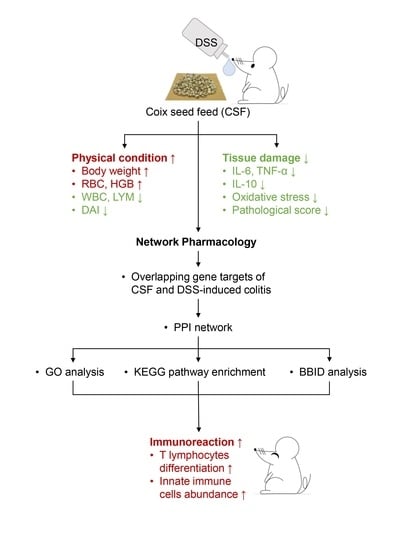
Coix Seed Diet Ameliorates Immune Function Disorders in Experimental Colitis Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Coix Seed Feed (CSF)
2.2. Animal Experiments
2.3. Histological Analysis
2.4. Enzyme Linked Immunosorbent Assay and Oxidative Damage Assay
2.5. Ultra-Performance Liquid Chromatography–Mass Spectrometry (UPLC–MS/MS) Analysis of CSF
2.6. Exploration of the Mechanism of CSF against Dextran Sulfate Sodium (DSS)-Induced Colitis
2.6.1. CSF Target Acquisition
2.6.2. DSS-Induced Colitis Target Acquisition
2.6.3. Protein–Protein Interaction Network Construction
2.6.4. Gene Ontology, Kyoto Encyclopedia of Genes and Genomes Pathway Enrichment Analysis, and Biological Biochemical Image Database Analysis
2.7. Flow Cytometry Analysis of Colonic Immune Cell Authentication
2.8. Statistics
3. Results
3.1. CSF Alleviated the DSS-Induced Colitis Mice’s Symptoms
3.2. CSF Ameliorated the Hemogram of the Colitis Mice
3.3. CSF Relieved the Inflammatory Cytokine Secretion and Oxidative Stress in the Colon Tissue of the Colitis Mice
3.4. The Protein–Protein Interaction (PPI) Network Construction of CSF and DSS-Induced Colitis Mice
3.5. The Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analyses of 53 Targets in CSF against DSS-Induced Colitis
3.6. CSF Altered the T-Lymphocyte Subsets in the Colon Tissue of the Colitis Mice
3.7. CSF Changed the Innate Immune Cell Abundance in the Colon of the Colitis Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, D.; Dong, M.; Dai, C.; Wu, S. Inflammation and Inflammatory Cytokine Contribute to the Initiation and Development of Ulcerative Colitis and Its Associated Cancer. Inflamm. Bowel Dis. 2019, 25, 1595–1602. [Google Scholar] [CrossRef]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and Functions of the IL-10 Family of Cytokines in Inflammation and Disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Knaus, U.G. ROS in gastrointestinal inflammation: Rescue Or Sabotage? Br. J. Pharmacol. 2017, 174, 1704–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jena, G.; Trivedi, P.P.; Sandala, B. Oxidative stress in ulcerative colitis: An old concept but a new concern. Free Radic. Res. 2012, 46, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Kobasyashi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 73. [Google Scholar]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J. Ulcerative colitis. Lancet 2017, 10080, 1756–1770. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Chen, H.-H.; Chiang, W. Adlay (yì yĭ; “soft-shelled job’s tears”; the seeds of Coix lachryma-jobi L. var. ma-yuen Stapf) is a Potential Cancer Chemopreventive Agent toward Multistage Carcinogenesis Processes. J. Tradit. Complement. Med. 2012, 2, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Shuang-xi, Z.; Xiao-feng, S.; Xiang-an, Z.; Yong-kang, A. Clinical effects of Yiyi Fuzi Baijiang Powder combined with mesalazine on patients with ulcerative colitis. Chin. Tradit. Pat. Med. 2019, 41, 2642–2646. [Google Scholar]
- DWang, D.; Yang, C.; Wang, Z.; Yang, Y.; Li, D.; Ding, X.; Xu, W.; Zheng, Q. Norcantharidin combined with Coix seed oil synergistically induces apoptosis and inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation. Sci. Rep. 2017, 7, 9373. [Google Scholar]
- Praengam, K.; Sahasakul, Y.; Kupradinun, P.; Sakarin, S.; Sanitchua, W.; Rungsipipat, A.; Rattanapinyopituk, K.; Angkasekwinai, P.; Changsri, K.; Mhuantong, W.; et al. Brown rice and retrograded brown rice alleviate inflammatory response in dextran sulfate sodium (DSS)-induced colitis mice. Food Funct. 2017, 8, 4630–46433. [Google Scholar] [CrossRef]
- Høivik, M.L.; Reinisch, W.; Cvancarova, M.; Moum, B.; The IBSEN Study Group. Anaemia in inflammatory bowel disease: A population-based 10-year follow-up. Aliment. Pharmacol. Ther. 2013, 39, 69–76. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, A.; Miyake, S.; Saga, R.; Chiba, A.; Mochizuki, H.; Yamamura, T. Gut environment-induced intraepithelial autoreactive CD4+ T cells suppress central nervous system autoimmunity via LAG-3. Nat. Commun. 2016, 7, 11639. [Google Scholar] [CrossRef] [Green Version]
- Noble, A.; Durant, L.; Hoyles, L.; McCartney, A.L.; Man, R.; Segal, J.; Costello, S.P.; Hendy, P.; Reddi, D.; Bouri, S.; et al. Deficient Resident Memory T Cell and CD8 T Cell Response to Commensals in Inflammatory Bowel Disease. J. Crohn’s Colitis 2020, 14, 525–537. [Google Scholar] [CrossRef]
- Jaeger, N.; Gamini, R.; Cella, M.; Schettini, J.L.; Bugatti, M.; Zhao, S.; Rosadini, C.V.; Esaulova, E.; Di Luccia, B.; Kinnett, B.; et al. Single-cell analyses of Crohn’s disease tissues reveal intestinal intraepithelial T cells heterogeneity and altered subset distributions. Nat. Commun. 2021, 12, 1921. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Qiu, Y.; Yang, H. CD4CD8αα IELs: They Have Something to Say. Front. Immunol. 2019, 10, 02269. [Google Scholar] [CrossRef] [PubMed]
- Sarrabayrouse, G.; Bossard, C.; Chauvin, J.-M.; Jarry, A.; Meurette, G.; Quévrain, E.; Bridonneau, C.; Preisser, L.; Asehnoune, K.; Labarrière, N.; et al. CD4CD8αα lymphocytes, a novel human regulatory T cell subset induced by colonic bacteria and deficient in patients with inflammatory bowel disease. PLoS Biol. 2014, 12, e1001833. [Google Scholar] [CrossRef] [Green Version]
- Marichal, T.; Boyman, O.; Reber, L.L. Editorial: Role of Neutrophils in Inflammatory Diseases. Front. Immunol. 2020, 11, 627939. [Google Scholar] [CrossRef] [PubMed]
- Sumagin, R.; Brazil, J.C.; Nava, P.; Nishio, H.; Alam, A.; Luissint, A.C.; Weber, D.A.; Neish, A.S.; Nusrat, A.; Parkos, C.A. Neutrophil interactions with epithelial-expressed ICAM-1 enhances intestinal mucosal wound healing. Mucosal Immunol. 2016, 9, 1151–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Worbs, T.; Hammerschmidt, S.I.; Förster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017, 17, 30–48. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2018, 19, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fournier, B.; Parkos, C.A. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012, 5, 354–366. [Google Scholar] [CrossRef]







| Ingredient | Maintenance Feed | Coix Seed Feed |
|---|---|---|
| crude protein (%) | 20.00 | 18.29 |
| crude fat (%) | 4.50 | 4.06 |
| crude fiber (%) | 3.70 | 3.69 |
| crude ash (%) | 6.53 | 3.12 |
| calcium (%) | 1.19 | 1.28 |
| phosphorus (%) | 0.77 | 0.79 |
| lysine (%) | 0.93 | 0.86 |
| methionine and cysteine (%) | 0.67 | 0.56 |
| arginine (%) | 1.02 | 0.96 |
| histidine (%) | 0.50 | 0.48 |
| tryptophan (%) | 0.21 | 0.21 |
| phenylalanine and phenylalanine (%) | 0.51 | 1.44 |
| threonine (%) | 0.78 | 0.64 |
| leucine (%) | 1.59 | 1.43 |
| isoleucine(%) | 0.76 | 0.72 |
| valine (%) | 0.90 | 0.87 |
| magnesium (%) | 0.26 | 0.26 |
| kalium (%) | 0.64 | 0.64 |
| Natrium (%) | 0.32 | 0.32 |
| vitamin K (mg/kg) | 6.15 | 6.15 |
| vitamin B1 (mg/kg) | 16.00 | 16.00 |
| vitamin B2 (mg/kg) | 16.03 | 16.03 |
| vitamin B6 (mg/kg) | 10.43 | 10.43 |
| nicotinic acid (mg/kg) | 89.00 | 89.00 |
| Pantothenic acid (mg/kg) | 30.09 | 30.09 |
| Folic acid (mg/kg) | 7.50 | 7.50 |
| Biotin (mg/kg) | 0.28 | 0.28 |
| vitamin B12 (mg/kg) | 0.03 | 0.03 |
| Choline (mg/kg) | 1900.00 | 1900.00 |
| iron (mg/kg) | 180.00 | 180.00 |
| manganese (mg/kg) | 123.50 | 123.50 |
| copper (mg/kg) | 17.80 | 17.80 |
| zinc (mg/kg) | 56.20 | 56.20 |
| iodine (mg/kg) | 0.61 | 0.61 |
| selenium (mg/kg) | 0.16 | 0.16 |
| vitamin A(KIU/kg) | 10.70 | 10.70 |
| vitamin D (KIU/kg) | 1.50 | 1.50 |
| vitamin E (KIU/kg) | 103.00 | 103.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Yu, R.; Liu, T.; Li, Y.; Zhong, J.; Zhang, T.; Liu, Z.; Hu, Y. Coix Seed Diet Ameliorates Immune Function Disorders in Experimental Colitis Mice. Nutrients 2022, 14, 123. https://doi.org/10.3390/nu14010123
Zhou Q, Yu R, Liu T, Li Y, Zhong J, Zhang T, Liu Z, Hu Y. Coix Seed Diet Ameliorates Immune Function Disorders in Experimental Colitis Mice. Nutrients. 2022; 14(1):123. https://doi.org/10.3390/nu14010123
Chicago/Turabian StyleZhou, Qilyu, Ruyang Yu, Tianlong Liu, Yeye Li, Jia Zhong, Tao Zhang, Zhongjie Liu, and Yusheng Hu. 2022. "Coix Seed Diet Ameliorates Immune Function Disorders in Experimental Colitis Mice" Nutrients 14, no. 1: 123. https://doi.org/10.3390/nu14010123
APA StyleZhou, Q., Yu, R., Liu, T., Li, Y., Zhong, J., Zhang, T., Liu, Z., & Hu, Y. (2022). Coix Seed Diet Ameliorates Immune Function Disorders in Experimental Colitis Mice. Nutrients, 14(1), 123. https://doi.org/10.3390/nu14010123