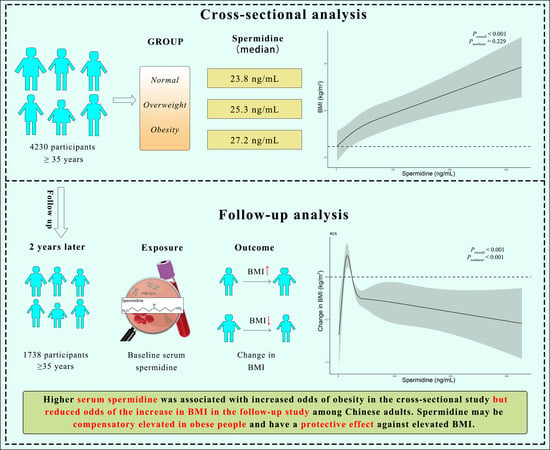
Elevation of Serum Spermidine in Obese Patients: Results from a Cross-Sectional and Follow-Up Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Detection of Spermidine in Serum Using High-Performance Liquid Chromatography with Fluorescence Detection (HPLC-FLD)
2.3. Definition of Obesity and Overweight
2.4. Assessment of Other Variables
2.5. Statistical Analysis
3. Results
3.1. Association between Serum Spermidine and Obesity as Well as BMI in a Cross-Sectional Study
3.2. Association of Serum Spermidine with the Change in BMI during a Follow-Up Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, B.; Zhao, Z.; Yang, L.; Zhang, M.; Jiang, Y.; Li, Y.; Zhou, M.; Wang, L.; Huang, Z.; et al. Body-mass index and obesity in urban and rural China: Findings from consecutive nationally representative surveys during 2004–18. Lancet 2021, 398, 53–63. [Google Scholar] [CrossRef]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [Green Version]
- Must, A.; Spadano, J.; Coakley, E.H.; Field, A.E.; Colditz, G.; Dietz, W.H. The disease burden associated with overweight and obesity. JAMA 1999, 282, 1523–1529. [Google Scholar] [CrossRef]
- Secord, A.A.; Hasselblad, V.; Von Gruenigen, V.E.; Gehrig, P.A.; Modesitt, S.C.; Bae-Jump, V.; Havrilesky, L.J. Body mass index and mortality in endometrial cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2016, 140, 184–190. [Google Scholar] [CrossRef]
- Park, B.; Kim, S.; Kim, H.; Cha, C.; Chung, M.S. Associations between obesity, metabolic health, and the risk of breast cancer in East Asian women. Br. J. Cancer 2021, 125, 1718–1725. [Google Scholar] [CrossRef]
- The Global BMI Mortality Collaboration; Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; Berrington de Gonzalez, A.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, K.; Kashiwagi, K. Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. 2010, 42, 39–51. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minois, N.; Carmona-Gutierrez, D.; Madeo, F. Polyamines in aging and disease. Aging 2011, 3, 716–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madeo, F.; Hofer, S.J.; Pendl, T.; Bauer, M.A.; Eisenberg, T.; Carmona-Gutierrez, D.; Kroemer, G. Nutritional Aspects of Spermidine. Annu. Rev. Nutr. 2020, 40, 135–159. [Google Scholar] [CrossRef] [PubMed]
- Kiechl, S.; Pechlaner, R.; Willeit, P.; Notdurfter, M.; Paulweber, B.; Willeit, K.; Werner, P.; Ruckenstuhl, C.; Iglseder, B.; Weger, S.; et al. Higher spermidine intake is linked to lower mortality: A prospective population-based study. Am. J. Clin. Nutr. 2018, 108, 371–380. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Chiba, F. Food polyamine and cardiovascular disease—An epidemiological study. Glob. J. Health Sci. 2012, 4, 170–178. [Google Scholar] [CrossRef]
- Pucciarelli, S.; Moreschini, B.; Micozzi, D.; De Fronzo, G.S.; Carpi, F.M.; Polzonetti, V.; Vincenzetti, S.; Mignini, F.; Napolioni, V. Spermidine and spermine are enriched in whole blood of nona/centenarians. Rejuvenation Res. 2012, 15, 590–595. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef]
- Wang, D.; Yin, J.; Zhou, Z.; Tao, Y.; Jia, Y.; Jie, H.; Zhao, J.; Li, R.; Li, Y.; Guo, C.; et al. Oral Spermidine Targets Brown Fat and Skeletal Muscle to Mitigate Diet-Induced Obesity and Metabolic Disorders. Mol. Nutr. Food Res. 2021, 65, e2100315. [Google Scholar] [CrossRef]
- Gao, M.; Zhao, W.; Li, C.; Xie, X.; Li, M.; Bi, Y.; Fang, F.; Du, Y.; Liu, X. Spermidine ameliorates non-alcoholic fatty liver disease through regulating lipid metabolism via AMPK. Biochem. Biophys. Res. Commun. 2018, 505, 93–98. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Hu, L.; Zhao, Y.; Zheng, L.; Yang, S.; Ni, L.; Fu, Z. Spermidine ameliorates high-fat diet-induced hepatic steatosis and adipose tissue inflammation in preexisting obese mice. Life Sci. 2021, 265, 118739. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Blin, M.G.; Song, J.; Wood, S.C.; Zhang, M.; Beard, D.A.; Goldstein, D.R. Age-Associated Mitochondrial Dysfunction Accelerates Atherogenesis. Circ. Res. 2020, 126, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C.F.; Kurdi, A.; Timmermans, J.P.; De Meyer, G.R.Y.; Martinet, W. Spermidine reduces lipid accumulation and necrotic core formation in atherosclerotic plaques via induction of autophagy. Atherosclerosis 2016, 251, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Ocaña-Wilhelmi, L.; Cardona, F.; Garrido-Sanchez, L.; Fernandez-Garcia, D.; Tinahones, F.J.; Ramos-Molina, B. Change in serum polyamine metabolome pattern after bariatric surgery in obese patients with metabolic syndrome. Surg. Obes. Relat. Dis. 2020, 16, 306–311. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Ben Hassen, C.; Fayosse, A.; Landré, B.; Raggi, M.; Bloomberg, M.; Sabia, S.; Singh-Manoux, A. Association between age at onset of multimorbidity and incidence of dementia: 30 year follow-up in Whitehall II prospective cohort study. BMJ Clin. Res. Ed. 2022, 376, e068005. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Sakuragi, M.; Takao, K.; Lefor, A.; Konishi, F. Long-term oral polyamine intake increases blood polyamine concentrations. J. Nutr. Sci. Vitaminol. 2009, 55, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Tavárez-Alonso, S.; Murria-Estal, R.; Herrera-Martín, G.; Alonso-Iglesias, E. Polyamines are increased in obese children and are related to markers of oxidative/nitrosative stress and angiogenesis. J. Clin. Endocrinol. Metab. 2011, 96, 2821–2825. [Google Scholar] [CrossRef] [Green Version]
- Fernández, Á.F.; Bárcena, C.; Martínez-García, G.G.; Tamargo-Gómez, I.; Suárez, M.F.; Pietrocola, F.; Castoldi, F.; Esteban, L.; Sierra-Filardi, E.; Boya, P.; et al. Autophagy couteracts weight gain, lipotoxicity and pancreatic β-cell death upon hypercaloric pro-diabetic regimens. Cell Death Dis. 2017, 8, e2970. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Normal (n = 1835) | Overweight (n = 1652) | Obesity (n = 743) | p-Value |
|---|---|---|---|---|
| Age, years | 60.3 ± 10.1 | 58.8 ± 9.7 | 57.7 ± 0.7 | <0.001 |
| Female sex, n (%) | 1120 (61.0%) | 1075 (65.1%) | 519 (69.9%) | <0.001 |
| Ethnicity, n (%) | 0.138 | |||
| Han | 1259 (68.6%) | 1059 (64.1%) | 452 (60.8%) | |
| Mongolian | 502 (27.4%) | 525 (31.8%) | 260 (35.0) | |
| Others | 74 (4.0%) | 68 (4.1%) | 31 (4.2%) | |
| Current smoking, n (%) | 782 (42.6%) | 574 (34.7%) | 212 (28.5%) | <0.001 |
| Current drinking, n (%) | 417 (22.7%) | 346 (20.9%) | 148 (19.9%) | 0.220 |
| Physical labor levels, n (%) | 0.841 | |||
| Low | 579 (31.6%) | 549 (33.2%) | 237 (31.9%) | |
| Moderate | 1190 (64.9%) | 1040 (63.0%) | 478 (64.3%) | |
| High | 66 (3.6%) | 63 (3.8%) | 28 (3.8%) | |
| Fruit/vegetables intake levels, n (%) | 0.300 | |||
| Few/day | 92 (5.0%) | 75 (4.5%) | 35 (4.7%) | |
| 250–500 g/day | 898 (48.9%) | 851 (51.5%) | 371 (49.9%) | |
| 500–1000 g/day | 732 (39.9%) | 634 (38.4%) | 278 (37.4%) | |
| >1000 g/day | 113 (6.2%) | 92 (5.6%) | 59 (7.9%) | |
| Whole grain intake levels, n (%) | 0.141 | |||
| Few/week | 410 (22.3%) | 306 (18.5%) | 145 (19.5%) | |
| 100–250 g/week | 393 (21.4%) | 370 (22.4%) | 171 (23.0%) | |
| 250–1000 g/week | 458 (25.0%) | 413 (25.0%) | 191 (25.7%) | |
| >1000 g/week | 574 (31.3%) | 563 (34.1%) | 236 (31.8%) | |
| Triglyceride, mmol/L | 1.3 ± 1.1 | 1.8 ± 1.7 | 2.0 ± 1.9 | <0.001 |
| Total cholesterol, mmol/L | 5.1 ± 0.9 | 5.2 ± 1.0 | 5.2 ± 1.0 | <0.001 |
| HDL-C, mmol/L | 1.2 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.2 | <0.001 |
| LDL-C, mmol/L | 3.2 ± 0.8 | 3.3 ± 0.8 | 3.3 ± 0.8 | 0.035 |
| Diabetes, n (%) | 203 (11.1%) | 264 (16.0%) | 119 (16.0%) | <0.001 |
| Stroke, n (%) | 175 (9.5%) | 222 (13.4%) | 93 (12.5%) | 0.001 |
| Spermidine, ng/mL | 23.8 (12.8–46.6) | 25.3 (13.8–50.5) | 27.2 (14.8–53.4) | 0.002 |
| Serum Spermidine | |||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||||
| OR | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Obesity | |||||||
| Model 1 | 1.000 (Ref.) | 1.009 (0.799, 1.275) | 0.938 | 1.254 (1.001, 1.572) | 0.049 | 1.307 (1.044, 1.636) | 0.019 |
| Model 2 | 1.000 (Ref.) | 0.992 (0.785, 1.254) | 0.946 | 1.263 (1.007, 1.584) | 0.044 | 1.375 (1.096, 1.725) | 0.006 |
| Model 3 | 1.000 (Ref.) | 0.993 (0.780, 1.264) | 0.953 | 1.327 (1.050, 1.678) | 0.018 | 1.417 (1.121, 1.791) | 0.004 |
| Overweight/Obesity | |||||||
| Model 1 | 1.000 (Ref.) | 1.096 (0.924, 1.301) | 0.293 | 1.193 (1.005. 1.416) | 0.044 | 1.312 (1.104, 1.559) | 0.002 |
| Model 2 | 1.000 (Ref.) | 1.079 (0.909, 1.282) | 0.385 | 1.200 (1.010, 1.426) | 0.038 | 1.374 (1.154, 1.636) | <0.001 |
| Model 3 | 1.000 (Ref.) | 1.106 (0.919, 1.330) | 0.288 | 1.315 (1.092, 1.582) | 0.004 | 1.449 (1.201, 1.748) | <0.001 |
| Serum Spermidine | |||||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||||
| OR | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Model 1 | 1.000 (Ref.) | 0.956 (0.726, 1.259) | 0.751 | 0.747 (0.567, 0.984) | 0.038 | 0.505 (0.383, 0.666) | <0.001 |
| Model 2 | 1.000 (Ref.) | 0.933 (0.707, 1.230) | 0.621 | 0.718 (0.544, 0.948) | 0.019 | 0.494 (0.374, 0.653) | <0.001 |
| Model 3 | 1.000 (Ref.) | 0.931 (0.702, 1.235) | 0.62 | 0.712 (0.535, 0.946) | 0.019 | 0.493 (0.370, 0.657) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Zhang, Q.; Xu, J.; Yuan, W.; Li, R.; Guo, H.; Gu, C.; Feng, W.; Ma, Y.; Sun, Z.; et al. Elevation of Serum Spermidine in Obese Patients: Results from a Cross-Sectional and Follow-Up Study. Nutrients 2022, 14, 2613. https://doi.org/10.3390/nu14132613
Gao H, Zhang Q, Xu J, Yuan W, Li R, Guo H, Gu C, Feng W, Ma Y, Sun Z, et al. Elevation of Serum Spermidine in Obese Patients: Results from a Cross-Sectional and Follow-Up Study. Nutrients. 2022; 14(13):2613. https://doi.org/10.3390/nu14132613
Chicago/Turabian StyleGao, Hanshu, Qianlong Zhang, Jiahui Xu, Wei Yuan, Ruixue Li, Hui Guo, Cuiying Gu, Wenjing Feng, Yanan Ma, Zhaoqing Sun, and et al. 2022. "Elevation of Serum Spermidine in Obese Patients: Results from a Cross-Sectional and Follow-Up Study" Nutrients 14, no. 13: 2613. https://doi.org/10.3390/nu14132613
APA StyleGao, H., Zhang, Q., Xu, J., Yuan, W., Li, R., Guo, H., Gu, C., Feng, W., Ma, Y., Sun, Z., & Zheng, L. (2022). Elevation of Serum Spermidine in Obese Patients: Results from a Cross-Sectional and Follow-Up Study. Nutrients, 14(13), 2613. https://doi.org/10.3390/nu14132613