2016, 6, 19076. [Google Scholar] [CrossRef] [PubMed]
- Kummen, M.; Vesterhus, M.; Troseid, M.; Moum, B.; Svardal, A.; Boberg, K.M.; Aukrust, P.; Karlsen, T.H.; Berge, R.K.; Hov, J.R. Elevated trimethylamine-N-oxide (TMAO) is associated with poor prognosis in primary sclerosing cholangitis patients with normal liver function. United Eur. Gastroenterol. J. 2017, 5, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, J.; Jiang, C.; Zhang, Y.; Xue, Y.; Li, Z.; Wang, J.; Xue, C.; Wang, Y. Fish oil ameliorates trimethylamine N-oxide-exacerbated glucose intolerance in high-fat diet-fed mice. Food Funct. 2015, 6, 1117–1125. [Google Scholar] [CrossRef]
- Shih, D.M.; Wang, Z.; Lee, R.; Meng, Y.; Che, N.; Charugundla, S.; Qi, H.; Wu, J.; Pan, C.; Brown, J.M.; et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J. Lipid Res. 2015, 56, 22–37. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez Malagon, S.G.; Melidoni, A.N.; Hernandez, D.; Omar, B.A.; Houseman, L.; Veeravalli, S.; Scott, F.; Varshavi, D.; Everett, J.; Tsuchiya, Y.; et al. The phenotype of a knockout mouse identifies flavin-containing monooxygenase 5 (FMO5) as a regulator of metabolic ageing. Biochem. Pharm. 2015, 96, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol attenuates Trimethylamine-N-Oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio 2016, 7, e02210–e02215. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.H.; Xin, F.Z.; Zhou, D.; Xue, Y.Q.; Liu, X.L.; Yang, R.X.; Pan, Q.; Fan, J.G. Trimethylamine N-oxide attenuates high-fat high-cholesterol diet-induced steatohepatitis by reducing hepatic cholesterol overload in rats. World J. Gastroenterol. 2019, 25, 2450–2462. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Peng, L.; Perez de Nanclares, M.; Trudeau, M.P.; Yao, D.; Cheng, Z.; Urriola, P.E.; Mydland, L.T.; Shurson, G.C.; Overland, M.; et al. Identification of sinapine-derived choline from a rapeseed diet as a source of serum Trimethylamine N-Oxide in pigs. J. Agric. Food Chem. 2019, 67, 7748–7754. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsukuma, K.E.; Bennett, M.K.; Huang, J.; Wang, L.; Gil, G.; Osborne, T.F. Coordinated control of bile acids and lipogenesis through FXR-dependent regulation of fatty acid synthase. J. Lipid Res. 2006, 47, 2754–2761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L.; Montezano, A.C. Oxidative stress: A unifying paradigm in hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ufnal, M.; Jazwiec, R.; Dadlez, M.; Drapala, A.; Sikora, M.; Skrzypecki, J. Trimethylamine-N-oxide: A carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can. J. Cardiol. 2014, 30, 1700–1705. [Google Scholar] [CrossRef] [Green Version]
- Achard, C.S.; Laybutt, D.R. Lipid-induced endoplasmic reticulum stress in liver cells results in two distinct outcomes: Adaptation with enhanced insulin signaling or insulin resistance. Endocrinology 2012, 153, 2164–2177. [Google Scholar] [CrossRef]
- Brunt, V.E.; Gioscia-Ryan, R.A.; Casso, A.G.; VanDongen, N.S.; Ziemba, B.P.; Sapinsley, Z.J.; Richey, J.J.; Zigler, M.C.; Neilson, A.P.; Davy, K.P.; et al. Trimethylamine-N-Oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension 2020, 76, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jiao, X.; Ma, Y.; Liu, Y.; Zhang, L.; He, Y.; Chen, Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2016, 481, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Li, D.; Zhao, M.; Liu, C.; Liu, J.; Zeng, A.; Shi, X.; Cheng, S.; Pan, B.; Zheng, L.; et al. Gut flora-dependent metabolite Trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radic. Biol. Med. 2018, 116, 88–100. [Google Scholar] [CrossRef]
- Wang, A.; Bolen, D.W. A naturally occurring protective system in urea-rich cells: Mechanism of osmolyte protection of proteins against urea denaturation. Biochemistry 1997, 36, 9101–9108. [Google Scholar] [CrossRef] [PubMed]
- Parkin, K.L.; Hultin, H.O. Characterization of trimethylamine-N-oxide (TMAO) demethylase activity from fish muscle microsomes. J. Biochem. 1986, 100, 77–86. [Google Scholar] [CrossRef]
- Rodriguez-Fuentes, G.; Aparicio-Fabre, R.; Li, Q.; Schlenk, D. Osmotic regulation of a novel flavin-containing monooxygenase in primary cultured cells from rainbow trout (Oncorhynchus mykiss). Drug Metab. Dispos. 2008, 36, 1212–1217. [Google Scholar] [CrossRef] [Green Version]
- Sackett, D.L. Natural osmolyte trimethylamine N-oxide stimulates tubulin polymerization and reverses urea inhibition. Am. J. Physiol. 1997, 273, R669–R676. [Google Scholar] [CrossRef]
- Song, J.L.; Chuang, D.T. Natural osmolyte trimethylamine N-oxide corrects assembly defects of mutant branched-chain alpha-ketoacid decarboxylase in maple syrup urine disease. J. Biol. Chem. 2001, 276, 40241–40246. [Google Scholar] [CrossRef] [Green Version]
- Larsen, B.K.; Schlenk, D. Effect of salinity on flavin-containing monooxygenase expression and activity in rainbow trout (Oncorhynchus mykiss). J. Comp. Physiol. B 2001, 171, 421–429. [Google Scholar] [CrossRef]
- Villalobos, A.R.; Renfro, J.L. Trimethylamine oxide suppresses stress-induced alteration of organic anion transport in choroid plexus. J. Exp. Biol. 2007, 210, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Yancey, P.H.; Siebenaller, J.F. Co-evolution of proteins and solutions: Protein adaptation versus cytoprotective micromolecules and their roles in marine organisms. J. Exp. Biol 2015, 218, 1880–1896. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, P.; Boserman, P.; van der Vegt, N.F.A.; Shea, J.E. Trimethylamine N-oxide counteracts urea denaturation by inhibiting protein-urea preferential interaction. J. Am. Chem. Soc. 2018, 140, 483–492. [Google Scholar] [CrossRef]
- Liao, Y.T.; Manson, A.C.; DeLyser, M.R.; Noid, W.G.; Cremer, P.S. Trimethylamine N-oxide stabilizes proteins via a distinct mechanism compared with betaine and glycine. Proc. Natl. Acad. Sci. USA 2017, 114, 2479–2484. [Google Scholar] [CrossRef] [Green Version]
- Mondal, J.; Stirnemann, G.; Berne, B.J. When does trimethylamine N-oxide fold a polymer chain and urea unfold it? J. Phys. Chem. B 2013, 117, 8723–8732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepshelovich, J.; Goldstein-Magal, L.; Globerson, A.; Yen, P.M.; Rotman-Pikielny, P.; Hirschberg, K. Protein synthesis inhibitors and the chemical chaperone TMAO reverse endoplasmic reticulum perturbation induced by overexpression of the iodide transporter pendrin. J. Cell Sci. 2005, 118, 1577–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.Y.; Luzuriaga, J.; Bensellam, M.; Biden, T.J.; Laybutt, D.R. Failure of the adaptive unfolded protein response in islets of obese mice is linked with abnormalities in beta-cell gene expression and progression to diabetes. Diabetes 2013, 62, 1557–1568. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, J.J.; Ma, J.H.; Jin, C.; Yu, Q.; Zhang, S.X. Activation of the UPR protects against cigarette smoke-induced RPE apoptosis through up-regulation of Nrf2. J. Biol. Chem. 2015, 290, 5367–5380. [Google Scholar] [CrossRef] [Green Version]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romine, I.C.; Wiseman, R.L. PERK signaling regulates extracellular proteostasis of an amyloidogenic protein during endoplasmic reticulum stress. Sci. Rep. 2019, 9, 410. [Google Scholar] [CrossRef] [Green Version]
- Lebeau, J.; Saunders, J.M.; Moraes, V.W.R.; Madhavan, A.; Madrazo, N.; Anthony, M.C.; Wiseman, R.L. The PERK arm of the unfolded protein response regulates mitochondrial morphology during acute endoplasmic reticulum stress. Cell Rep. 2018, 22, 2827–2836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullinan, S.B.; Diehl, J.A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004, 279, 20108–20117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M.; Sadri, N.; Yun, C.; Popko, B.; Paules, R.; et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 2003, 11, 619–633. [Google Scholar] [CrossRef]
- Rainbolt, T.K.; Atanassova, N.; Genereux, J.C.; Wiseman, R.L. Stress-regulated translational attenuation adapts mitochondrial protein import through Tim17A degradation. Cell Metab. 2013, 18, 908–919. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, U.; Yilmaz, E.; Ozcan, L.; Furuhashi, M.; Vaillancourt, E.; Smith, R.O.; Gorgun, C.Z.; Hotamisligil, G.S. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006, 313, 1137–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruppen, E.G.; Garcia, E.; Connelly, M.A.; Jeyarajah, E.J.; Otvos, J.D.; Bakker, S.J.L.; Dullaart, R.P.F. TMAO is Associated with Mortality: Impact of modestly impaired renal function. Sci. Rep. 2017, 7, 13781. [Google Scholar] [CrossRef]
- Robinson-Cohen, C.; Newitt, R.; Shen, D.D.; Rettie, A.E.; Kestenbaum, B.R.; Himmelfarb, J.; Yeung, C.K. Association of FMO3 variants and Trimethylamine N-Oxide concentration, disease progression, and mortality in CKD patients. PLoS ONE 2016, 11, e0161074. [Google Scholar] [CrossRef]
- Pelletier, C.C.; Croyal, M.; Ene, L.; Aguesse, A.; Billon-Crossouard, S.; Krempf, M.; Lemoine, S.; Guebre-Egziabher, F.; Juillard, L.; Soulage, C.O. Elevation of Trimethylamine-N-Oxide in chronic kidney disease: Contribution of decreased glomerular filtration rate. Toxins 2019, 11, 635. [Google Scholar] [CrossRef] [Green Version]
- Rhee, E.P.; Clish, C.B.; Ghorbani, A.; Larson, M.G.; Elmariah, S.; McCabe, E.; Yang, Q.; Cheng, S.; Pierce, K.; Deik, A.; et al. A combined epidemiologic and metabolomic approach improves CKD prediction. J. Am. Soc. Nephrol. 2013, 24, 1330–1338. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.Z.; Dou, P.; Gao, M.; Kong, X.J.; Li, C.W.; Liu, Z.H.; Huang, T. Assessment of causal direction between gut microbiota-dependent metabolites and cardiometabolic health: A bidirectional mendelian randomization analysis. Diabetes 2019, 68, 1747–1755. [Google Scholar] [CrossRef]
- Kaysen, G.A.; Johansen, K.L.; Chertow, G.M.; Dalrymple, L.S.; Kornak, J.; Grimes, B.; Dwyer, T.; Chassy, A.W.; Fiehn, O. Associations of Trimethylamine N-Oxide with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J. Ren. Nutr. 2015, 25, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Gawrys-Kopczynska, M.; Konop, M.; Maksymiuk, K.; Kraszewska, K.; Derzsi, L.; Sozanski, K.; Holyst, R.; Pilz, M.; Samborowska, E.; Dobrowolski, L.; et al. TMAO, a seafood-derived molecule, produces diuresis and reduces mortality in heart failure rats. eLife 2020, 9, e57028. [Google Scholar] [CrossRef]
- Liu, J.; Lai, L.; Lin, J.; Zheng, J.; Nie, X.; Zhu, X.; Xue, J.; Liu, T. Ranitidine and finasteride inhibit the synthesis and release of trimethylamine N-oxide and mitigates its cardiovascular and renal damage through modulating gut microbiota. Int. J. Biol. Sci. 2020, 16, 790–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Rio, D.; Zimetti, F.; Caffarra, P.; Tassotti, M.; Bernini, F.; Brighenti, F.; Zini, A.; Zanotti, I. The gut microbial metabolite Trimethylamine-N-Oxide is present in human cerebrospinal fluid. Nutrients 2017, 9, 1053. [Google Scholar] [CrossRef] [Green Version]
- Vogt, N.M.; Romano, K.A.; Darst, B.F.; Engelman, C.D.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Blennow, K.; Zetterberg, H.; Bendlin, B.B.; et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers Res. Ther. 2018, 10, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Meng, G.; Huang, B.; Zhou, X.; Stavrakis, S.; Wang, M.; Li, X.; Zhou, L.; Wang, Y.; Wang, M.; et al. A potential relationship between gut microbes and atrial fibrillation: Trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int. J. Cardiol. 2018, 255, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Ntranos, A.; Casaccia, P. The microbiome-gut-behavior axis: Crosstalk between the gut microbiome and oligodendrocytes modulates behavioral responses. Neurotherapeutics 2018, 15, 31–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609, 609.e1–609.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, S.M.; Mohajeri, M.H. The role of gut bacterial metabolites in brain development, aging and disease. Nutrients 2021, 13, 732. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, S.; Wang, S.; Nagpal, R.; Wang, B.; Jain, S.; Razazan, A.; Mishra, S.P.; Zhu, X.; Wang, Z.; Kavanagh, K.; et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight 2020, 5, e132055. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Jeffery, I.B. Gut microbiota and aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef]
- Meng, F.; Li, N.; Li, D.; Song, B.; Li, L. The presence of elevated circulating trimethylamine N-oxide exaggerates postoperative cognitive dysfunction in aged rats. Behav. Brain Res. 2019, 368, 111902. [Google Scholar] [CrossRef]
- Du, D.; Tang, W.; Zhou, C.; Sun, X.; Wei, Z.; Zhong, J.; Huang, Z. Fecal microbiota transplantation is a promising method to restore gut microbiota dysbiosis and relieve neurological deficits after traumatic brain injury. Oxid. Med. Cell Longev. 2021, 2021, 5816837. [Google Scholar] [CrossRef]
- Olek, R.A.; Samulak, J.J.; Sawicka, A.K.; Hartmane, D.; Grinberga, S.; Pugovics, O.; Lysiak-Szydlowska, W. Increased Trimethylamine N-Oxide is not associated with oxidative stress markers in healthy aged women. Oxid. Med. Cell Longev. 2019, 2019, 6247169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schirra, H.J.; Anderson, C.G.; Wilson, W.J.; Kerr, L.; Craik, D.J.; Waters, M.J.; Lichanska, A.M. Altered metabolism of growth hormone receptor mutant mice: A combined NMR metabonomics and microarray study. PLoS ONE 2008, 3, e2764. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zhang, C.; Cao, G.; Dong, X.; Li, D.; Jiang, L. Higher circulating Trimethylamine N-oxide sensitizes sevoflurane-induced cognitive dysfunction in aged rats probably by downregulating hippocampal methionine sulfoxide reductase, A. Neurochem. Res. 2019, 44, 2506–2516. [Google Scholar] [CrossRef] [PubMed]
- Lupachyk, S.; Watcho, P.; Stavniichuk, R.; Shevalye, H.; Obrosova, I.G. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes 2013, 62, 944–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Theije, C.G.; Wopereis, H.; Ramadan, M.; van Eijndthoven, T.; Lambert, J.; Knol, J.; Garssen, J.; Kraneveld, A.D.; Oozeer, R. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav. Immun. 2014, 37, 197–206. [Google Scholar] [CrossRef]
- Li, Q.; Han, Y.; Dy, A.B.C.; Hagerman, R.J. The gut microbiota and autism spectrum disorders. Front. Cell Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Siniscalco, D.; Schultz, S.; Brigida, A.L.; Antonucci, N. Inflammation and neuro-immune dysregulations in autism spectrum disorders. Pharmaceuticals 2018, 11, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, L.J.; Yi, J.P.; Zhao, Y.; Zhang, F.; Shi, X.T.; Feng, Z.; Miller, H.L. Plasma trimethylamine N-oxide, a gut microbe generated phosphatidylcholine metabolite, is associated with autism spectrum disorders. Neurotoxicology 2020, 76, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Lv, H.; Wang, H.; Yang, H.; Li, Y.; Qian, J. Aging increases the severity of colitis and the related changes to the gut barrier and gut microbiota in humans and mice. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1284–1292. [Google Scholar] [CrossRef]
- Qi, Z.; Qi, S.; Gui, L.; Shen, L.; Feng, Z. Daphnetin protects oxidative stress-induced neuronal apoptosis via regulation of MAPK signaling and HSP70 expression. Oncol. Lett. 2016, 12, 1959–1964. [Google Scholar] [CrossRef] [Green Version]
- Reeg, S.; Jung, T.; Castro, J.P.; Davies, K.J.A.; Henze, A.; Grune, T. The molecular chaperone Hsp70 promotes the proteolytic removal of oxidatively damaged proteins by the proteasome. Free Radic. Biol. Med. 2016, 99, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Uversky, V.N.; Li, J.; Fink, A.L. Trimethylamine-N-oxide-induced folding of alpha-synuclein. FEBS Lett. 2001, 509, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Yoshizawa, T.; Shibasaki, F.; Shoji, S.; Kanazawa, I. Chemical chaperones reduce aggregate formation and cell death caused by the truncated Machado-Joseph disease gene product with an expanded polyglutamine stretch. Neurobiol. Dis. 2002, 10, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Paul, S. Polyglutamine-mediated neurodegeneration: Use of chaperones as prevention strategy. Biochemistry 2007, 72, 359–366. [Google Scholar] [CrossRef]
- Bartolini, M.; Andrisano, V. Strategies for the inhibition of protein aggregation in human diseases. Chembiochem 2010, 11, 1018–1035. [Google Scholar] [CrossRef] [PubMed]
- Lisabeth, E.M.; Falivelli, G.; Pasquale, E.B. Eph receptor signaling and ephrins. Cold Spring Harb. Perspect. Biol. 2013, 5, a009159. [Google Scholar] [CrossRef] [Green Version]
- Chang, Q.; Jorgensen, C.; Pawson, T.; Hedley, D.W. Effects of dasatinib on EphA2 receptor tyrosine kinase activity and downstream signalling in pancreatic cancer. Br. J. Cancer 2008, 99, 1074–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantinova, I.; Nikolova, G.; Ohara-Imaizumi, M.; Meda, P.; Kucera, T.; Zarbalis, K.; Wurst, W.; Nagamatsu, S.; Lammert, E. EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell 2007, 129, 359–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Ke, Y.; Zhan, R.; Liu, C.; Zhao, M.; Zeng, A.; Shi, X.; Ji, L.; Cheng, S.; Pan, B.; et al. Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 2018, 17, e12768. [Google Scholar] [CrossRef] [PubMed]
- D’Orio, B.; Fracassi, A.; Ceru, M.P.; Moreno, S. Targeting PPARalpha in alzheimer’s disease. Curr. Alzheimer Res. 2018, 15, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as novel potential biomarker of early predictors of metabolic syndrome. Nutrients 2018, 10, 1917. [Google Scholar] [CrossRef] [Green Version]
- Erickson, M.L.; Malin, S.K.; Wang, Z.; Brown, J.M.; Hazen, S.L.; Kirwan, J.P. Effects of lifestyle intervention on plasma Trimethylamine N-Oxide in obese adults. Nutrients 2019, 11, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumas, M.E.; Rothwell, A.R.; Hoyles, L.; Aranias, T.; Chilloux, J.; Calderari, S.; Noll, E.M.; Pean, N.; Boulange, C.L.; Blancher, C.; et al. Microbial-Host Co-metabolites are prodromal markers predicting phenotypic heterogeneity in behavior, obesity, and impaired glucose tolerance. Cell Rep. 2017, 20, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Parks, B.W.; Nam, E.; Org, E.; Kostem, E.; Norheim, F.; Hui, S.T.; Pan, C.; Civelek, M.; Rau, C.D.; Bennett, B.J.; et al. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 2013, 17, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Veeravalli, S.; Omar, B.A.; Houseman, L.; Hancock, M.; Gonzalez Malagon, S.G.; Scott, F.; Janmohamed, A.; Phillips, I.R.; Shephard, E.A. The phenotype of a flavin-containing monooyxgenase knockout mouse implicates the drug-metabolizing enzyme FMO1 as a novel regulator of energy balance. Biochem. Pharm. 2014, 90, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, X.; Xu, J.; Xue, C.; Xue, Y.; Wang, Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J. Biosci. Bioeng. 2014, 118, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Flier, J.S. Adipose tissue as an endocrine organ. Trends Endocrinol. Met. 2000, 11, 327–332. [Google Scholar] [CrossRef]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.-i.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Knauf, C.; Neynhck, A.; Alessi, M.C.; Burcelin, R.; Delzenne, N. Metabolic endotoxemia initiates obesity and insulin resistance. Ann. Nutr. Metab. 2007, 51, 79. [Google Scholar] [CrossRef] [Green Version]
- Lagathu, C.; Bastard, J.P.; Auclair, M.; Maachi, M.; Capeau, J.; Caron, M. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: Prevention by rosiglitazone. Biochem. Biophys. Res. Commun. 2003, 311, 372–379. [Google Scholar] [CrossRef]
- Yancey, P.H.; Gerringer, M.E.; Drazen, J.C.; Rowden, A.A.; Jamieson, A. Marine fish may be biochemically constrained from inhabiting the deepest ocean depths. Proc. Natl. Acad. Sci. USA 2014, 111, 4461–4465. [Google Scholar] [CrossRef] [Green Version]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef] [Green Version]
- Baskakov, I.; Wang, A.; Bolen, D.W. Trimethylamine-N-oxide counteracts urea effects on rabbit muscle lactate dehydrogenase function: A test of the counteraction hypothesis. Biophys. J. 1998, 74, 2666–2673. [Google Scholar] [CrossRef] [Green Version]
- Baskakov, I.; Bolen, D.W. Time-dependent effects of trimethylamine-N-oxide/urea on lactate dehydrogenase activity: An unexplored dimension of the adaptation paradigm. Biophys. J. 1998, 74, 2658–2665. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Costa, S.; Sorenson, M.M.; Sola-Penna, M. Counteracting effects of urea and methylamines in function and structure of skeletal muscle myosin. Arch. Biochem. Biophys. 2002, 408, 272–278. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Z.; Yan, J.; Liu, H.; Liu, Q.; Deng, Y.; Ou, C.; Chen, M. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab. Investig. 2019, 99, 346–357. [Google Scholar] [CrossRef]
- Li, X.; Geng, J.; Zhao, J.; Ni, Q.; Zhao, C.; Zheng, Y.; Chen, X.; Wang, L. Trimethylamine N-Oxide exacerbates cardiac fibrosis via activating the NLRP3 inflammasome. Front. Physiol. 2019, 10, 866. [Google Scholar] [CrossRef]
- Chen, K.; Zheng, X.; Feng, M.; Li, D.; Zhang, H. Gut microbiota-dependent metabolite Trimethylamine N-Oxide contributes to cardiac dysfunction in western diet-induced obese mice. Front. Physiol. 2017, 8, 139. [Google Scholar] [CrossRef]
- Savi, M.; Bocchi, L.; Bresciani, L.; Falco, A.; Quaini, F.; Mena, P.; Brighenti, F.; Crozier, A.; Stilli, D.; Del Rio, D. Trimethylamine-N-Oxide (TMAO)-induced impairment of cardiomyocyte function and the protective role of urolithin B-Glucuronide. Molecules 2018, 23, 549. [Google Scholar] [CrossRef] [Green Version]
- Piacentino, V., 3rd; Weber, C.R.; Chen, X.; Weisser-Thomas, J.; Margulies, K.B.; Bers, D.M.; Houser, S.R. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ. Res. 2003, 92, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Oakley, C.I.; Vallejo, J.A.; Wang, D.; Gray, M.A.; Tiede-Lewis, L.M.; Shawgo, T.; Daon, E.; Zorn, G., 3rd; Stubbs, J.R.; Wacker, M.J. Trimethylamine-N-oxide acutely increases cardiac muscle contractility. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1272–H1282. [Google Scholar] [CrossRef]
- Videja, M.; Vilskersts, R.; Korzh, S.; Cirule, H.; Sevostjanovs, E.; Dambrova, M.; Makrecka-Kuka, M. Microbiota-derived metabolite Trimethylamine N-Oxide protects mitochondrial energy metabolism and cardiac functionality in a rat model of right ventricle heart failure. Front. Cell Dev. Biol. 2020, 8, 622741. [Google Scholar] [CrossRef]
- Jaworska, K.; Bielinska, K.; Gawrys-Kopczynska, M.; Ufnal, M. TMA (trimethylamine), but not its oxide TMAO (trimethylamine-oxide), exerts haemodynamic effects: Implications for interpretation of cardiovascular actions of gut microbiome. Cardiovasc. Res. 2019, 115, 1948–1949. [Google Scholar] [CrossRef]
- Frey, N.; Olson, E.N. Cardiac hypertrophy: The good, the bad and the ugly. Annu. Rev. Physiol. 2003, 65, 45–79. [Google Scholar] [CrossRef]
- Richter, K.; Kietzmann, T. Reactive oxygen species and fibrosis: Further evidence of a significant liaison. Cell Tissue Res. 2016, 365, 591–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, Y.; Jin, L.; Kee, H.J.; Piao, Z.H.; Cho, J.Y.; Kim, G.R.; Choi, S.Y.; Lin, M.Q.; Jeong, M.H. Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and Smad3 binding activity. Sci. Rep. 2016, 6, 34790. [Google Scholar] [CrossRef] [Green Version]
- Makrecka-Kuka, M.; Volska, K.; Antone, U.; Vilskersts, R.; Grinberga, S.; Bandere, D.; Liepinsh, E.; Dambrova, M. Trimethylamine N-oxide impairs pyruvate and fatty acid oxidation in cardiac mitochondria. Toxicol. Lett. 2017, 267, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, M. A novel action of insulin on cardiac membrane. Circ. Res. 2003, 92, 707–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doenst, T.; Pytel, G.; Schrepper, A.; Amorim, P.; Farber, G.; Shingu, Y.; Mohr, F.W.; Schwarzer, M. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc. Res. 2010, 86, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Niizuma, S.; Inuzuka, Y.; Kawashima, T.; Okuda, J.; Tamaki, Y.; Iwanaga, Y.; Narazaki, M.; Matsuda, T.; Soga, T.; et al. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ. Heart Fail. 2010, 3, 420–430. [Google Scholar] [CrossRef] [Green Version]
- Mori, J.; Basu, R.; McLean, B.A.; Das, S.K.; Zhang, L.; Patel, V.B.; Wagg, C.S.; Kassiri, Z.; Lopaschuk, G.D.; Oudit, G.Y. Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: A metabolic contribution to heart failure with normal ejection fraction. Circ. Heart Fail. 2012, 5, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Osorio, J.C.; Stanley, W.C.; Linke, A.; Castellari, M.; Diep, Q.N.; Panchal, A.R.; Hintze, T.H.; Lopaschuk, G.D.; Recchia, F.A. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation 2002, 106, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kappaB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef] [Green Version]
- Querio, G.; Antoniotti, S.; Levi, R.; Gallo, M.P. Trimethylamine N-Oxide does not impact viability, ROS production, and mitochondrial membrane potential of adult rat cardiomyocytes. Int. J. Mol. Sci. 2019, 20, 3045. [Google Scholar] [CrossRef] [Green Version]
- Huc, T.; Drapala, A.; Gawrys, M.; Konop, M.; Bielinska, K.; Zaorska, E.; Samborowska, E.; Wyczalkowska-Tomasik, A.; Paczek, L.; Dadlez, M.; et al. Chronic, low-dose TMAO treatment reduces diastolic dysfunction and heart fibrosis in hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1805–H1820. [Google Scholar] [CrossRef]
- Steiner, D.J.; Kim, A.; Miller, K.; Hara, M. Pancreatic islet plasticity: Interspecies comparison of islet architecture and composition. Islets 2010, 2, 135–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ionescu-Tirgoviste, C.; Gagniuc, P.A.; Gubceac, E.; Mardare, L.; Popescu, I.; Dima, S.; Militaru, M. A 3D map of the islet routes throughout the healthy human pancreas. Sci. Rep. 2015, 5, 14634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Misawa, R.; Zielinski, M.C.; Cowen, P.; Jo, J.; Periwal, V.; Ricordi, C.; Khan, A.; Szust, J.; Shen, J.; et al. Regional differences in islet distribution in the human pancreas--preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS ONE 2013, 8, e67454. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krueger, E.S.; Lloyd, T.S.; Tessem, J.S. The Accumulation and Molecular Effects of Trimethylamine N-Oxide on Metabolic Tissues: It’s Not All Bad. Nutrients 2021, 13, 2873. https://doi.org/10.3390/nu13082873
Krueger ES, Lloyd TS, Tessem JS. The Accumulation and Molecular Effects of Trimethylamine N-Oxide on Metabolic Tissues: It’s Not All Bad. Nutrients. 2021; 13(8):2873. https://doi.org/10.3390/nu13082873
Chicago/Turabian StyleKrueger, Emily S., Trevor S. Lloyd, and Jeffery S. Tessem. 2021. "The Accumulation and Molecular Effects of Trimethylamine N-Oxide on Metabolic Tissues: It’s Not All Bad" Nutrients 13, no. 8: 2873. https://doi.org/10.3390/nu13082873
APA StyleKrueger, E. S., Lloyd, T. S., & Tessem, J. S. (2021). The Accumulation and Molecular Effects of Trimethylamine N-Oxide on Metabolic Tissues: It’s Not All Bad. Nutrients, 13(8), 2873. https://doi.org/10.3390/nu13082873