The Effect of Vitamin D Supplementation on the Severity of Symptoms and the Quality of Life in Irritable Bowel Syndrome Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Data Sources & Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction
2.6. Risk of Bias and Quality Assessment
2.7. Statistical Analysis
3. Results
3.1. Search Results and Study Selection
3.2. Characteristics of Included Studies
3.3. Risk of Bias and Quality of Evidence
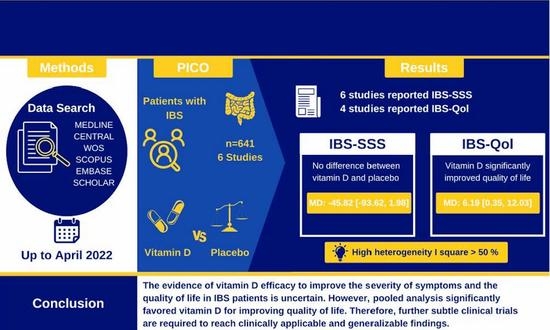
3.4. Primary Outcome
IBS-SSS
3.5. Secondary Outcomes
3.5.1. IBS-Qol
3.5.2. Serum 25(OH)D
4. Discussion
4.1. Strengths
4.2. Limitations
4.3. Implications for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 25(OH)D | Calcifediol, 25-hydroxycholecalciferol monohydrate, prohormone of the vitamin D system |
| CI | Confidence interval, the lower and upper limits of significance |
| IBS | Irritable bowel syndrome, a common gastrointestinal condition affecting 7–12% of the general population [1], characterized by fluctuating severity of symptoms, including abdominal discomfort, pain, bloating, and alternating bowel habits |
| IBS-QoL | Irritable bowel syndrome quality of life [36] |
| IBS-SSS | Irritable Bowel Severity Scoring System [35] |
| IU | International unit |
| MD | Mean difference |
| N/A | Not available |
| p | Probability |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses [33] |
| RCT | Randomized controlled trial |
| SD | Standard deviation |
| WOS | Web of Science |
References
- Chey, W.D.; Kurlander, J.; Eswaran, S. Irritable Bowel Syndrome: A Clinical Review. JAMA J. Am. Med. Assoc. 2015, 313, 949–958. [Google Scholar] [CrossRef]
- Spiller, R.C.; Jenkins, D.; Thornley, J.P.; Hebden, J.M.; Wright, T.; Skinner, M.; Neal, K.R. Increased Rectal Mucosal Enteroendocrine Cells, T Lymphocytes, and Increased Gut Permeability Following Acute Campylobacter Enteritis and in Post-Dysenteric Irritable Bowel Syndrome. Gut 2000, 47, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Lasch, K.; Zhou, W. Irritable Bowel Syndrome: Methods, Mechanisms, and Pathophysiology. The Confluence of Increased Permeability, Inflammation, and Pain in Irritable Bowel Syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G775–G785. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Chang, L. Diagnosis and Management of IBS. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Smart, H.L.; Mayberry, J.F.; Atkinson, M. Alternative Medicine Consultations and Remedies in Patients with the Irritable Bowel Syndrome. Gut 1986, 27, 826–828. [Google Scholar] [CrossRef] [Green Version]
- Choi, R.; Cho, S.-E.; Lee, S.G.; Lee, E.H. Recent Information on Vitamin D Deficiency in an Adult Korean Population Visiting Local Clinics and Hospitals. Nutrients 2022, 14, 1978. [Google Scholar] [CrossRef]
- De Giuseppe, R.; Tomasinelli, C.E.; Cena, H.; Braschi, V.; Giampieri, F.; Preatoni, G.; Centofanti, D.; Princis, M.P.; Bartoletti, E.; Biino, G. Development of a Short Questionnaire for the Screening for Vitamin D Deficiency in Italian Adults: The EVIDENCe-Q Project. Nutrients 2022, 14, 1772. [Google Scholar] [CrossRef]
- Hussain, T.; Latif, A.H.E.; Malik, S.; Raza, S.; Saeed, T.; Zahid, A.S.; Nazary, K.; Arshad, M.M.; Khan, R.; Walizada, K.; et al. Vitamin D Deficiency and Associated Risk Factors in Muslim Housewives of Quetta, Pakistan: A Cross-Sectional Study. Cureus 2021, 13, e17643. [Google Scholar] [CrossRef]
- Mansur, J.L.; Oliveri, B.; Giacoia, E.; Fusaro, D.; Costanzo, P.R. Vitamin D: Before, during and after Pregnancy: Effect on Neonates and Children. Nutrients 2022, 14, 1900. [Google Scholar] [CrossRef]
- Acharya, P.; Dalia, T.; Ranka, S.; Sethi, P.; Oni, O.A.; Safarova, M.S.; Parashara, D.; Gupta, K.; Barua, R.S. The Effects of Vitamin D Supplementation and 25-Hydroxyvitamin D Levels on the Risk of Myocardial Infarction and Mortality. J. Endocr. Soc. 2021, 5, bvab124. [Google Scholar] [CrossRef]
- Park, D.; Lee, J.; Park, C.Y.; Shin, M.-J. Low Vitamin D Status Is Associated with Increased Risk of Mortality in Korean Men and Adults with Hypertension: A Population-Based Cohort Study. Nutrients 2022, 14, 1849. [Google Scholar] [CrossRef] [PubMed]
- Aladel, A.; Murphy, A.M.; Abraham, J.; Shah, N.; Barber, T.M.; Ball, G.; Menon, V.; Piya, M.K.; McTernan, P.G. Vitamin D Levels as an Important Predictor for Type 2 Diabetes Mellitus and Weight Regain Post-Sleeve Gastrectomy. Nutrients 2022, 14, 2052. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, L.; De Lorenzo, R.; Giustina, A.; Rovere-Querini, P.; Conte, C. Vitamin D in Osteosarcopenic Obesity. Nutrients 2022, 14, 1816. [Google Scholar] [CrossRef] [PubMed]
- Tucker, L.A. Serum, Dietary, and Supplemental Vitamin D Levels and Insulin Resistance in 6294 Randomly Selected, Non-Diabetic U.S. Adults. Nutrients 2022, 14, 1844. [Google Scholar] [CrossRef]
- Yousef, S.; Colman, I.; Papadimitropoulos, M.; Manuel, D.; Hossain, A.; Faris, M.; Wells, G.A. Vitamin D and Chronic Diseases among First-Generation Immigrants: A Large-Scale Study Using Canadian Health Measures Survey (CHMS) Data. Nutrients 2022, 14, 1760. [Google Scholar] [CrossRef]
- Sprake, E.F.; Grant, V.A.; Corfe, B.M. Vitamin D3 as a Novel Treatment for Irritable Bowel Syndrome: Single Case Leads to Critical Analysis of Patient-Centred Data. BMJ Case Rep. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Dussik, C.M.; Hockley, M.; Grozić, A.; Kaneko, I.; Zhang, L.; Sabir, M.S.; Park, J.; Wang, J.; Nickerson, C.A.; Yale, S.H.; et al. Gene Expression Profiling and Assessment of Vitamin D and Serotonin Pathway Variations in Patients with Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2018, 24, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Coussens, A.K.; Martineau, A.R.; Wilkinson, R.J. Anti-Inflammatory and Antimicrobial Actions of Vitamin D in Combating TB/HIV. Scientifica 2014, 2014, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2009, 357, 266–281. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, Z.; Musch, M.W.; Ning, G.; Sun, J.; Hart, J.; Bissonnette, M.; Yan, C.L. Novel Role of the Vitamin D Receptor in Maintaining the Integrity of the Intestinal Mucosal Barrier. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 294. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, A.N.; Khalili, H.; Higuchi, L.M.; Bao, Y.; Korzenik, J.R.; Giovannucci, E.L.; Richter, J.M.; Fuchs, C.S.; Chan, A.T. Higher Predicted Vitamin D Status Is Associated with Reduced Risk of Crohn’s Disease. Gastroenterology 2012, 142, 482–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khayyat, Y.; Attar, S. Vitamin D Deficiency in Patients with Irritable Bowel Syndrome: Does It Exist? Oman Med. J. 2015, 30, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.E.; Williams, E.A.; Corfe, B.M. Vitamin D Status in Irritable Bowel Syndrome and the Impact of Supplementation on Symptoms: What Do We Know and What Do We Need to Know? Eur. J. Clin. Nutr. 2018, 72, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, B.U.; Maranda, L.; Candela, N. Vitamin D Status in Pediatric Irritable Bowel Syndrome. PLoS ONE 2017, 12, e0172183. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, D.J.; Meenagh, G.K.; Bickle, I.; Lee, A.S.; Curran, E.-S.; Finch, M.B. Vitamin D Deficiency Is Associated with Anxiety and Depression in Fibromyalgia. Clin. Rheumatol. 2007, 26, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, W.E.; Palsson, O.; Jones, K.R. Systematic Review of the Comorbidity of Irritable Bowel Syndrome with Other Disorders: What Are the Causes and Implications? Gastroenterology 2002, 122, 1140–1156. [Google Scholar] [CrossRef]
- Abbasnezhad, A.; Amani, R.; Hajiani, E.; Alavinejad, P.; Cheraghian, B.; Ghadiri, A. Effect of Vitamin D on Gastrointestinal Symptoms and Health-Related Quality of Life in Irritable Bowel Syndrome Patients: A Randomized Double-Blind Clinical Trial. Neurogastroenterol. Motil. 2016, 28, 1533–1544. [Google Scholar] [CrossRef]
- El Amrousy, D.; Hassan, S.; El Ashry, H.; Yousef, M.; Hodeib, H. Vitamin D Supplementation in Adolescents with Irritable Bowel Syndrome: Is It Useful? A Randomized Controlled Trial. Saudi J. Gastroenterol. 2018, 24, 109–114. [Google Scholar] [CrossRef]
- Jalili, M.; Vahedi, H.; Poustchi, H.; Hekmatdoost, A. Effects of Vitamin D Supplementation in Patients with Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Int. J. Prev. Med. 2019, 10, 16. [Google Scholar] [CrossRef]
- Sikaroudi, K.M.; Mokhtare, M.; Janani, L.; Faghihi Kashani, A.H.; Masoodi, M.; Agah, S.; Abbaspour, N.; Dehnad, A.; Shidfar, F. Vitamin D3 Supplementation in Diarrhea-Predominant Irritable Bowel Syndrome Patients: The Effects on Symptoms Improvement, Serum Corticotropin-Releasing Hormone, and Interleukin-6—A Randomized Clinical Trial. Complement. Med. Res. 2020, 27, 302–309. [Google Scholar] [CrossRef]
- Williams, C.E.; Williams, E.A.; Corfe, B.M. Vitamin D Supplementation in People with IBS Has No Effect on Symptom Severity and Quality of Life: Results of a Randomised Controlled Trial. Eur. J. Nutr. 2022, 61, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Zeid, W.; Ezzeldeen, E.; Khattab, M.; Ahmed, S.; Abdo, M. Effect of Vitamin D3 (Cholecalciferol) Supplementation on Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome Attending El-Mahsama Family Practice Center, Ismailia, Egypt: A Randomized Clinical Trial. Al-Azhar Int. Med. J. 2020, 1, 37–42. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley: Chichester, UK, 2019; pp. 1–694. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The Irritable Bowel Severity Scoring System: A Simple Method of Monitoring Irritable Bowel Syndrome and Its Progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drossman, D.A.; Patrick, D.L.; Whitehead, W.E.; Toner, B.B.; Diamant, N.E.; Hu, Y.; Jia, H.; Bangdiwala, S.I. Further Validation of the IBS-QOL: A Disease-Specific Quality-of-Life Questionnaire. Am. J. Gastroenterol. 2000, 95, 999–1007. [Google Scholar] [CrossRef]
- Covidence. Available online: https://www.covidence.org (accessed on 18 May 2022).
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Vist, G.E.; Falck-Ytter, Y.; Schünemann, H.J. Rating Quality of Evidence and Strength of Recommendations: What Is “Quality of Evidence” and Why Is It Important to Clinicians? BMJ 2008, 336, 995. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. Rating Quality of Evidence and Strength of Recommendations: GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ Br. Med. J. 2008, 336, 924. [Google Scholar] [CrossRef] [Green Version]
- Hultcrantz, M.; Rind, D.; Akl, E.A.; Treweek, S.; Mustafa, R.A.; Iorio, A.; Alper, B.S.; Meerpohl, J.J.; Murad, M.H.; Ansari, M.T.; et al. The GRADE Working Group clarifies the construct of certainty of evidence. J. Clin. Epidemiol. 2017, 87, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Cochrane RevMan. Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman (accessed on 18 May 2022).
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, S.M.; Piche, T.; Rampal, P. The Putative Role of Inflammation in the Irritable Bowel Syndrome. Gut 2001, 49, 743–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbara, G.; Cremon, C.; Carini, G.; Bellacosa, L.; Zecchi, L.; De Giorgio, R.; Corinaldesi, R.; Stanghellini, V. The Immune System in Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2011, 17, 349–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbara, G.; Stanghellini, V.; De Giorgio, R.; Cremon, C.; Cottrell, G.S.; Santini, D.; Pasquinelli, G.; Morselli-Labate, A.M.; Grady, E.F.; Bunnett, N.W.; et al. Activated Mast Cells in Proximity to Colonic Nerves Correlate with Abdominal Pain in Irritable Bowel Syndrome. Gastroenterology 2004, 126, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Mahon, B.D.; Wittke, A.; Weaver, V.; Cantorna, M.T. The Targets of Vitamin D Depend on the Differentiation and Activation Status of CD4 Positive T Cells. J. Cell. Biochem. 2003, 89, 922–932. [Google Scholar] [CrossRef]
- Patrick, R.P.; Ames, B.N. Vitamin D Hormone Regulates Serotonin Synthesis. Part 1: Relevance for Autism. FASEB J. 2014, 28, 2398–2413. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.C.; Chen, Y.; Du, J. Critical Roles of Intestinal Epithelial Vitamin D Receptor Signaling in Controlling Gut Mucosal Inflammation. J. Steroid Biochem. Mol. Biol. 2015, 148, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Drossman, D.A. The Functional Gastrointestinal Disorders and the Rome III Process. Gastroenterology 2006, 130, 1377–1390. [Google Scholar] [CrossRef]
- Silva, M.C.; Furlanetto, T.W. Intestinal Absorption of Vitamin D: A Systematic Review. Nutr. Rev. 2018, 76, 60–76. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine: What Clinicians Need to Know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Hathcock, J.N.; Shao, A.; Vieth, R.; Heaney, R. Risk Assessment for Vitamin D. Am. J. Clin. Nutr. 2007, 85, 6–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, R.I.H.; Yaow, C.Y.L.; Loh, C.Y.L.; Teoh, S.E.; Masuda, Y.; Ng, W.K.; Lim, Y.L.; Ng, Q.X. Vitamin D Supplementation for Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. J. Gastroenterol. Hepatol. 2022, 33, 993–1003. [Google Scholar] [CrossRef]
- Tazzyman, S.; Richards, N.; Trueman, A.R.; Evans, A.L.; Grant, V.A.; Garaiova, I.; Plummer, S.F.; Williams, E.A.; Corfe, B.M. Vitamin D Associates with Improved Quality of Life in Participants with Irritable Bowel Syndrome: Outcomes from a Pilot Trial. BMJ Open Gastroenterol. 2015, 2, e000052. [Google Scholar] [CrossRef]
- Jalili, M.; Hekmatdoost, A.; Vahedi, H.; Poustchi, H.; Khademi, B.; Saadi, M.; Zemestani, M.; Janani, L. Co-Administration of Soy Isoflavones and Vitamin D in Management of Irritable Bowel Disease. PLoS ONE 2016, 11, e0158545. [Google Scholar] [CrossRef] [PubMed]
- Flik, C.E.; Bakker, L.; Laan, W.; Van Rood, Y.R.; Smout, A.J.P.M.; De Wit, N.J. Systematic Review: The Placebo Effect of Psychological Interventions in the Treatment of Irritable Bowel Syndrome. World J. Gastroenterol. 2017, 23, 2223–2233. [Google Scholar] [CrossRef]
- Abuelazm, M.; Abdelazeem, B. Vitamin D Supplementation for Irritable Bowel Syndrome: Concerns About the Meta-Analysis. J. Gastroenterol. Hepatol. 2022. [Google Scholar] [CrossRef]
- Huang, H.; Lu, L.; Chen, Y.; Zeng, Y.; Xu, C. The Efficacy of Vitamin D Supplementation for Irritable Bowel Syndrome: A Systematic Review with Meta-Analysis. Nutr. J. 2022, 21, 1–11. [Google Scholar] [CrossRef]
- Pérez-Castrillon, J.-L.; Usategui-Martín, R.; Pludowski, P. Treatment of Vitamin D Deficiency with Calcifediol: Efficacy and Safety Profile and Predictability of Efficacy. Nutrients 2022, 14, 1943. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S. Systematic reviews and meta-analysis in nutrition research. Br. J. Nutr. 2019, 122, 1279–1294. [Google Scholar] [CrossRef] [Green Version]





| Database | Search Terms | Search Field | Search Results |
|---|---|---|---|
| PubMed | (“Vitamin D” OR “Cholecalciferol” OR “Hydroxycholecalciferols” OR “Ergocalciferols” OR “25 Hydroxyvitamin D” OR “Dihydrotachysterol” OR “25(OH)D” OR “25-hydroxyvitamin D” OR calcifediol OR calciferol OR “Vitamin D”) AND (“Colonic Diseases, Functional”[Mesh] OR IBS OR irritable bowel syndrome OR”functional abdominal pain” OR “functional gastrointestinal” OR FGID OR “irritable colon” OR Colitis, Mucous OR Colitides, Mucous OR Mucous Colitides OR Mucous Colitis) | All Field | 137 |
| Cochrane | ((Vitamin D) OR (Cholecalciferol) OR (Hydroxycholecalciferols) OR (Ergocalciferols) OR (25 Hydroxyvitamin D) (Word variations have been searched)) AND ((irritable bowel syndrome) OR (IBS) OR (functional abdominal pain) OR (Functional Colonic Diseases) OR (irritable colon) (Word variations have been searched)) | All Field | 127 |
| WOS | (“Vitamin D” OR “Cholecalciferol” OR “Hydroxycholecalciferols” OR “Ergocalciferols” OR “25 Hydroxyvitamin D” OR “Dihydrotachysterol” OR “25(OH)D” OR “25-hydroxyvitamin D” OR calcifediol OR calciferol OR “Vitamin D”) AND (“Colonic Diseases, Functional”[Mesh] OR IBS OR irritable bowel syndrome OR “functional abdominal pain” OR “functional gastrointestinal” OR FGID OR “irritable colon” OR Colitis, Mucous OR Colitides, Mucous OR Mucous Colitides OR Mucous Colitis) | All Field | 196 |
| SCOPUS | (TITLE-ABSKEY ((vitamin AND d) OR (cholecalciferol) OR (hydroxycholecalciferols) OR (ergocalciferols) OR (25 hydroxyvitamin AND d) OR (dihydrotachysterol) OR (25(OH)D) OR (25-hydroxyvitamin AND d) OR (calcifediol) OR (calciferol) OR (vitamin AND d)) AND TITLE-ABS-KEY ((functional AND colonic AND diseases) OR (irritable AND bowel AND syndrome) OR (ibs) OR (functional AND abdominal AND pain) OR (functional AND gastrointestinal) OR (fgid) OR (mucous AND colitides) OR (mucous AND colitis)) AND (LIMIT-TO (DOCTYPE, “ar”)) | Title, Abstract, Keywords | 879 |
| EMBASE | (“vitamin deficiency”/exp OR “vitamin deficiency” OR cholecalciferol OR hydroxycholecalciferols OR ergocalciferols OR (25 AND hydroxyvitamin AND d) OR dihydrotachysterol OR (25 AND oh AND d) OR (“25 hydroxyvitamin” AND d) OR calcifediol OR calciferol OR (vitamin AND d)) AND (“irritable colon”/exp OR “irritable colon” OR ibs OR (functional AND abdominal AND pain) OR (functional AND gastrointestinal) OR fgid OR (irritable AND colon) OR (mucous AND colitis) OR (mucous AND colitides)) AND “randomized controlled trial”/de | All Field | 88 |
| Google Scholar | (“Vitamin D” OR Cholecalciferol OR Hydroxycholecalciferol OR Ergocalciferol OR 25 “Hydroxyvitamin D”) AND (“irritable bowel syndrome” OR IBS OR “functional abdominal pain” OR “Functional Colonic Diseases” OR “irritable colon”) | All Field | 1430 Ext. (first 200 only) |
| Study ID | Country | Study Design | Total Participants | IBS Sub-type | Follow-up Duration (Months) | Vitamin D | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Female n (%) | Age (Years) Mean (SD) | Baseline Serum Vitamin D Mean (SD) | Dose | Number | Female n (%) | Age (Years) Mean (SD) | Baseline Serum Vitamin D Mean (SD) | ||||||
| Abbasnezhad et al., 2016 [27] | Iran | Single-center double blinded RCT | 85 | BS-D IBS-A IBS-C | 6 | 44 | 28 (63.6) | 37.45 (8.11) | 19.65 (10.35) | 50,000 IU fortnightly | 41 | 29 (70.7) | 38.45 (9.85) | 18.62 (11.23) |
| Zeid et al., 2020 [32] | Egypt | Single-center double blinded RCT | 80 | N/A | 3 | 40 | N/A | 37.64 (11.13) | N/A | 4000 IU daily | 40 | N/A | 38.03 (6.37) | N/A |
| Williams et al., 2021 [31] | UK | Single-center double blinded RCT | 135 | N/A | 3 | 68 | 55 (80.9) | 28.94 (10.03) | 48.75 (27.91) | 3000 IU daily | 67 | 51 (76.1) | 31.1 (10.85) | 49.71 (27.05) |
| Sikaroudi et al., 2020 [30] | Iran | Single-center double blinded RCT | 88 | IBS-D | 2 | 44 | 25 (56.8) | 35.07 (11.73) | 17.68 (7.69) | 50,000 IU weekly | 44 | 22 (50) | 35.61 (8) | 17.83 (7.84) |
| Jalili et al., 2019 [29] | Iran | Multi-center Double blinded RCT | 116 | N/A | 1.5 | 58 | 58 (100) | 52.24 (12.26) | N/A | 50,000 IU weekly | 58 | 58 (100) | 40.06 (13.37) | N/A |
| El Amrousy et al., 2018 [28] | Egypt | Single-center double blinded RCT | 112 | IBS-C IBS-U IBS-M IBS-D | 6 | 56 | 29 (52) | 16.4 (1.5) | 17.2 (1.3) | 2000 IU daily | 56 | 33 (59) | 16.2 (1.1) | 17.5 (1.1) |
| Certainty Assessment | No. of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Vitamin D | Placebo | Relative (95% CI) | Absolute (95% CI) | ||
| IBS-SSS | ||||||||||||
| 6 | randomized trials | not serious | very serious a | not serious | very serious b | none | 310 | 306 | - | MD 45.86 lower (93.65 lower to 1.93 higher) | ⨁◯◯◯ Very low | Critical |
| IBS-Qol | ||||||||||||
| 4 | randomized trials | serious c | very serious a | not serious | serious d | none | 226 | 222 | - | MD 6.19 higher (0.35 higher to 12.03 higher) | ⨁◯◯◯ Very low | Important |
| Serum 25(OH)D | ||||||||||||
| 4 | randomized trials | not serious | very serious a | serious e | not serious | very strong association | 168 | 164 | - | MD 30.03 higher (20.72 higher to 39.34 higher) | ⨁⨁⨁◯ Moderate | Important |
| Outcome | No. of participants (Vitamin D/Placebo) | No. of Trials | Quantitative Data Synthesis | Heterogeneity Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| MD | 95% CI | Z Value | p-Value | df | p-Value | I2 (%) | |||
| IBS-SSS | |||||||||
| All studies | 310/306 | 6 | −45.82 | [−93.62, 1.98] | 1.88 | 0.06 | 5 | 0.00001 | 95 |
| Omitting Abbasnezhad et al., 2016 [27] | 266/265 | 5 | −47.23 | [−118.33, 23.88] | 1.3 | 0.19 | 4 | 0.00001 | 95 |
| Omitting El Amrousy et al., 2018 [28] | 254/250 | 5 | −42.28 | [−99.7, 15.13] | 1.44 | 0.15 | 4 | 0.00001 | 96 |
| Omitting Jalili et al., 2019 [29] | 252/248 | 5 | −59.82 | [−111.85, −7.79] | 2.25 | 0.02 | 4 | 0.00001 | 95 |
| Omitting Sikaroudi et al., 2020 [30] | 266/262 | 5 | −43.01 | [−98.52, 12.49] | 1.52 | 0.13 | 4 | 0.00001 | 96 |
| Omitting Williams et al., 2021 [31] | 242/239 | 5 | −57.82 | [−110.94, −4.71] | 2.13 | 0.03 | 4 | 0.00001 | 95 |
| Omitting Zeid et al., 2020 [32] | 270/266 | 5 | −24.33 | [−54.85, 6.19] | 1.56 | 0.12 | 4 | 0.0003 | 81 |
| IBS-Qol | |||||||||
| All studies | 226/222 | 4 | 6.19 | [0.35, 12.03] | 2.08 | 0.04 | 3 | 0.03 | 66 |
| Omitting Abbasnezhad et al., 2016 [27] | 182/181 | 3 | 8.23 | [0.15, 16.31] | 2 | 0.05 | 2 | 0.13 | 51 |
| Omitting El Amrousy et al., 2018 [28] | 170/166 | 3 | 3.26 | [2.14, 4.39] | 5.67 | 0.00001 | 2 | 0.98 | 0 |
| Omitting Jalili et al., 2019 [29] | 168/164 | 3 | 6.41 | [−0.37, 13.18] | 1.85 | 0.06 | 2 | 0.01 | 77 |
| Omitting Williams et al., 2021 [31] | 158/155 | 3 | 7.41 | [−0.96, 15.77] | 1.73 | 0.08 | 2 | 0.01 | 77 |
| Serum 25(OH)D | |||||||||
| All studies | 226/222 | 4 | 25.20 | [18.41, 31.98] | 7.28 | 0.00001 | 3 | 0.00001 | 93 |
| Omitting Abbasnezhad et al., 2016 [27] | 182/181 | 3 | 22.58 | [15.39, 29.76] | 6.16 | 0.00001 | 2 | 0.0002 | 88 |
| Omitting El Amrousy et al., 2018 [28] | 170/166 | 3 | 28.16 | [14.87, 41.46] | 4.15 | 0.0001 | 2 | 0.00001 | 95 |
| Omitting Williams et al., 2021 [31] | 158/155 | 3 | 22.54 | [15.80, 29.29] | 6.55 | 0.00001 | 2 | 0.00001 | 94 |
| Omitting Jalili et al., 2019 [29] | 168/164 | 3 | 29.33 | [20.29, 38.38] | 6.36 | 0.00001 | 2 | 0.00001 | 94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuelazm, M.; Muhammad, S.; Gamal, M.; Labieb, F.; Amin, M.A.; Abdelazeem, B.; Brašić, J.R. The Effect of Vitamin D Supplementation on the Severity of Symptoms and the Quality of Life in Irritable Bowel Syndrome Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 2618. https://doi.org/10.3390/nu14132618
Abuelazm M, Muhammad S, Gamal M, Labieb F, Amin MA, Abdelazeem B, Brašić JR. The Effect of Vitamin D Supplementation on the Severity of Symptoms and the Quality of Life in Irritable Bowel Syndrome Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2022; 14(13):2618. https://doi.org/10.3390/nu14132618
Chicago/Turabian StyleAbuelazm, Mohamed, Shoaib Muhammad, Mohamed Gamal, Fatma Labieb, Mostafa Atef Amin, Basel Abdelazeem, and James Robert Brašić. 2022. "The Effect of Vitamin D Supplementation on the Severity of Symptoms and the Quality of Life in Irritable Bowel Syndrome Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 14, no. 13: 2618. https://doi.org/10.3390/nu14132618
APA StyleAbuelazm, M., Muhammad, S., Gamal, M., Labieb, F., Amin, M. A., Abdelazeem, B., & Brašić, J. R. (2022). The Effect of Vitamin D Supplementation on the Severity of Symptoms and the Quality of Life in Irritable Bowel Syndrome Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 14(13), 2618. https://doi.org/10.3390/nu14132618