Early Childhood Diet in Relation to Toddler Nighttime Sleep Duration Trajectories
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
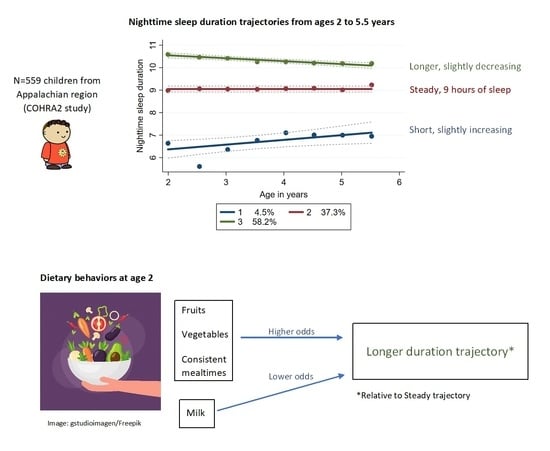
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reynaud, E.; Forhan, A.; Heude, B.; Charles, M.A.; Plancoulaine, S. Night-sleep Duration Trajectories and Behavior in Preschoolers: Results from a Prospective Birth Cohort Study. Behav. Sleep Med. 2021, 19, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Smithson, L.; Baird, T.; Tamana, S.K.; Lau, A.; Mariasine, J.; Chikuma, J.; Lefebvre, D.L.; Subbarao, P.; Becker, A.B.; Turvey, S.E.; et al. Shorter sleep duration is associated with reduced cognitive development at two years of age. Sleep Med. 2018, 48, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Touchette, É.; Petit, D.; Tremblay, R.E.; Boivin, M.; Falissard, B.; Genolini, C.; Montplaisir, J.Y. Associations between sleep duration patterns and overweight/obesity at age 6. Sleep 2008, 31, 1507–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health J. Natl. Sleep Found. 2015, 1, 40–43. [Google Scholar] [CrossRef]
- Singareddy, R.; Moole, S.; Calhoun, S.; Vocalan, P.; Tsaoussoglou, M.; Vgontzas, A.N.; Bixler, E.O. Medical Complaints Are More Common in Young School-Aged Children with Parent Reported Insomnia Symptoms. J. Clin. Sleep Med. 2009, 5, 549. [Google Scholar] [CrossRef] [Green Version]
- Williamson, A.A.; Mindell, J.A.; Hiscock, H.; Quach, J. Longitudinal sleep problem trajectories are associated with multiple impairments in child well-being. J. Child Psychol. Psychiatry 2020, 61, 1092–1103. [Google Scholar] [CrossRef]
- Seegers, V.; Touchette, E.; Dionne, G.; Petit, D.; Seguin, J.R.; Montplaisir, J.; Vitaro, F.; Falissard, B.; Boivin, M.; Tremblay, R.E. Short persistent sleep duration is associated with poor receptive vocabulary performance in middle childhood. J. Sleep Res. 2016, 25, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Shetty, J.; Newton, A.; Reid, G. Parenting Practices, Bedtime Routines, and Consistency: Associations with Pediatric Sleep Problems. J. Pediatr. Psychol. 2022, 47, 49–58. [Google Scholar] [CrossRef]
- Zhang, Z.; Sousa-Sá, E.; Pereira, J.R.; Okely, A.D.; Feng, X.; Santos, R. Correlates of Sleep Duration in Early Childhood: A Systematic Review. Behav. Sleep Med. 2020, 19, 407–425. [Google Scholar] [CrossRef]
- Janssen, X.; Martin, A.; Hughes, A.R.; Hill, C.M.; Kotronoulas, G.; Hesketh, K.R. Associations of screen time, sedentary time and physical activity with sleep in under 5s: A systematic review and meta-analysis. Sleep Med. Rev. 2020, 49, 101226. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Eckel-Mahan, K. Interdependence of nutrient metabolism and the circadian clock system: Importance for metabolic health. Mol. Metab. 2016, 5, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.C.; Prather, A.; Leung, C.W. Associations between sleep duration and dietary quality: Results from a nationally-representative survey of US adults. Appetite 2020, 153, 104748. [Google Scholar] [CrossRef] [PubMed]
- Plancoulaine, S.; Lioret, S.; Regnault, N.; Heude, B.; Charles, M.A. Gender-specific factors associated with shorter sleep duration at age 3 years. J. Sleep Res. 2015, 24, 610–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, E.C.; Peterson, K.E.; Lumeng, J.C.; Kaciroti, N.; LeBourgeois, M.K.; Chen, K.; Miller, A.L. Associations between Sleep and Dietary Patterns among Low-Income Children Attending Preschool. J. Acad. Nutr. Diet. 2019, 119, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.L.; Reynolds, A.N.; Kuroko, S.; Fangupo, L.J.; Galland, B.C.; Taylor, R.W. Bidirectional associations between sleep and dietary intake in 0–5 year old children: A systematic review with evidence mapping. Sleep Med. Rev. 2020, 49, 101231. [Google Scholar] [CrossRef]
- Plancoulaine, S.; Reynaud, E.; Forhan, A.; Lioret, S.; Heude, B.; Charles, M.-A.; Annesi-Maesano, I.; Bernard, J.; Botton, J.; Dargent-Molina, P.; et al. Night sleep duration trajectories and associated factors among preschool children from the EDEN cohort. Sleep Med. 2018, 48, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Adam, E.K.; Snell, E.K.; Pendry, P. Sleep timing and quantity in ecological and family context: A nationally representative time-diary study. J. Fam. Psychol. 2007, 21, 4–19. [Google Scholar] [CrossRef] [Green Version]
- Hendryx, M. Poverty and Mortality Disparities in Central Appalachia: Mountaintop Mining and Environmental Justice. J. Health Dispar. Res. Pract. 2012, 4, 6. Available online: https://digitalscholarship.unlv.edu/jhdrp/vol4/iss3/6 (accessed on 12 October 2021).
- Neiswanger, K.; McNeil, D.W.; Foxman, B.; Govil, M.; Cooper, M.E.; Weyant, R.J.; Shaffer, J.R.; Crout, R.J.; Simhan, H.N.; Beach, S.R.; et al. Oral health in a sample of pregnant women from Northern Appalachia (2011–2015). Int. J. Dent. 2015, 2015, 469376. [Google Scholar] [CrossRef] [Green Version]
- Andruff, H.; Carraro, N.; Thompson, A.; Gaudreau, P. Latent Class Growth Modelling: A Tutorial. Tutor. Quant. Methods Psychol. 2009, 5, 11–24. [Google Scholar] [CrossRef]
- Kachurak, A.; Bailey, R.L.; Davey, A.; Dabritz, L.; Fisher, J.O. Daily snacking occasions, snack size, and snack energy density as predictors of diet quality among us children aged 2 to 5 years. Nutrients 2019, 11, 1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhage, C.L.; Gillebaart, M.; van der Veek, S.M.C.; Vereijken, C.M.J.L. The relation between family meals and health of infants and toddlers: A review. Appetite 2018, 127, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Zerón-Rugerio, M.F.; Hernáez, Á.; Porras-Loaiza, A.P.; Cambras, T.; Izquierdo-Pulido, M. Eating jet lag: A marker of the variability in meal timing and its association with body mass index. Nutrients 2019, 11, 2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarem, N.; Sears, D.D.; St-Onge, M.; Zuraikat, F.M.; Gallo, L.C.; Talavera, G.A.; Castaneda, S.F.; Lai, Y.; Aggarwal, B. Variability in Daily Eating Patterns and Eating Jetlag Are Associated With Worsened Cardiometabolic Risk Profiles in the American Heart Association Go Red for Women Strategically Focused Research Network. J. Am. Heart Assoc. 2021, 10, e022024. [Google Scholar] [CrossRef]
- Jansen, E.C.; Dolinoy, D.; Peterson, K.E.; O’Brien, L.M.; Chervin, R.D.; Cantoral, A.; Tellez-Rojo, M.M.; Solano-Gonzalez, M.; Goodrich, J. Adolescent sleep timing and dietary patterns in relation to DNA methylation of core circadian genes: A pilot study of Mexican youth. Epigenetics 2020, 16, 894–907. [Google Scholar] [CrossRef]
- Lopez-Minguez, J.; Gómez-Abellán, P.; Garaulet, M. Circadian rhythms, food timing and obesity. Proc. Nutr. Soc. 2016, 75, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Loth, K.A.; Tate, A.; Trofholz, A.; Fisher, J.O.; Miller, L.; Neumark-Sztainer, D.; Berge, J. Ecological momentary assessment of the snacking environments of children from racially/ethnically diverse households. Appetite 2020, 145, 104497. [Google Scholar] [CrossRef]
- Jansen, E.C.; She, R.; Rukstalis, M.; Alexander, G.L. Changes in fruit and vegetable consumption in relation to changes in sleep characteristics over a 3-month period among young adults. Sleep Health 2021, 7, 345–352. [Google Scholar] [CrossRef]
- Godos, J.; Ferri, R.; Caraci, F.; Cosentino, F.I.I.; Castellano, S.; Shivappa, N.; Hebert, J.R.; Galvano, F.; Grosso, G. Dietary inflammatory index and sleep quality in Southern Italian Adults. Nutrients 2019, 11, 1324. [Google Scholar] [CrossRef] [Green Version]
- St-Onge, M.P.; Roberts, A.; Shechter, A.; Choudhury, A.R. Fiber and saturated fat are associated with sleep arousals and slow wave sleep. J. Clin. Sleep Med. 2016, 12, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Jansen, E.C.; Corcoran, K.; Perng, W.; Dunietz, G.L.; Cantoral, A.; Zhou, L.; Téllez-Rojo, M.M.; Peterson, K.E. Relationships of beverage consumption and actigraphy-assessed sleep parameters among urban-dwelling youth from Mexico. Public Health Nutr. 2021, 25, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.-P.; Tremblay, M.S.; Katzmarzyk, P.T.; Fogelholm, M.; Hu, G.; Maher, C.; Maia, J.; Olds, T.; Onywera, V.; Sarmiento, O.L.; et al. Sleep patterns and sugar-sweetened beverage consumption among children from around the world. Public Health Nutr. 2018, 21, 2385–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheaton, A.G. Short Sleep Duration Among Infants, Children, and Adolescents Aged 4 Months–17 Years—United States, 2016–2018. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Bird, A.; Peterson, E.; Underwood, L.; Morton, S.M.B.; Grant, C.C. Maternal Antenatal Depression and Early Childhood Sleep: Potential Pathways Through Infant Temperament. J. Pediatr. Psychol. 2020, 45, 203–217. [Google Scholar] [CrossRef]

| Nightly Sleep Duration Trajectories | ||||
|---|---|---|---|---|
| Group 1 Short Duration n = 25 1 | Group 2 Steady n = 201 1 | Group 3 Longer Duration n = 333 1 | p 3 | |
| Baseline nightly sleep duration | 6.5 (1.5) 2 | 8.9 (1.1) | 10.6 (1.0) | 0.0001 |
| Daytime nap duration | 2.9 (1.9) | 2.2 (1.2) | 2.0 (0.8) | 0.10 |
| Age, months | 2.00 (0.07) | 2.00 (0.06) | 1.99 (0.06) | 0.79 |
| Breastfeeding, any | 0.48 | 0.39 | 0.39 | 0.65 |
| Daycare attendance | 0.44 | 0.44 | 0.51 | 0.29 |
| Sleep problem (any at all) | 0.44 | 0.16 | 0.15 | 0.001 |
| Mother’s age at birth | 28.3 (6.2) | 29.7 (5.6) | 30.6 (4.4) | 0.02 |
| Gestational week at delivery | 39.6 (1.1) | 39.4 (1.4) | 39.5 (1.3) | 0.76 |
| Male sex | 0.52 | 0.50 | 0.57 | 0.28 |
| Vaginal delivery | 0.60 | 0.66 | 0.78 | 0.005 |
| Birth weight, kg | 3.38 (0.41) | 3.42 (0.50) | 3.49 (0.46) | 0.19 |
| Household income | <0.0001 | |||
| $0–14,999 | 0.24 | 0.21 | 0.08 | |
| $15,000–34,999 | 0.36 | 0.22 | 0.11 | |
| $35,000–49,999 | 0.16 | 0.11 | 0.16 | |
| $50,000–74,999 | 0.16 | 0.18 | 0.15 | |
| $75,000–99,999 | 0.08 | 0.13 | 0.23 | |
| $100,000 or more | 0 | 0.14 | 0.27 | |
| Maternal education | <0.0001 | |||
| 8th grade-completed high school | 0.12 | 0.52 | 0.36 | |
| Some college/Associate’s | 0.07 | 0.51 | 0.42 | |
| Bachelor’s degree | 0.02 | 0.26 | 0.72 | |
| Graduate degree | 0 | 0.23 | 0.77 | |
| Nightly Sleep Duration Trajectories, Percentage in Each Category | ||||
|---|---|---|---|---|
| % | Short Duration | Steady | Longer Duration | |
| Typical meal pattern | ||||
| Meals and snacks at same time every day | 81.1 | 3.77 | 31.93 | 64.30 |
| Meals and snacks at different times every day | 14.0 | 7.69 | 52.56 | 39.74 |
| Snacking throughout the day, few meals | 4.9 | 7.41 | 51.85 | 40.74 |
| p value | <0.0001 | |||
| Fruit consumption | ||||
| Never to every few days | 9.4 | 7.69 | 57.69 | 34.62 |
| Once per day | 19.2 | 8.41 | 48.60 | 42.99 |
| Several times per day | 71.4 | 3.02 | 29.47 | 67.51 |
| p value | <0.0001 | |||
| Vegetable consumption | ||||
| Never to every few days | 17.8 | 2.02 | 51.52 | 46.46 |
| Once per day | 37.8 | 8.10 | 33.33 | 58.57 |
| Several times per day | 44.4 | 2.43 | 31.58 | 65.99 |
| p value | <0.0001 | |||
| Water | ||||
| Never or once in last week | 3.8 | 14.29 | 47.62 | 38.1 |
| Every few days | 3.4 | 5.26 | 73.68 | 21.05 |
| Once a day | 14.0 | 8.97 | 41.03 | 50.00 |
| Several times a day | 78.9 | 3.17 | 32.88 | 63.95 |
| p value | <0.0001 | |||
| Milk | ||||
| Never or once in last week | 14.0 | 5.13 | 24.36 | 70.51 |
| Every few days | 5.6 | 6.45 | 35.48 | 58.06 |
| Once a day | 14.9 | 1.20 | 37.35 | 61.45 |
| Several times a day | 65.7 | 4.9 | 38.15 | 56.95 |
| p value | 0.239 | |||
| Juice | ||||
| Never or once in last week | 42.8 | 2.09 | 31.80 | 66.11 |
| Every few days | 18.6 | 6.73 | 34.62 | 58.65 |
| Once a day | 19.5 | 6.42 | 31.19 | 62.39 |
| Several times a day | 19.0 | 5.66 | 51.89 | 42.45 |
| p value | 0.001 | |||
| Soda | ||||
| Never or once in last week | 93.7 | 4.01 | 34.73 | 61.26 |
| At least every few days | 6.3 | 11.43 | 54.29 | 34.29 |
| p value | 0.637 | |||
| Short Duration vs. Longer Duration, OR (95% CI) 1 | p | Steady vs. Longer Duration, OR (95% CI) 1 | p | |
|---|---|---|---|---|
| Typical meal pattern | ||||
| Meals and snacks at same time every day | Reference | Reference | ||
| Meals and snacks at different times every day | 1.89 (0.65, 5.49) | 0.24 | 1.98 (1.12, 3.52) | 0.02 |
| Snacking throughout the day, few meals | 2.23 (0.41, 12.09) | 0.35 | 2.08 (0.83, 5.18) | 0.12 |
| Fruit consumption | ||||
| Never to every few days | Reference | Reference | ||
| Once per day | 0.91 (0.23, 3.63) | 0.90 | 0.65 (0.31, 1.40) | 0.27 |
| Several times per day | 0.38 (0.10, 1.40) | 0.15 | 0.35 (0.18, 0.69) | 0.002 |
| Vegetable consumption | ||||
| Never to every few days | Reference | Reference | ||
| Once per day | 4.64 (0.97, 22.19) | 0.06 | 0.59 (0.34, 1.03) | 0.06 |
| Several times per day | 1.15 (0.21, 6.26) | 0.87 | 0.47 (0.27, 0.80) | 0.006 |
| Water | ||||
| Never or once in last week | Reference | Reference | ||
| Every few days | 1.16 (0.08, 17.08) | 0.91 | 3.87 (0.82, 18.19) | 0.09 |
| Once a day | 0.71 (0.13, 3.78) | 0.69 | 0.79 (0.26, 2.47) | 0.69 |
| Several times a day | 0.30 (0.06, 1.40) | 0.13 | 0.65 (0.23, 1.86) | 0.42 |
| Milk | ||||
| Never or once in last week | Reference | Reference | ||
| Every few days | 1.66 (0.25, 11.18) | 0.60 | 1.93 (0.73, 5.11) | 0.19 |
| Once a day | 0.29 (0.03, 2.83) | 0.29 | 1.82 (0.87, 3.82) | 0.11 |
| Several times a day | 0.99 (0.30, 3.31) | 0.99 | 1.89 (1.03, 3.50) | 0.04 |
| Juice | ||||
| Never or once in last week | Reference | Reference | ||
| Every few days | 2.55 (0.73, 8.88) | 0.14 | 1.04 (0.61, 1.77) | 0.90 |
| Once a day | 1.58 (0.45, 5.61) | 0.48 | 0.67 (0.38, 1.16) | 0.15 |
| Several times a day | 1.75 (0.47, 6.53) | 0.41 | 1.58 (0.92, 2.73) | 0.10 |
| Soda | ||||
| Never or once in last week | Reference | Reference | ||
| At least every few days | 2.44 (0.64, 9.37) | 0.19 | 1.89 (0.81, 4.43) | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, E.C.; Zhao, W.; Jones, A.D.; Marshall, T.A.; Neiswanger, K.; Shaffer, J.R.; McNeil, D.W.; Marazita, M.L.; Foxman, B. Early Childhood Diet in Relation to Toddler Nighttime Sleep Duration Trajectories. Nutrients 2022, 14, 3059. https://doi.org/10.3390/nu14153059
Jansen EC, Zhao W, Jones AD, Marshall TA, Neiswanger K, Shaffer JR, McNeil DW, Marazita ML, Foxman B. Early Childhood Diet in Relation to Toddler Nighttime Sleep Duration Trajectories. Nutrients. 2022; 14(15):3059. https://doi.org/10.3390/nu14153059
Chicago/Turabian StyleJansen, Erica C., Wentong Zhao, Andrew D. Jones, Teresa A. Marshall, Katherine Neiswanger, John R. Shaffer, Daniel W. McNeil, Mary L. Marazita, and Betsy Foxman. 2022. "Early Childhood Diet in Relation to Toddler Nighttime Sleep Duration Trajectories" Nutrients 14, no. 15: 3059. https://doi.org/10.3390/nu14153059
APA StyleJansen, E. C., Zhao, W., Jones, A. D., Marshall, T. A., Neiswanger, K., Shaffer, J. R., McNeil, D. W., Marazita, M. L., & Foxman, B. (2022). Early Childhood Diet in Relation to Toddler Nighttime Sleep Duration Trajectories. Nutrients, 14(15), 3059. https://doi.org/10.3390/nu14153059