Diet Quality Is Associated with Glucose Regulation in a Cohort of Young Adults
Abstract
:1. Introduction
2. Materials and Methods
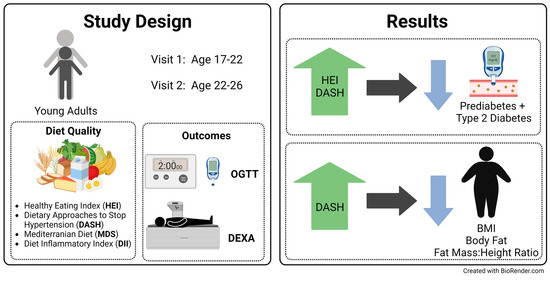
2.1. Cohort
2.2. Glucose Outcomes
2.3. Adiposity Outcomes
2.4. Diet Assessment
2.5. Covariates
2.6. Statistical Analysis
2.7. Sensitivity Analyses
3. Results
3.1. Prediabetes/T2D
3.2. Fasting Glucose and Glucose Tolerance
3.3. Hemoglobin A1c
3.4. Body Composition
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef]
- Andes, L.J.; Cheng, Y.J.; Rolka, D.B.; Gregg, E.W.; Imperatore, G. Prevalence of Prediabetes Among Adolescents and Young Adults in the United States, 2005–2016. JAMA Pediatr. 2020, 174, e194498. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y.; Zhang, D.; Yi, S.S.; Liu, J. Trends in Prediabetes Among Youths in the US From 1999 Through 2018. JAMA Pediatr. 2022, 176, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.H.; Khunti, K.; Achana, F.; Srinivasan, B.; Gray, L.J.; Davies, M.J.; Webb, D. Progression rates from HbA1c 6.0–6.4% and other prediabetes definitions to type 2 diabetes: A meta-analysis. Diabetologia 2013, 56, 1489–1493. [Google Scholar] [CrossRef]
- Divers, J.; Mayer-Davis, E.J.; Lawrence, J.M.; Isom, S.; Dabelea, D.; Dolan, L.; Imperatore, G.; Marcovina, S.; Pettitt, D.J.; Pihoker, C.; et al. Trends in Incidence of Type 1 and Type 2 Diabetes Among Youths-Selected Counties and Indian Reservations, United States, 2002–2015. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 161–165. [Google Scholar] [CrossRef]
- Shah, A.S.; Nadeau, K.J. The changing face of paediatric diabetes. Diabetologia 2020, 63, 683–691. [Google Scholar] [CrossRef]
- Magliano, D.J.; Sacre, J.W.; Harding, J.L.; Gregg, E.W.; Zimmet, P.Z.; Shaw, J.E. Young-onset type 2 diabetes mellitus-implications for morbidity and mortality. Nat. Rev. Endocrinol. 2020, 16, 321–331. [Google Scholar] [CrossRef]
- Lascar, N.; Brown, J.; Pattison, H.; Barnett, A.H.; Bailey, C.J.; Bellary, S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018, 6, 69–80. [Google Scholar] [CrossRef]
- American Diabetes Association. 3. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43 (Suppl. 1), S32–S36. [Google Scholar] [CrossRef]
- Neuenschwander, M.; Ballon, A.; Weber, K.S.; Norat, T.; Aune, D.; Schwingshackl, L.; Schlesinger, S. Role of diet in type 2 diabetes incidence: Umbrella review of meta-analyses of prospective observational studies. BMJ 2019, 366, l2368. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef]
- Toi, P.L.; Anothaisintawee, T.; Chaikledkaew, U.; Briones, J.R.; Reutrakul, S.; Thakkinstian, A. Preventive Role of Diet Interventions and Dietary Factors in Type 2 Diabetes Mellitus: An Umbrella Review. Nutrients 2020, 12, 2722. [Google Scholar] [CrossRef]
- De Koning, L.; Chiuve, S.E.; Fung, T.T.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care 2011, 34, 1150–1156. [Google Scholar] [CrossRef]
- Jannasch, F.; Kröger, J.; Schulze, M.B. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J. Nutr. 2017, 147, 1174–1182. [Google Scholar] [CrossRef]
- Qian, F.; Liu, G.; Hu, F.B.; Bhupathiraju, S.N.; Sun, Q. Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 1335–1344. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; Estruch, R.; Ros, E.; Covas, M.I.; Ibarrola-Jurado, N.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; et al. Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Ann. Intern. Med. 2014, 160, 1–10. [Google Scholar] [CrossRef]
- Vahid, F.; Shivappa, N.; Karamati, M.; Naeini, A.J.; Hebert, J.R.; Davoodi, S.H. Association between Dietary Inflammatory Index (DII) and risk of prediabetes: A case-control study. Appl. Physol. Nutr. Metab. 2017, 42, 399–404. [Google Scholar] [CrossRef]
- Laouali, N.; Mancini, F.R.; Hajji-Louati, M.; El Fatouhi, D.; Balkau, B.; Boutron-Ruault, M.-C.; Bonnet, F.; Fagherazzi, G. Dietary inflammatory index and type 2 diabetes risk in a prospective cohort of 70,991 women followed for 20 years: The mediating role of BMI. Diabetologia 2019, 62, 2222–2232. [Google Scholar] [CrossRef]
- Esposito, K.; Chiodini, P.; Maiorino, M.I.; Bellastella, G.; Panagiotakos, D.; Giugliano, D. Which diet for prevention of type 2 diabetes? A meta-analysis of prospective studies. Endocrine 2014, 47, 107–116. [Google Scholar] [CrossRef]
- Alhazmi, A.; Stojanovski, E.; McEvoy, M.; Garg, M.L. The association between dietary patterns and type 2 diabetes: A systematic review and meta-analysis of cohort studies. J. Hum. Nutr. Diet. 2014, 27, 251–260. [Google Scholar] [CrossRef]
- Barnes, T.L.; Crandell, J.L.; Bell, R.A.; Mayer-Davis, E.J.; Dabelea, D.; Liese, A.D.; for the SEARCH for Diabetes in Youth Study Group. Change in DASH diet score and cardiovascular risk factors in youth with type 1 and type 2 diabetes mellitus: The SEARCH for Diabetes in Youth Study. Nutr. Diabetes 2013, 3, e91. [Google Scholar] [CrossRef] [PubMed]
- Gow, M.L.; Garnett, S.P.; Baur, L.A.; Lister, N.B. The Effectiveness of Different Diet Strategies to Reduce Type 2 Diabetes Risk in Youth. Nutrients 2016, 8, 486. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.J.; Lopez, R.; Snair, M.; Tang, A. Mediterranean diet adherence and metabolic syndrome in US adolescents. Int. J. Food Sci. Nutr. 2021, 72, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Cañete, R.; Gil-Campos, M.; Aguilera, C.M.; Gil, A. Development of insulin resistance and its relation to diet in the obese child. Eur. J. Nutr. 2007, 46, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Hirahatake, K.M.; Jacobs, D.R., Jr.; Shikany, J.M.; Jiang, L.; Wong, N.D.; Odegaard, A.O. Cumulative average dietary pattern scores in young adulthood and risk of incident type 2 diabetes: The CARDIA study. Diabetologia 2019, 62, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Cha, E.; Pasquel, F.J.; Yan, F.; Jacobs, D.R., Jr.; Dunbar, S.B.; Umpierrez, G.; Choi, Y.; Shikany, J.M.; Bancks, M.P.; Reis, J.P.; et al. Characteristics associated with early- vs. later-onset adult diabetes: The CARDIA study. Diabetes Res. Clin. Pract. 2021, 182, 109144. [Google Scholar] [CrossRef] [PubMed]
- Bantle, A.E.; Chow, L.S.; Steffen, L.M.; Wang, Q.; Hughes, J.; Durant, N.H.; Ingram, K.H.; Reis, J.P.; Schreiner, P.J. Association of Mediterranean diet and cardiorespiratory fitness with the development of pre-diabetes and diabetes: The Coronary Artery Risk Development in Young Adults (CARDIA) study. BMJ Open Diabetes Res. Care 2016, 4, e000229. [Google Scholar] [CrossRef]
- Choi, Y.; Larson, N.; Gallaher, D.D.; Odegaard, A.O.; Rana, J.S.; Shikany, J.M.; Steffen, L.M.; Jacobs, D.R., Jr. A Shift Toward a Plant-Centered Diet From Young to Middle Adulthood and Subsequent Risk of Type 2 Diabetes and Weight Gain: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes Care 2020, 43, 2796–2803. [Google Scholar] [CrossRef]
- Nelson, M.C.; Story, M.; Larson, N.I.; Neumark-Sztainer, D.; Lytle, L.A. Emerging Adulthood and College-aged Youth: An Overlooked Age for Weight-related Behavior Change. Obesity 2008, 16, 2205–2211. [Google Scholar] [CrossRef]
- McConnell, R.; Shen, E.; Gilliland Frank, D.; Jerrett, M.; Wolch, J.; Chang, C.-C.; Lurmann, F.; Berhane, K. A Longitudinal Cohort Study of Body Mass Index and Childhood Exposure to Secondhand Tobacco Smoke and Air Pollution: The Southern California Children’s Health Study. Environ. Health Perspect. 2015, 123, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Chen, Z.; Alderete, T.L.; Toledo-Corral, C.; Lurmann, F.; Berhane, K.; Gilliland, F.D. Associations of air pollution, obesity and cardiometabolic health in young adults: The Meta-AIR study. Environ. Int. 2019, 133, 105180. [Google Scholar] [CrossRef]
- Floch, J.-P.L.; Escuyer, P.; Baudin, E.; Baudon, D.; Perlemuter, L. Blood Glucose Area Under the Curve: Methodological Aspects. Diabetes Care 1990, 13, 172–175. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2020, 44 (Suppl. 1), S15–S33. [Google Scholar] [CrossRef]
- Hoffmann, K.; Boeing, H.; Dufour, A.; Volatier, J.L.; Telman, J.; Virtanen, M.; Becker, W.; De Henauw, S.; for the EFCOSUM Group. Estimating the distribution of usual dietary intake by short-term measurements. Eur. J. Clin. Nutr. 2002, 56, S53–S62. [Google Scholar] [CrossRef]
- Park, Y.; Dodd, K.W.; Kipnis, V.; Thompson, F.E.; Potischman, N.; Schoeller, D.A.; Baer, D.J.; Midthune, D.; Troiano, R.P.; Bowles, H.; et al. Comparison of self-reported dietary intakes from the Automated Self-Administered 24-h recall, 4-d food records, and food-frequency questionnaires against recovery biomarkers. Am. J. Clin. Nutr. 2018, 107, 80–93. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- Mellen, P.B.; Gao, S.K.; Vitolins, M.Z.; Goff, D.C., Jr. Deteriorating Dietary Habits Among Adults with Hypertension: DASH Dietary Accordance, NHANES 1988–1994 and 1999–2004. Arch. Intern. Med. 2008, 168, 308–314. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Food and Drug Administration. Final determination regarding partially hydrogenated oils. Fed. Regist. 2015, 80, 34650–34670. [Google Scholar]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [Green Version]
- Schisterman, E.F.; Cole, S.R.; Platt, R.W. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009, 20, 488–495. [Google Scholar] [CrossRef]
- Winpenny, E.M.; Penney, T.L.; Corder, K.; White, M.; van Sluijs, E.M.F. Change in diet in the period from adolescence to early adulthood: A systematic scoping review of longitudinal studies. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 60. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Pulgaron, E.R.; Delamater, A.M. Obesity and type 2 diabetes in children: Epidemiology and treatment. Curr. Diab. Rep. 2014, 14, 508. [Google Scholar] [CrossRef]
- Maggio, C.A.; Pi-Sunyer, F.X. Obesity and type 2 diabetes. Endocrinol. Metab. Clin. N. Am. 2003, 32, 805–822. [Google Scholar] [CrossRef]
- Zhao, T.; Lin, Z.; Zhu, H.; Wang, C.; Jia, W. Impact of body fat percentage change on future diabetes in subjects with normal glucose tolerance. IUBMB Life 2017, 69, 947–955. [Google Scholar] [CrossRef]
- Chen, Y.; He, D.; Yang, T.; Zhou, H.; Xiang, S.; Shen, L.; Wen, J.; Chen, S.; Peng, S.; Gan, Y. Relationship between body composition indicators and risk of type 2 diabetes mellitus in Chinese adults. BMC Public Health 2020, 20, 452. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Lanca, C.; Gan, A.T.L.; Soh, P.; Thakur, S.; Tao, Y.; Kumari, N.; Man, R.E.K.; Fenwick, E.K.; Lamoureux, E.L. The Association between Body Composition using Dual energy X-ray Absorptiometry and Type-2 Diabetes: A Systematic Review and Meta-Analysis of Observational studies. Sci. Rep. 2019, 9, 12634. [Google Scholar] [CrossRef] [PubMed]
- Jesudason, D.R.; Dunstan, K.; Leong, D.; Wittert, G.A. Macrovascular Risk and Diagnostic Criteria for Type 2 Diabetes: Implications for the use of FPG and HbA1c for cost-effective screening. Diabetes Care 2003, 26, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.; Albano, D.; Gitto, S.; Tofanelli, L.; Bazzocchi, A.; Ulivieri, F.M.; Guglielmi, G.; Sconfienza, L.M. Body composition with dual energy X-ray absorptiometry: From basics to new tools. Quant. Imaging Med. Surg. 2020, 10, 1687–1698. [Google Scholar] [CrossRef]
- Bartoli, E.; Fra, G.P.; Carnevale Schianca, G.P. The oral glucose tolerance test (OGTT) revisited. Eur. J. Intern. Med. 2011, 22, 8–12. [Google Scholar] [CrossRef]
- Maitre, L.; de Bont, J.; Casas, M.; Robinson, O.; Aasvang, G.M.; Agier, L.; Andrušaitytė, S.; Ballester, F.; Basagaña, X.; Borràs, E.; et al. Human Early Life Exposome (HELIX) study: A European population-based exposome cohort. BMJ Open 2018, 8, e021311. [Google Scholar] [CrossRef]
- Smith-Warner, S.A.; Spiegelman, D.; Ritz, J.; Albanes, D.; Beeson, W.L.; Bernstein, L.; Berrino, F.; van den Brandt, P.A.; Buring, J.E.; Cho, E.; et al. Methods for pooling results of epidemiologic studies: The Pooling Project of Prospective Studies of Diet and Cancer. Am. J. Epidemiol. 2006, 163, 1053–1064. [Google Scholar] [CrossRef]
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J. Neuroimmune Pharmacol. 2020, 15, 359–386. [Google Scholar] [CrossRef]
- Karatzi, K.; Poulia, K.A.; Papakonstantinou, E.; Zampelas, A. The Impact of Nutritional and Lifestyle Changes on Body Weight, Body Composition and Cardiometabolic Risk Factors in Children and Adolescents during the Pandemic of COVID-19: A Systematic Review. Children 2021, 8, 1130. [Google Scholar] [CrossRef]
- Kreutz, R.; Dobrowolski, P.; Prejbisz, A.; Algharably, E.A.E.; Bilo, G.; Creutzig, F.; Grassi, G.; Kotsis, V.; Lovic, D.; Lurbe, E.; et al. Lifestyle, psychological, socioeconomic and environmental factors and their impact on hypertension during the coronavirus disease 2019 pandemic. J. Hypertens. 2021, 39, 1077–1089. [Google Scholar] [CrossRef]
- Millen, B.E.; Abrams, S.; Adams-Campbell, L.; Anderson, C.A.; Brenna, J.T.; Campbell, W.W.; Clinton, S.; Hu, F.; Nelson, M.; Neuhouser, M.L.; et al. The 2015 Dietary Guidelines Advisory Committee Scientific Report: Development and Major Conclusions. Adv. Nutr. 2016, 7, 438–444. [Google Scholar] [CrossRef]
- Gardner, C.D.; Trepanowski, J.F.; Del Gobbo, L.C.; Hauser, M.E.; Rigdon, J.; Ioannidis, J.P.A.; Desai, M.; King, A.C. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight Loss in Overweight Adults and the Association With Genotype Pattern or Insulin Secretion: The DIETFITS Randomized Clinical Trial. JAMA 2018, 319, 667–679. [Google Scholar] [CrossRef]
- Koliaki, C.; Spinos, T.; Spinou, Μ.; Brinia, Μ.-E.; Mitsopoulou, D.; Katsilambros, N. Defining the Optimal Dietary Approach for Safe, Effective and Sustainable Weight Loss in Overweight and Obese Adults. Healthcare 2018, 6, 73. [Google Scholar] [CrossRef] [Green Version]


| Baseline (n = 155) | Follow-Up (n = 88) 1 | Baseline vs. Follow-Up p-Value 2 | |
|---|---|---|---|
| Age (years), Mean (SD) | 19.7 (1.2) | 24.1 (0.8) | - |
| Sex, n (%) Female Male | 71 (45.8) 84 (54.2) | 46 (52.3) 42 (47.7) | 0.40 |
| Ethnicity, n (%) Hispanic/Latino Non-Hispanic White Other | 94 (60.6) 52 (33.5) 9 (5.8) | 50 (56.8) 30 (34.1) 8 (9.1) | 0.60 |
| Parental Education, n (%) Did not complete high school Completed high school More than high school Don’t know | 31 (20.0) 23 (14.8) 96 (61.9) 5 (3.2) | 15 (17.0) 12 (13.6) 56 (63.6) 5 (5.7) | 0.76 |
| Exercise 3, n (%) Yes No | 118 (76.1) 37 (23.9) | - | - |
| Physical Activity Category, n (%) High Moderate Low Missing, n (%) | - | 50 (56.8) 21 (23.9) 16 (18.2) 1 (1.1) | - |
| Baseline (n = 155) | Follow-Up (n = 88) | Change between Baseline and Follow-Up (n = 85) 1 | Baseline vs. Follow-Up p-Value 2 | |
|---|---|---|---|---|
| HEI, Mean (SD) Range: 0–100 | 52.7 (13.0) | 49.7 (12.5) | −4.9 (13.2) | <0.001 |
| MDS, Mean (SD) Range: 0–9 | 5.03 (1.23) | 4.92 (1.53) | −0.22 (1.79) | 0.25 |
| DASH, Mean (SD) Range: 0–8 | 2.26 (1.51) | 1.74 (1.31) | −0.45 (1.53) | 0.009 |
| DII, Mean (SD) | 0.81 (1.56) | 0.29 (2.05) | −0.44 (1.98) | 0.044 |
| Energy (kcal), Mean (SD) | 2053 (630) | 2223 (773) | 158 (792) | 0.070 |
| Baseline (n = 155) | Follow-Up (n = 88) | Change between Baseline and Follow-Up (n = 85) 1 | Baseline vs. Follow-Up p-Value 2 | |
|---|---|---|---|---|
| Fasting Glucose, Mean (SD) Missing: n (%) | 91. (14) 1 (0.6) | 95 (16) 1 (1.1) | 5 (15) 1 (1.2%) | 0.003 |
| 2-h Glucose, Mean (SD) Missing: n (%) | 123 (37) 1 (0.6) | 122 (35) 4 (4.5) | 3 (32) 4 (4.7) | 0.39 |
| HbA1c, Mean (SD) Missing: n (%) | 5.25 (0.53) 1 (0.6) | 5.26 (0.51) | 0.042 (0.46) | 0.35 |
| Glucose AUC, Mean (SD) Missing: n (%) | 267 (59) 1 (0.6) | 269 (44) 6 (6.8) | 11 (40) 6 (7.1) | 0.023 |
| Diabetes, n (%) | 0.17 | |||
| No Diabetes | 109 (70.3) | 54 (61.4) | ||
| Prediabetes | 42 (27.1) | 30 (34.1) | ||
| Type 2 Diabetes | 3 (1.9) | 4 (4.5) | ||
| Missing | 1 (0.6) |
| Baseline (n = 155) | Follow-Up (n = 88) | Change between Baseline and Follow-Up (n = 85) 1 | Baseline vs. Follow-Up p-Value 2,3 | |
|---|---|---|---|---|
| BMI Category, n (%) Normal Weight Overweight Obese | 24 (15.5) 73 (47.1) 58 (37.4) | 12 (13.6) 34 (38.6) 42 (47.7) | 0.47 | |
| BMI (kg/m2), Mean (SD) | 29.9 (5.1) | 31.7 (7.0) | 1.8 (4.3) | <0.001 |
| Body Fat %, Mean (SD) Missing: n (%) | 34.8 (8.6) - | 38.5 (8.3) 2 (2.3) | 3.1 (4.7) 2 (2.4) | <0.001 |
| FFMI (kg/m2), Mean (SD) Missing: n (%) | 18.5 (2.5) - | 17.7 (2.9) 2 (2.3) | −0.6 (1.5) 2 (2.4) | 0.001 |
| Fat Mass:Height Ratio, Mean (SD) Missing: n (%) | 10.8 (4.3) 98 (63.2) | 12.2 (4.7) 2 (2.3) | 1.6 (2.1) 47 (55.3) | <0.001 |
| Android:Gynoid Ratio, Mean (SD) Missing: n (%) | (0.14) 98 (63.2) | 1.01 (0.15) 2 (2.3) | 0.015 (0.085) 47 (55.3) | 0.30 |
| Trunk:Leg Ratio, Mean (SD) Missing: n (%) | 0.95 (0.13) 98 (63.3) | 0.97 (0.13) 2 (2.3) | 0.016 (0.077) 47 (55.3) | 0.20 |
| Trunk:Limb Ratio, Mean (SD) Missing: n (%) | 1.05 (0.20) 98 (63.3) | 1.10 (0.23) 2 (2.3) | 0.051 (0.11) 47 (55.3) | 0.005 |
| VAT Volume (in3), Mean (SD) Missing: n (%) | 592 (301) 98 (63.3) | 633 (325) 2 (2.3) | 88 (148) 47 (55.3) | <0.001 |
| Diet | Outcome | Effect Estimate, β (95% CI) | ||
|---|---|---|---|---|
| Baseline 1 | Follow-Up 1 | Change between Visits 2 | ||
| Healthy Eating Index—2015 (HEI) | ||||
| BMI (kg/m2) | −0.62 (−1.45, 0.21) | −1.33 (−2.89, 0.24) | −0.38 (−1.62, 0.85) | |
| Body Fat (%) | −0.85 (−1.86, 0.16) | −1.09 (−2.37, 0.18) | 0.40 (−0.92, 1.73) | |
| FFMI (kg/m2) | −0.14 (−0.46, 0.17) | −0.46 (−1.04, 0.12) | −0.23 (−0.64, 0.18) | |
| Fat Mass:Height Ratio | −0.56 (−1.74, 0.62) | −0.73 (−1.68, 0.22) | −0.36 (−1.50, 0.78) | |
| Android:Gynoid Ratio | −0.045 (−0.087, −0.0036) | −0.043 (−0.071, −0.014) | −0.014 (−0.061, 0.034) | |
| Trunk:Leg Ratio | −0.040 (−0.077, −0.0028) | −0.035 (−0.060, −0.0087) | −0.0013 (−0.043, 0.041) | |
| Trunk:Limb Ratio | −0.052 (−0.11, 0.010) | −0.052 (−0.099, −0.0048) | −0.036 (−0.092, 0.020) | |
| VAT (in3) | −65.78 (−161.45, 29.49) | −60.54 (−132.21, 11.13) | −48.05 (−123.33, 27.23) | |
| Dietary Approaches to Stop Hypertension (DASH) Score | ||||
| BMI (kg/m2) | 0.067 (−0.80, 0.94) | −1.64 (−3.17, −0.11) | −1.63 (−2.91, −0.35) | |
| Body Fat (%) | 0.12 (−0.94, 1.18) | −1.79 (−3.01, −0.57) | −1.61 (−3.01, −0.21) | |
| FFMI (kg/m2) | −0.036 (−0.36, 0.29) | −0.49 (−1.06, 0.088) | −0.41 (−0.85, 0.024) | |
| Fat Mass:Height Ratio | 0.50 (−0.89, 1.88) | −1.09 (−2.02, −0.17) | −1.50 (−2.73, −0.27) | |
| Android:Gynoid Ratio | −0.015 (−0.066, 0.035) | −0.043 (−0.071, −0.015) | −0.047 (−0.098, 0.0045) | |
| Trunk:Leg Ratio | −0.023 (−0.068, 0.022) | −0.039 (−0.064, −0.014) | −0.037 (−0.084, 0.0097) | |
| Trunk:Limb Ratio | −0.018 (−0.093, 0.057) | −0.052 (−0.099, −0.0057) | −0.073 (−0.13, −0.011) | |
| VAT (in3) | 42.25 (−70.97, 155.46) | −76.57 (−146.46, −6.68) | −100.39 (−183.62, −17.17) | |
| Mediterranean Diet Score (MDS) | ||||
| BMI (kg/m2) | −0.090 (−0.91, 0.73) | −0.71 (−2.28, 0.86) | 0.27 (−0.95, 1.49) | |
| Body Fat (%) | −0.45 (−1.69, 0.79) | −0.48 (−2.35, 1.39) | 1.24 (−0.062, 2.55) | |
| FFMI (kg/m2) | 0.078 (−0.32, 0.47) | 0.075 (−0.57, 0.72) | −0.00040 (−0.42, 0.42) | |
| Fat Mass:Height Ratio | −0.37 (−1.49, 0.75) | −0.28 (−1.38, 0.83) | −0.081 (−1.11, 0.95) | |
| Android:Gynoid Ratio | 0.00054 (−0.042, 0.043) | −0.0049 (−0.039, 0.030) | 0.021 (−0.015, 0.057) | |
| Trunk:Leg Ratio | −0.030 (−0.065, 0.0037) | −0.0042 (−0.035, 0.027) | −0.0030 (−0.041, 0.035) | |
| Trunk:Limb Ratio | −0.044 (−0.10, 0.014) | −0.0073 (−0.062, 0.047) | −0.011 (−0.064, 0.042) | |
| VAT (in3) | −21.86 (−109.41, 65.68) | −17.16 (−92.10, 57.79) | −25.82 (−98.45, 46.81) | |
| Dietary Inflammatory Index (DII) | ||||
| BMI (kg/m2) | 0.86 (0.044, 1.67) | −0.67 (−2.32, 0.97) | −0.21 (−1.24, 0.83) | |
| Body Fat (%) | 2.04 (1.09, 2.99) | 1.13 (−0.19, 2.45) | 0.44 (−0.66, 1.54) | |
| FFMI (kg/m2) | −0.073 (−0.38, 0.23) | −0.60 (−1.20, −0.0068) | −0.16 (−0.50, 0.18) | |
| Fat Mass:Height Ratio | 0.88 (−0.23, 1.99) | −0.17 (−1.17, 0.84) | 0.52 (−0.33, 1.37) | |
| Android:Gynoid Ratio | 0.031 (−0.010, 0.072) | 0.014 (−0.017, 0.045) | 0.035 (0.0025, 0.068) | |
| Trunk:Leg Ratio | 0.027 (−0.010, 0.063) | 0.021 (−0.0070, 0.048) | 0.017 (−0.014, 0.048) | |
| Trunk:Limb Ratio | 0.028 (−0.033, 0.089) | 0.023 (−0.027, 0.074) | 0.029 (−0.014, 0.071) | |
| VAT (in3) | 47.00 (−44.96, 138.95) | −22.50 (−97.94, 52.94) | 17.77 (−42.53, 78.08) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costello, E.; Goodrich, J.; Patterson, W.B.; Rock, S.; Li, Y.; Baumert, B.; Gilliland, F.; Goran, M.I.; Chen, Z.; Alderete, T.L.; et al. Diet Quality Is Associated with Glucose Regulation in a Cohort of Young Adults. Nutrients 2022, 14, 3734. https://doi.org/10.3390/nu14183734
Costello E, Goodrich J, Patterson WB, Rock S, Li Y, Baumert B, Gilliland F, Goran MI, Chen Z, Alderete TL, et al. Diet Quality Is Associated with Glucose Regulation in a Cohort of Young Adults. Nutrients. 2022; 14(18):3734. https://doi.org/10.3390/nu14183734
Chicago/Turabian StyleCostello, Elizabeth, Jesse Goodrich, William B. Patterson, Sarah Rock, Yiping Li, Brittney Baumert, Frank Gilliland, Michael I. Goran, Zhanghua Chen, Tanya L. Alderete, and et al. 2022. "Diet Quality Is Associated with Glucose Regulation in a Cohort of Young Adults" Nutrients 14, no. 18: 3734. https://doi.org/10.3390/nu14183734
APA StyleCostello, E., Goodrich, J., Patterson, W. B., Rock, S., Li, Y., Baumert, B., Gilliland, F., Goran, M. I., Chen, Z., Alderete, T. L., Conti, D. V., & Chatzi, L. (2022). Diet Quality Is Associated with Glucose Regulation in a Cohort of Young Adults. Nutrients, 14(18), 3734. https://doi.org/10.3390/nu14183734