Korean Red Ginseng Ameliorates Fatigue via Modulation of 5-HT and Corticosterone in a Sleep-Deprived Mouse Model
Abstract
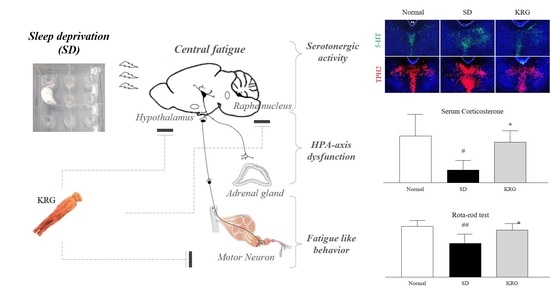
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Fingerprinting of KRG
2.3. Animals and Care
2.4. Apparatus for Inducing Sleep Deprivation
2.5. Experimental Design
2.6. Rotarod Test
2.7. Grip Strength Test
2.8. Blood Collection and Brain Tissue Preparation
2.9. Determination of the Corticosterone Level in Serum
2.10. Western Blot Analysis
2.11. Immunofluorescence Staining
2.12. Statistical Analysis
3. Results
3.1. Development of an SD-Induced Fatigue Model
3.2. Effects of KRG on Fatigue-Like Behaviors
3.3. Effects of KRG on the Levels of Corticosterone and/or Its Receptor in Serum and Brain Tissues
3.4. Effects of KRG on the CREB–BDNF Pathway in the Brain
3.5. Effects of KRG on the Levels of 5-HT, TPH2, and 5-HT Autoreceptors and Transporters in the Brain
3.6. Effects of KRG on DA and TH Levels in the Brain
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Finsterer, J.; Mahjoub, S.Z. Fatigue in healthy and diseased individuals. Am. J. Hosp. Palliat. Med. 2014, 31, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Son, C.-G. Differential diagnosis between “chronic fatigue” and “chronic fatigue syndrome”. Integr. Med. Res. 2019, 8, 89. [Google Scholar] [CrossRef]
- Wessely, S.; Chalder, T.; Hirsch, S.; Wallace, P.; Wright, D. The prevalence and morbidity of chronic fatigue and chronic fa-tigue syndrome: A prospective primary care study. Am. J. Public Health 1997, 87, 1449–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hvidberg, M.F.; Brinth, L.; Olesen, A.V.; Petersen, K.D.; Ehlers, L.H. The Health-Related Quality of Life for Patients with Myalgic Encephalomyelitis / Chronic Fatigue Syndrome (ME/CFS). PLoS ONE 2015, 10, e0132421. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; Watson, P.; Hasegawa, H.; Roelands, B.; Piacentini, M.F. Central fatigue. Sports Med. 2006, 36, 881–909. [Google Scholar] [CrossRef]
- Cotel, F.; Exley, R.; Cragg, S.J.; Perrier, J.-F. Serotonin spillover onto the axon initial segment of motoneurons induces cen-tral fatigue by inhibiting action potential initiation. Proc. Natl. Acad. Sci. USA 2013, 110, 4774–4779. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, M.; Yamamoto, T. Tryptophan and Kynurenic Acid May Produce an Amplified Effect in Central Fatigue Induced by Chronic Sleep Disorder. Int. J. Tryptophan Res. 2014, 7, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Cleare, A.J. The Neuroendocrinology of Chronic Fatigue Syndrome. Endocr. Rev. 2003, 24, 236–252. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Fossey, M.; Libman, E.; Bailes, S.; Baltzan, M.; Schondorf, R.; Amsel, R.; Fichten, C.S. Sleep quality and psychological ad-justment in chronic fatigue syndrome. J. Behav. Med. 2004, 27, 581–605. [Google Scholar] [CrossRef] [Green Version]
- Saper, C.B.; Scammell, T.E.; Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005, 437, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, A.S.; Cleare, A.J. Hypothalamic–pituitary–adrenal axis dysfunction in chronic fatigue syndrome. Nat. Rev. Endocrinol. 2012, 8, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Shergis, J.; Zhang, A.L.; Zhou, W.; Xue, C.C. Panax ginseng in Randomised Controlled Trials: A Systematic Review. Phytother. Res. 2012, 27, 949–965. [Google Scholar] [CrossRef] [PubMed]
- Arring, N.M.; Millstine, D.; Marks, L.A.; Nail, L.M. Ginseng as a treatment for fatigue: A systematic review. J. Altern. Complementary Med. 2018, 24, 624–633. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Huang, X.; Liu, S.; Guo, C.; Xie, Y.; Meijer, A.H.; Wang, M. The Difference between White and Red Ginseng: Variations in Ginsenosides and Immunomodulation. Planta Med. 2018, 84, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-M.; Yoon, H.; Park, H.-M.; Song, B.C.; Yeum, K.-J. Implications of red Panax ginseng in oxidative stress associated chronic diseases. J. Ginseng Res. 2017, 41, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.-Y.; Rong, P.-Q.; Liang, H.-Y.; Zhang, P.-P.; Zheng, G.-Q.; Lin, Y. Clinical and Preclinical Systematic Review of Panax ginseng CA Mey and Its Compounds for Fatigue. Front. Pharmacol. 2020, 11, 1031. [Google Scholar] [CrossRef]
- In, G.; Ahn, N.-G.; Bae, B.-S.; Lee, M.-W.; Park, H.-W.; Jang, K.H.; Cho, B.-G.; Han, C.K.; Park, C.K.; Kwak, Y.-S. In situ analy-sis of chemical components induced by steaming between fresh ginseng, steamed ginseng, and red ginseng. J. Ginseng Res. 2017, 41, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Suchecki, D.; Palma, B.D.; Tufik, S. Sleep rebound in animals deprived of paradoxical sleep by the modified multiple plat-form method. Brain Res. 2000, 875, 14–22. [Google Scholar] [CrossRef]
- Weaver, S.A.; Janal, M.N.; Aktan, N.; Ottenweller, J.E.; Natelson, B.H. Sex differences in plasma prolactin response to tryp-tophan in chronic fatigue syndrome patients with and without comorbid fibromyalgia. J. Women’s Health 2010, 19, 951–958. [Google Scholar] [CrossRef] [Green Version]
- Kashi, A.A.; Davis, R.W.; Phair, R.D. The IDO Metabolic Trap Hypothesis for the Etiology of ME/CFS. Diagnostics 2019, 9, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, D.; Lima, N.; Coimbra, C.; Marubayashi, U. Evidence that tryptophan reduces mechanical efficiency and running performance in rats. Pharmacol. Biochem. Behav. 2003, 74, 357–362. [Google Scholar] [CrossRef]
- Potter, G.; Skene, D.; Arendt, J.; Cade, J.; Grant, P.J.; Hardie, L.J. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr. Rev. 2016, 37, 584–608. [Google Scholar] [CrossRef] [Green Version]
- Clow, A.; Hucklebridge, F.; Stalder, T.; Evans, P.; Thorn, L. The cortisol awakening response: More than a measure of HPA axis function. Neurosci. Biobehav. Rev. 2010, 35, 97–103. [Google Scholar] [CrossRef]
- Jain, F.A.; Connolly, C.G.; Reus, V.I.; Meyerhoff, D.J.; Yang, T.T.; Mellon, S.H.; Mackin, S.; Hough, C.M.; Morford, A.; Wolkowitz, O.M. Cortisol, moderated by age, is associated with antidepressant treatment outcome and memory improvement in Major Depressive Disorder: A retrospective analysis. Psychoneuroendocrinology 2019, 109, 104386. [Google Scholar] [CrossRef]
- Clayton, E.W. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: An IOM report on redefining an illness. JAMA 2015, 313, 1101–1102. [Google Scholar] [CrossRef]
- Klaver-Krol, E.; Hermens, H.; Vermeulen, R.; Klaver, M.; Luyten, H.; Henriquez, N.; Zwarts, M. Chronic fatigue syndrome: Abnormally fast muscle fiber conduction in the membranes of motor units at low static force load. Clin. Neurophysiol. 2021, 132, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Nagata, A.; Nakayama, K.; Nakamura, S.; Mochizuki, A.; Gemba, C.; Aoki, R.; Dantsuji, M.; Maki, K.; Inoue, T. Serotonin1B receptor-mediated presynaptic inhibition of proprioceptive sensory inputs to jaw-closing motoneurons. Brain Res. Bull. 2019, 149, 260–267. [Google Scholar] [CrossRef]
- Cristina-Souza, G.; Santos-Mariano, A.C.; Lima-Silva, A.E.; Costa, P.L.; Domingos, P.R.; Silva, S.F.; Abreu, W.C.; De-Oliveira, F.R.; Osiecki, R. Panax ginseng Supplementation Increases Muscle Recruitment, Attenuates Perceived Effort, and Accelerates Muscle Force Recovery After an Eccentric-Based Exercise in Athletes. J. Strength Cond. Res. 2020. [Google Scholar] [CrossRef]
- Jones, B.J.; Roberts, D.J. The quantitative measurement of motor inco-ordination in naive mice using an accelerating rotarod. J. Pharm. Pharmacol. 1968, 20, 302–304. [Google Scholar] [CrossRef]
- Oikonomou, G.; Altermatt, M.; Zhang, R.-W.; Coughlin, G.M.; Montz, C.; Gradinaru, V.; Prober, D.A. The Serotonergic Raphe Promote Sleep in Zebrafish and Mice. Neuron 2019, 103, 686–701.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, S.K.; Ang, J.E.; Revell, V.L.; Holmes, B.; Mann, A.; Robertson, F.P.; Cui, N.; Middleton, B.; Ackermann, K.; Kayser, M. Effect of sleep deprivation on the human metabolome. Proc. Natl. Acad. Sci. USA 2014, 111, 10761–10766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evrard, A.; Barden, N.; Hamon, M.; Adrien, J. Glucocorticoid Receptor-Dependent Desensitization of 5-HT1A Autoreceptors by Sleep Deprivation: Studies in GR-i Transgenic Mice. Sleep 2006, 29, 31–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudnick, G.; Sandtner, W. Serotonin transport in the 21st century. J. Gen. Physiol. 2019, 151, 1248–1264. [Google Scholar] [CrossRef] [Green Version]
- Mariman, A.N.; Vogelaers, D.P.; Tobback, E.; Delesie, L.M.; Hanoulle, I.P.; Pevernagie, D.A. Sleep in the chronic fatigue syndrome. Sleep Med. Rev. 2013, 17, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Ross, I.L.; Thomas, K.G.F. Reduced slow-wave sleep and altered diurnal cortisol rhythms in patients with Addi-son’s disease. Eur. J. Endocrinol. 2018, 179, 319–330. [Google Scholar] [CrossRef] [Green Version]
- Russell, G.; Lightman, S. The human stress response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef] [Green Version]
- Nijhof, S.L.; Rutten, J.M.; Uiterwaal, C.S.; Bleijenberg, G.; Kimpen, J.L.; van de Putte, E.M. The role of hypocortisolism in chronic fatigue syndrome. Psychoneuroendocrinology 2014, 42, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Bains, J.S.; Cusulin, J.I.W.; Inoue, W. Stress-related synaptic plasticity in the hypothalamus. Nat. Rev. Neurosci. 2015, 16, 377–388. [Google Scholar] [CrossRef]
- Tapia-Arancibia, L.; Rage, F.; Givalois, L.; Arancibia, S. Physiology of BDNF: Focus on hypothalamic function. Front. Neuroendocr. 2004. [CrossRef]
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef]
- Cho, J.R.; Treweek, J.B.; Robinson, J.E.; Xiao, C.; Bremner, L.R.; Greenbaum, A.; Gradinaru, V. Dorsal raphe dopamine neu-rons modulate arousal and promote wakefulness by salient stimuli. Neuron 2017, 94, 1205–1219.e1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, S.-Y.; Bae, W.K.; Ahn, H.-Y.; Choi, S.-E.; Jung, G.-C.; Yeom, C.H. Intravenous Vitamin C administration reduces fatigue in office workers: A double-blind randomized controlled trial. Nutr. J. 2012, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafat, A.; Butler, P.; Jensen, R.L.; Donnelly, A. Effects of dietary supplementation with vitamins C and E on muscle function during and after eccentric contractions in humans. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 93, 196–202. [Google Scholar] [CrossRef]
- Adams, D.; Wu, T.; Yang, X.; Tai, S.; Vohra, S. Traditional Chinese medicinal herbs for the treatment of idiopathic chronic fatigue and chronic fatigue syndrome. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.; Cheng, Y.; Chen, Q.; Tan, H.; Son, D.; Chang, D.; Bian, Z.-X.; Fang, H.; Xu, H. Safety and antifatigue effect of Korean Red Ginseng: A randomized, double-blind, and placebo-controlled clinical trial. J. Ginseng Res. 2019, 43, 676–683. [Google Scholar] [CrossRef]
- Sung, W.S.; Kang, H.R.; Jung, C.Y.; Park, S.S.; Lee, S.H.; Kim, E.J. Efficacy of Korean red ginseng (Panax ginseng) for mid-dle-aged and moderate level of chronic fatigue patients: A randomized, double-blind, placebo-controlled trial. Complementary Ther. Med. 2020, 48, 102246. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, B.; Kim, H.; Kim, M. Effects of Red Ginseng on Exercise Capacity and Peripheral Fatigue in Mice. Phys. Ther. Rehabil. Sci. 2021, 10, 175–184. [Google Scholar] [CrossRef]
- Lee, S.M.; Bae, B.-S.; Park, H.-W.; Ahn, N.-G.; Cho, B.-G.; Cho, Y.-L.; Kwak, Y.-S. Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J. Ginseng Res. 2015, 39, 384–391. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.-Y.; Kim, D.-Y.; Lee, J.-S.; Hwang, S.-J.; Kim, G.-H.; Hyun, S.-H.; Son, C.-G. Korean Red Ginseng Ameliorates Fatigue via Modulation of 5-HT and Corticosterone in a Sleep-Deprived Mouse Model. Nutrients 2021, 13, 3121. https://doi.org/10.3390/nu13093121
Kang J-Y, Kim D-Y, Lee J-S, Hwang S-J, Kim G-H, Hyun S-H, Son C-G. Korean Red Ginseng Ameliorates Fatigue via Modulation of 5-HT and Corticosterone in a Sleep-Deprived Mouse Model. Nutrients. 2021; 13(9):3121. https://doi.org/10.3390/nu13093121
Chicago/Turabian StyleKang, Ji-Yun, Do-Young Kim, Jin-Seok Lee, Seung-Ju Hwang, Geon-Ho Kim, Sun-Hee Hyun, and Chang-Gue Son. 2021. "Korean Red Ginseng Ameliorates Fatigue via Modulation of 5-HT and Corticosterone in a Sleep-Deprived Mouse Model" Nutrients 13, no. 9: 3121. https://doi.org/10.3390/nu13093121
APA StyleKang, J.-Y., Kim, D.-Y., Lee, J.-S., Hwang, S.-J., Kim, G.-H., Hyun, S.-H., & Son, C.-G. (2021). Korean Red Ginseng Ameliorates Fatigue via Modulation of 5-HT and Corticosterone in a Sleep-Deprived Mouse Model. Nutrients, 13(9), 3121. https://doi.org/10.3390/nu13093121