Ameliorative Effect of Citrus junos Tanaka Waste (By-Product) Water Extract on Particulate Matter 10-Induced Lung Damage
Abstract
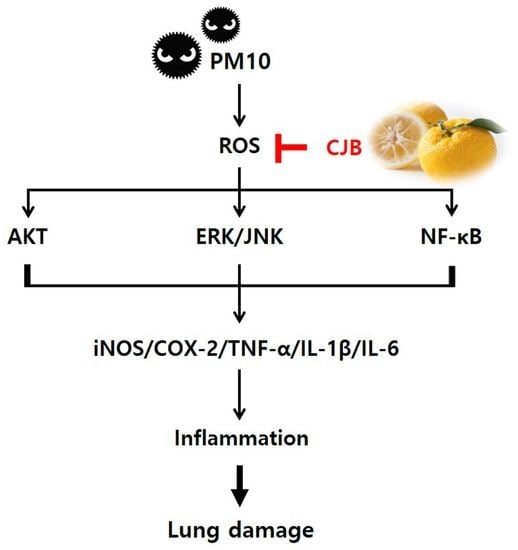
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Antioxidant Capacity and Component Assay
2.2.1. ABTS Radical Scavenging Assay
2.2.2. DPPH Radical Scavenging Assay
2.2.3. Total Polyphenol Content
2.2.4. Total Flavonoid Content
2.3. Analysis of Naringin and Hesperidin Content
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Intracellular Anti-Oxidative Assay
2.6.1. Nitric Oxide (NO) Analysis
2.6.2. Intracellular Levels of ROS
2.7. Cell Treatment for Protein Expression
2.8. Animals and Treatment
2.9. Histological Analysis
2.10. ELISA Assay
2.11. Immunoblot Analysis
2.12. Statistical Analysis
3. Results
3.1. Antioxidant Capacity and Phytochemicals in CJBs
3.2. Effects of CJB on PM10-Induced Pulmonary Damage
3.3. Effects of CJBs on PM10-Induced Pulmonary Inflammation
3.4. Effects of CJBs on PM10-Induced Signaling Pathways
3.5. Effects of CJBs on Nitric Oxide Production and Inflammation in RAW264.7 Macrophages
3.6. Effects of CJBs on PM10-Induced ROS Generation in A549 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abe, H.; Ishioka, M.; Fujita, Y.; Umeno, A.; Yasunaga, M.; Sato, A.; Ohnishi, S.; Suzuki, S.; Ishida, N.; Shichiri, M.; et al. Yuzu (Citrus junos Tanaka) Peel Attenuates Dextran Sulfate Sodium-induced Murine Experimental Colitis. J. Oleo Sci. 2018, 67, 335–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.W.; Jo, E.H.; Moon, J.E.; Cha, H.; Chang, M.H.; Cho, H.T.; Lee, M.K.; Jung, W.S.; Lee, J.H.; Heo, W.; et al. In Vitro and In Vivo Inhibitory Effect of Citrus Junos Tanaka Peel Extract against Oxidative Stress-Induced Apoptotic Death of Lung Cells. Antioxidants 2020, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.; Jo, A.; Shin, J.; Lim, E.H.; Lee, Y.E.; Jeong, D.E.; Lee, M. Anti-inflammatory activities of isogosferol, a furano-coumarin isolated from Citrus Junos seed shells through bioactivity-guided fractionation. Molecules 2019, 24, 4088. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.; Hirakawa, E.; Umeki, M.; Sakai, K.; Koya, M.; Oda, H.; Mochizuki, S.; Nobuoka, K.; Ishikawa, Y. Yuzu, Citrus junos, peels extract ameliorated hepatic steatosis induced by chloretone in rats. Food Sci. Technol. Res. 2021, 27, 281–292. [Google Scholar] [CrossRef]
- Shim, J.; Chae, J.; Cho, S. Identification and extraction optimization of active constituents in Citrus junos Sieb ex Tanaka peel and its biological evaluation. Molecules 2019, 24, 680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuraya, E.; Nakada, S.; Touyama, A.; Itoh, S. Improving the antioxidant functionality of Citrus junos Tanaka (yuzu) fruit juice by underwater shockwave pretreatment. Food Chem. 2016, 216, 123–129. [Google Scholar] [CrossRef]
- Nam, S.-H.; Cho, H.-S.; Jeong, H.; Lee, B.-B.; Cho, Y.-S.; Rameeza, F.; Eun, J.-B. Physiochemical properties, dietary fibers, and functional characterization of three yuzu cultivars at five harvesting times. Food Sci. Biotechnol. 2021, 30, 117–127. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Kim, H.-J.; Ko, M.-J.; Chung, M.-S. Recovery of hesperidin and narirutin from waste Citrus unshiu peel using subcritical water extraction aided by pulsed electric field treatment. Food Sci. Biotechnol. 2021, 30, 217–226. [Google Scholar] [CrossRef]
- Jo, A.; Lee, Y.E.; Jeong, D.E.; Oh, S.H.; Mun, M.J.; Lee, M. Simultaneous determination of the flavonoids and limonoids in Citrus Junos seed shells using a UPLC-DAD-ESI/MS. Nat. Prod. Sci. 2020, 26, 64–70. [Google Scholar]
- El-Toumy, S.A.; Hussein, A.A. Cold pressed yuzu (Citrus junos Sieb. ex Tanaka) oil. Cold Pressed Oils 2020, 2020, 711–718. [Google Scholar] [CrossRef]
- Fusaro, L.; Salvatori, E.; Winkler, A.; Frezzini, M.A.; De Santis, E.; Sagnotti, L.; Canepari, S.; Manes, F. Urban trees for bio-monitoring atmospheric particulate matter: An integrated approach combining plant functional traits, magnetic and chemical properties. Ecol. Ind. 2021, 126, 107707. [Google Scholar] [CrossRef]
- Zhu, C.; Maharajan, K.; Liu, K.; Zhang, Y. Role of atmospheric particulate matter exposure in COVID-19 and other health risks in human: A review. Environ. Res. 2021, 198, 111281. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, L.; Chen, K. The impact of ambient particulate matter on hospital outpatient visits for respiratory and cir-culatory system disease in an urban Chinese population. Sci. Total Environ. 2019, 666, 672–679. [Google Scholar] [CrossRef]
- Lee, D.C.; Oh, J.; Choi, H.; Kim, S.W.; Kim, S.W.; Kim, B.G.; Cho, J.H.; Lee, J.; Kim, J. Eupatilin inhibits reactive oxygen species generation via Akt/NF-κB/MAPK signaling pathways in particulate matter-exposed human bronchial epithelial cells. Toxics 2021, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, Y.; Qu, S.; Li, K.; Yang, T.; Yang, Y.; Zheng, Z.; Liu, H.; Wang, X.; Deng, S.; et al. The Protective Effects of Shengmai Formula Against Myocardial Injury Induced by Ultrafine Particulate Matter Exposure and Myocardial Ischemia are Mediated by the PI3K/AKT/p38 MAPK/Nrf2 Pathway. Front. Pharmacol. 2021, 12, 619311. [Google Scholar] [CrossRef]
- Pintha, K.; Chaiwangyen, W.; Yodkeeree, S.; Suttajit, M.; Tantipaiboonwong, P. Suppressive Effects of Rosmarinic Acid Rich Fraction from Perilla on Oxidative Stress, Inflammation and Metastasis Ability in A549 Cells Exposed to PM via C-Jun, P-65-Nf-Κb and Akt Signaling Pathways. Biomolecules 2021, 11, 1090. [Google Scholar] [CrossRef]
- Ullah, H.M.A.; Lee, Y.Y.; Kim, S.D.; Rhee, M.H. Duchesnea indica Extract Attenuates Coal Fly Ash-Induced Inflammation in Murine Alveolar Macrophages through the NF-Kappa B Pathway. Evid. Based Complement. Altern. Med. 2021, 2021, 5546052. [Google Scholar] [CrossRef]
- Lyu, Y.R.; Yang, W.-K.; Lee, S.-W.; Kim, S.-H.; Kim, D.-S.; Son, E.; Jung, I.C.; Park, Y.-C. Inhibitory effects of modified gamgil-tang in a particulate matter-induced lung injury mouse model. J. Ethnopharmacol. 2021, 284, 114789. [Google Scholar] [CrossRef]
- Yan, H.; Gulimire, A.; Zhu, J.; Liang, H.; Gu, Z. Punicalagin Suppresses LPS-induced Inflammatory Responses in Murine Macrophages via JAK/STAT Signaling Pathway and Zymosan-induced Mice Paw Edema. Indian J. Pharm. Educ. Res. 2021, 55, 550–555. [Google Scholar] [CrossRef]
- Lee, D.-H.; Woo, J.-K.; Heo, W.; Huang, W.-Y.; Kim, Y.; Chung, S.; Lee, G.-H.; Park, J.-W.; Han, B.-K.; Shin, E.-C.; et al. Citrus junos Tanaka Peel Extract and Its Bioactive Naringin Reduce Fine Dust-Induced Respiratory Injury Markers in BALB/c Male Mice. Nutrients 2022, 14, 1101. [Google Scholar] [CrossRef]
- Shalaby, E.A.; Shanab, S.M. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J. Geo. Mar. Sci. 2013, 42, 556–564. [Google Scholar]
- Singh, R.; Verma, P.; Singh, G. Total phenolic, flavonoids and tannin contents in different extracts of Artemisia absinthium. J. Intercult. Ethnopharmacol. 2012, 1, 101–104. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Qiu, X.; Xu, F.; Chen, R.; Gu, W.; Zhang, L.; Yang, S.; Cai, Z.; Liu, C. Difference on oxidative stress in lung epithelial cells and macrophages induced by ambient fine particulate matter (PM2.5). Air Qual. Atmosphere Heal. 2020, 13, 789–796. [Google Scholar] [CrossRef]
- Keum, H.; Kim, J.; Yoo, D.; Kim, T.W.; Seo, C.; Kim, D.; Jon, S. Biomimetic lipid Nanocomplexes incorporating STAT3-inhibiting peptides effectively infiltrate the lung barrier and ameliorate pulmonary fibrosis. J. Control. Release 2021, 332, 160–170. [Google Scholar] [CrossRef]
- Park, C.-H.; Min, S.-Y.; Yu, H.-W.; Kim, K.; Kim, S.; Lee, H.-J.; Kim, J.-H.; Park, Y.-J. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef]
- Xuan, T.; Gong, G.; Du, H.; Liu, C.; Wu, Y.; Bao, G.; Ma, Q.; Zhen, D. Protective effect of Pteryxin on LPS-induced acute lung injury via modulating MAPK/NF-κB pathway and NLRP3 inflammasome activation. J. Ethnopharmacol. 2022, 286, 114924. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; El-Ansary, A.E.; Mostafa, M.A.; Kamel, T.A.; Safwat, G. Evaluation of the phytochemical, antioxidant, anti-bacterial and anticancer activity of Prunus domestica fruit. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Sarker, U.; Oba, S.; Daramy, M.A. Nutrients, minerals, antioxidant pigments and phytochemicals, and antioxidant capacity of the leaves of stem amaranth. Sci. Rep. 2020, 10, 3892. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.; Li, H.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R. Citrus fla-vonoids as promising phytochemicals targeting diabetes and related complications: A systematic review of in vitro and in vivo studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An Overview of Bioactive Flavonoids from Citrus Fruits. Appl. Sci. 2021, 12, 29. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, D.; Tan, C.; Hu, Y.; Sundararajan, B.; Zhou, Z. Profiling of Flavonoid and Antioxidant Activity of Fruit Tissues from 27 Chinese Local Citrus Cultivars. Plants 2020, 9, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuyama, S.; Yamamoto, K.; Mori, H.; Sawamoto, A.; Amakura, Y.; Yoshimura, M.; Tamanaha, A.; Ohkubo, Y.; Sugawara, K.; Sudo, M. Neuroprotective effect of Citrus kawachiensis (Kawachi Bankan) peels, a rich source of naringin, against global cerebral ischemia/reperfusion injury in mice. Biosci. Biotechnol. Biochem. 2018, 82, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Piragine, E.; Piano, I.; Flori, L.; Da Pozzo, E.; Miragliotta, V.; Pirone, A.; Citi, V.; Mannelli, L.D.C.; Brogi, S.; et al. The Citrus Flavonoid Naringenin Protects the Myocardium from Ageing-Dependent Dysfunction: Potential Role of SIRT1. Oxidative Med. Cell. Longev. 2020, 2020, 4650207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, L.; Zhang, L.; Wang, X.; Xie, T.; Zhou, S.; Lu, S.; Liu, X.; Lu, H.; Xiao, K.; Wang, W.; et al. Oxidative Potential Induced by Ambient Particulate Matters with Acellular assays: A Review. Processes 2020, 8, 1410. [Google Scholar] [CrossRef]
- Ho, C.; Tsai, M.; Chen, Y.; Kuo, C.; Lin, P. Persistent elevation of blood pressure by ambient coarse particulate matter after recovery from pulmonary inflammation in mice. Environ. Toxicol. 2019, 34, 814–824. [Google Scholar] [CrossRef]
- Misiukiewicz-Stepien, P.; Paplinska-Goryca, M. Biological effect of PM10 on airway epithelium-focus on obstructive lung diseases. Clin. Immunol. 2021, 227, 108754. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Min, D.; Park, S.-Y.; Lee, J.; Bae, H. Standardized herbal extract PM014 alleviates fine dust-induced lung inflammation in mice. BMC Complement. Med. Ther. 2020, 20, 270. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Guascito, M.R.; Giordano, M.E.; Caricato, R.; De Bartolomeo, A.R.; Romano, M.P.; Conte, M.; Dinoi, A.; Contini, D. Oxidative potential, cytotoxicity, and intracellular oxidative stress generating capacity of PM10: A case study in South of Italy. Atmosphere 2021, 12, 464. [Google Scholar] [CrossRef]
- Seok, J.K.; Lee, J.; Kim, Y.M.; Boo, Y.C. Punicalagin and (–)-Epigallocatechin-3-Gallate Rescue Cell Viability and Attenuate In-flammatory Responses of Human Epidermal Keratinocytes Exposed to Airborne Particulate Matter PM10. Skin Pharmacol. Physiol. 2018, 31, 134–143. [Google Scholar] [CrossRef]
- García-Cuellar, C.M.; Chirino, Y.I.; Morales-Bárcenas, R.; Soto-Reyes, E.; Quintana-Belmares, R.; Santibáñez-Andrade, M.; Sánchez-Pérez, Y. Airborne particulate matter (PM10) inhibits apoptosis through PI3K/AKT/FoxO3a pathway in lung epi-thelial cells: The role of a second oxidant stimulus. Int. J. Mol. Sci. 2020, 21, 473. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-J.; Kim, D.-K. Effect of Airborne Particulate Matter on the Immunologic Characteristics of Chronic Rhinosinusitis with Nasal Polyps. Int. J. Mol. Sci. 2022, 23, 1018. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, P.S.; Jain, S.; Shrestha, S.; Senapati, S.; Puppala, S.P. Ambient endotoxin in PM10 and association with inflammatory activity, air pollutants, and meteorology, in Chitwan, Nepal. Sci. Total Environ. 2018, 618, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- van Leenen, K.; Jouret, J.; Demeyer, P.; Vermeir, P.; Leenknecht, D.; Van Driessche, L.; De Cremer, L.; Masmeijer, C.; Boyen, F.; Deprez, P. Particulate matter and airborne endotoxin concentration in Calf Barns and their association with lung consolida-tion, inflammation, and infection. J. Dairy Sci. 2021, 104, 5932–5947. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Hasan, A.; Shenouda, S.; Wilson, A.; Kochumon, S.; Ali, S.; Tuomilehto, J.; Sindhu, S.; Ahmad, R. TLR4/MyD88-Mediated CCL2 production by lipopolysaccharide (endotoxin): Implications for metabolic inflammation. J. Diabetes Metab. Disord. 2018, 17, 77–84. [Google Scholar] [CrossRef]








| ABTS Assay | DPPH Assay | Total Polyphenol Content | Total Flavonoid Content | Naringin | Hesperidin |
|---|---|---|---|---|---|
| mg VCE/g sample | mg GAE/g sample | mg RE/g sample | mg/g sample/retention time (min) | ||
| 23.47 ± 0.34 | 28.98 ± 0.42 | 36.68 ± 0.30 | 21.11 ± 0.49 | 4.00 ± 0.09 (23.8) | 9.00 ± 0.03 (25.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-Y.; Heo, W.; Jeong, I.; Kim, M.-J.; Han, B.-K.; Shin, E.-C.; Kim, Y.-J. Ameliorative Effect of Citrus junos Tanaka Waste (By-Product) Water Extract on Particulate Matter 10-Induced Lung Damage. Nutrients 2022, 14, 2270. https://doi.org/10.3390/nu14112270
Huang W-Y, Heo W, Jeong I, Kim M-J, Han B-K, Shin E-C, Kim Y-J. Ameliorative Effect of Citrus junos Tanaka Waste (By-Product) Water Extract on Particulate Matter 10-Induced Lung Damage. Nutrients. 2022; 14(11):2270. https://doi.org/10.3390/nu14112270
Chicago/Turabian StyleHuang, Wen-Yan, Wan Heo, Inhye Jeong, Mi-Jeong Kim, Bok-Kyung Han, Eui-Cheol Shin, and Young-Jun Kim. 2022. "Ameliorative Effect of Citrus junos Tanaka Waste (By-Product) Water Extract on Particulate Matter 10-Induced Lung Damage" Nutrients 14, no. 11: 2270. https://doi.org/10.3390/nu14112270
APA StyleHuang, W. -Y., Heo, W., Jeong, I., Kim, M. -J., Han, B. -K., Shin, E. -C., & Kim, Y. -J. (2022). Ameliorative Effect of Citrus junos Tanaka Waste (By-Product) Water Extract on Particulate Matter 10-Induced Lung Damage. Nutrients, 14(11), 2270. https://doi.org/10.3390/nu14112270