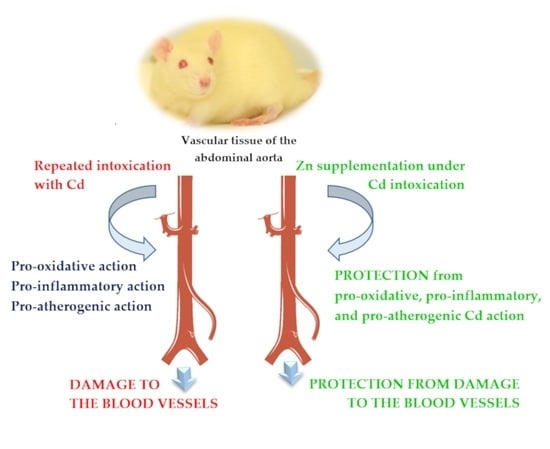
The Beneficial Impact of Zinc Supplementation on the Vascular Tissue of the Abdominal Aorta under Repeated Intoxication with Cadmium: A Study in an In Vivo Experimental Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Exposure to Cd
2.3. Supplementation with Zn
2.4. Experimental Model
- Control group: received drinking water without Cd and Zn addition;
- Zn30 group: received Zn at the concentration of 30 mg/L of drinking water;
- Zn60 group: received Zn at the concentration of 60 mg/L of drinking water;
- Cd5 group: intoxicated with Cd at the concentration of 5 mg/L of drinking water;
- Cd5 + Zn30 group: received drinking water containing 5 mg Cd/L and 30 mg Zn/L;
- Cd5 + Zn60 group: received drinking water containing 5 mg Cd/L and 60 mg Zn/L;
- Cd50 group: intoxicated with Cd at the concentration of 50 mg/L of drinking water;
- Cd50 + Zn30 group: received drinking water containing 50 mg Cd/L and 30 mg Zn/L;
- Cd50 + Zn60 group: received drinking water containing 50 mg Cd/L and 60 mg Zn/L.
2.5. Laboratory Procedures
2.5.1. Measurements in the Vascular Tissue
Preparation of the Homogenates of the Vascular Tissue
Biochemical Measurements in the Homogenates of the Vascular Tissue
2.5.2. Evaluation of Biomarkers of Inflammation in the Serum
2.5.3. Estimation of the Expression of Adhesive Molecules on the Endothelial Cells of the Abdominal Aorta and Leukocytes in the Blood
2.6. Statistical Analysis
3. Results
3.1. The Impact of Zn and/or Cd on the Oxidative/Antioxidative Status of the Vascular Tissue of the Abdominal Aorta
3.2. The Impact of Zn and/or Cd on the Concentrations of TC, TG, and eNOS in the Vascular Tissue of the Abdominal Aorta
3.3. The Impact of Zn and/or Cd on the Concentrations of Biomarkers of Inflammation in the Vascular Tissue of the Abdominal Aorta and Serum
3.4. The Impact of Zn and/or Cd on the Concentration of VEGF in the Serum
3.5. The Impact of Zn and/or Cd on the Expression of Adhesive Molecules on the Endothelial Cells of the Abdominal Aorta and Leukocytes in the Blood
3.6. Mutual Relationships between the Investigated Parameters, as Well as between These Parameters and the Concentrations of Cd in the Blood and Zn in the Serum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Skalny, A.V.; Aschner, M.; Tinkov, A.A. Zinc. Adv. Food Nutr. Res. 2021, 96, 251–310. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, H. Aberrance of Zinc Metalloenzymes-Induced Human Diseases and Its Potential Mechanisms. Nutrients 2021, 13, 4456. [Google Scholar] [CrossRef]
- Ho, E.; Wong, C.P.; King, J.C. Impact of zinc on DNA integrity and age-related inflammation. Free Radic. Biol. Med. 2022, 178, 391–397. [Google Scholar] [CrossRef]
- Oyagbemi, A.A.; Ajibade, T.O.; Aboua, Y.G.; Gbadamosi, I.T.; Adedapo, A.D.A.; Aro, A.O.; Adejumobi, O.A.; Thamahane-Katengua, E.; Omobowale, T.O.; Falayi, O.O.; et al. Potential health benefits of zinc supplementation for the management of COVID-19 pandemic. J. Food Biochem. 2021, 45, 13604. [Google Scholar] [CrossRef]
- Brzóska, M.; Kozłowska, M.; Rogalska, J.; Gałażyn-Sidorczuk, M.; Roszczenko, A.; Smereczański, N. Enhanced Zinc Intake Protects against Oxidative Stress and Its Consequences in the Brain: A Study in an In Vivo Rat Model of Cadmium Exposure. Nutrients 2021, 13, 478. [Google Scholar] [CrossRef]
- Rogalska, J.; Pilat-Marcinkiewicz, B.; Brzóska, M.M. Protective effect of zinc against cadmium hepatotoxicity depends on this bioelement intake and level of cadmium exposure: A study in a rat model. Chem. Biol. Interact. 2011, 193, 191–203. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Rogalska, J.; Galażyn-Sidorczuk, M.; Jurczuk, M.; Roszczenko, A.; Kulikowska-Karpińska, E.; Moniuszko-Jakoniuk, J. Effect of zinc supplementation on bone metabolism in male rats chronically exposed to cadmium. Toxicology 2007, 237, 89–103. [Google Scholar] [CrossRef]
- Galażyn-Sidorczuk, M.; Brzóska, M.M.; Rogalska, J.; Roszczenko, A.; Jurczuk, M. Effect of zinc supplementation on glutathione peroxidase activity and selenium concentration in the serum, liver and kidney of rats chronically exposed to cadmium. J. Trace Elem. Med. Biol. 2012, 26, 46–52. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Rogalska, J. Protective effect of zinc supplementation against cadmium-induced oxidative stress and the RANK/RANKL/OPG system imbalance in the bone tissue of rats. Toxicol. Appl. Pharmacol. 2013, 272, 208–220. [Google Scholar] [CrossRef]
- Rogalska, J.; Brzóska, M.M.; Roszczenko, A.; Moniuszko-Jakoniuk, J. Enhanced zinc consumption prevents cadmium-induced alterations in lipid metabolism in male rats. Chem. Biol. Interact. 2009, 177, 142–152. [Google Scholar] [CrossRef]
- Rahman, M.; Hossain, K.F.B.; Banik, S.; Sikder, T.; Akter, M.; Bondad, S.E.C.; Rahaman, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. Selenium and zinc protections against metal-(loids)-induced toxicity and disease manifestations: A review. Ecotoxicol. Environ. Saf. 2019, 30, 146–163. [Google Scholar] [CrossRef]
- Mezynska, M.; Brzóska, M.M. Environmental exposure to cadmium—A risk for health of the general population in industrialized countries and preventive strategies. Environ. Sci. Pollut. Res. 2018, 25, 3211–3232. [Google Scholar] [CrossRef]
- Ruczaj, A.; Brzóska, M.M. Environmental exposure of the general population to cadmium as a risk factor of the damage to the nervous system—A critical review of current data. J. Appl. Toxicol. 2022, 1–23. [Google Scholar] [CrossRef]
- ATSDR. Substance Priority List|ATSDR. Agency for Toxic Substances and Disease Registry. 2019. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 12 April 2022).
- Chen, X.; Zhu, G.; Wang, Z.; Zhou, H.; He, P.; Liu, Y.; Jin, T. The association between lead and cadmium co-exposure and renal dysfunction. Ecotoxicol. Environ. Saf. 2019, 173, 429–435. [Google Scholar] [CrossRef]
- Djordjevic, V.R.; Wallace, D.R.; Schweitzer, A.; Boricic, N.; Knezevic, D.; Matic, S.; Grubor, N.; Kerkez, M.; Radenkovic, D.; Bulat, Z.; et al. Environmental cadmium exposure and pancreatic cancer: Evidence from case control, animal and in vitro studies. Environ. Int. 2019, 128, 353–361. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E. Chronic cadmium exposure and cardiovascular disease in adults. J. Environ. Sci. Health Part A Tox. Hazard. Subst. Environ. Eng. 2020, 55, 726–729. [Google Scholar] [CrossRef]
- Tellez-Plaza, M.; Guallar, E.; Howard, B.V.; Umans, J.G.; Francesconi, K.A.; Goessler, W.; Silbergeld, E.K.; Devereux, R.B.; Navas-Acien, A. Cadmium Exposure and Incident Cardiovascular Disease. Epidemiology 2013, 24, 421–429. [Google Scholar] [CrossRef]
- Barregard, L.; Sallsten, G.; Harari, F.; Andersson, E.M.; Forsgard, N.; Hjelmgren, O.; Angerås, O.; Fagman, E.; Persson, M.; Lundh, T.; et al. Cadmium Exposure and Coronary Artery Atherosclerosis: A Cross-Sectional Population-Based Study of Swedish Middle-Aged Adults. Environ. Health Perspect. 2021, 129, 067007. [Google Scholar] [CrossRef]
- Lin, H.-C.; Hao, W.-M.; Chu, P.-H. Cadmium and cardiovascular disease: An overview of pathophysiology, epidemiology, therapy, and predictive value. Rev. Port. Cardiol. 2021, 40, 611–617. [Google Scholar] [CrossRef]
- Fagerberg, B.; Barregard, L. Review of cadmium exposure and smoking-independent effects on atherosclerotic cardiovascular disease in the general population. J. Intern. Med. 2021, 290, 1153–1179. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Edwards, J.R.; Nebert, D.W.; Woods, J.M.; Barchowsky, A.; Atchison, W.D. The Vascular System as a Target of Metal Toxicity. Toxicol. Sci. 2007, 102, 207–218. [Google Scholar] [CrossRef]
- Almenara, C.C.P.; Broseghini-Filho, G.B.; Vescovi, M.V.A.; Angeli, J.K.; Faria, T.D.O.; Stefanon, I.; Vassallo, D.; Padilha, A.S. Chronic Cadmium Treatment Promotes Oxidative Stress and Endothelial Damage in Isolated Rat Aorta. PLoS ONE 2013, 8, e68418. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Edwards, J.R.; Woods, J.M. The vascular endothelium as a target of cadmium toxicity. Life Sci. 2006, 79, 1493–1506. [Google Scholar] [CrossRef]
- Szuster-Ciesielska, A.; Stachura, A.; Słotwińska, M.; Kamińska, T.; Śnieżko, R.; Paduch, R.; Abramczyk, D.; Filar, J.; Kandefer-Szerszeń, M. The inhibitory effect of zinc on cadmium-induced cell apoptosis and reactive oxygen species (ROS) production in cell cultures. Toxicology 2000, 145, 159–171. [Google Scholar] [CrossRef]
- Oliveira, T.F.; Batista, P.R.; Leal, M.A.; Campagnaro, B.P.; Nogueira, B.V.; Vassallo, D.V.; Meyrelles, S.S.; Padilha, A.S. Chronic Cadmium Exposure Accelerates the Development of Atherosclerosis and Induces Vascular Dysfunction in the Aorta of ApoE−/− Mice. Biol. Trace Elem. Res. 2019, 187, 163–171. [Google Scholar] [CrossRef]
- Angeli, J.K.; Pereira, C.A.C.; Faria, T.D.O.; Stefanon, I.; Padilha, A.S.; Vassallo, D.V. Cadmium exposure induces vascular injury due to endothelial oxidative stress: The role of local angiotensin II and COX-2. Free Radic. Biol. Med. 2013, 65, 838–848. [Google Scholar] [CrossRef]
- Díaz, M.F.P.; Pignatari, M.G.P.; Filippa, V.P.; Mohamed, F.H.; Marchevsky, E.J.; Gimenez, M.S.; Ramirez, D.C.; Pignatari, G.P. A soybean-based diet modulates cadmium-induced vascular apoptosis. J. Trace Elements Med. Biol. 2019, 52, 239–246. [Google Scholar] [CrossRef]
- Fittipaldi, S.; Bimonte, V.M.; Soricelli, A.; Aversa, A.; Lenzi, A.; Greco, E.A.; Migliaccio, S. Cadmium exposure alters steroid receptors and proinflammatory cytokine levels in endothelial cells in vitro: A potential mechanism of endocrine disruptor atherogenic effect. J. Endocrinol. Investig. 2019, 42, 727–739. [Google Scholar] [CrossRef]
- Kumar, S.K.; Prakash, T.; Vetriselvan, M.; Mani, K.P. Trehalose protects the endothelium from cadmium-induced dysfunction. Cell Biol. Int. 2021, 45, 957–964. [Google Scholar] [CrossRef]
- Amini, M.; Zayeri, F.; Salehi, M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: Results from global burden of disease study 2017. BMC Public Health 2021, 21, 401. [Google Scholar] [CrossRef]
- Zalewski, P.D.; Beltrame, J.F.; Wawer, A.A.; Abdo, A.I.; Murgia, C. Roles for endothelial zinc homeostasis in vascular physiology and coronary artery disease. Crit. Rev. Food Sci. Nutr. 2019, 59, 3511–3525. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.; Foster, M.; Samman, S. Zinc Status and Risk of Cardiovascular Diseases and Type 2 Diabetes Mellitus—A Systematic Review of Prospective Cohort Studies. Nutrients 2016, 8, 707. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Huang, L.; Zhao, J.; Wang, Z.; Yao, W.; Wu, X.; Huang, J.; Bian, B. The Relationship between Serum Zinc Level and Heart Failure: A Meta-Analysis. BioMed Res. Int. 2018, 2018, 2739014. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, S.; Bergdahl, A. Zinc Homeostasis in Diabetes Mellitus and Vascular Complications. Biomedicines 2022, 10, 139. [Google Scholar] [CrossRef]
- Yu, H.-T.; Zhen, J.; Leng, J.-Y.; Cai, L.; Ji, H.-L.; Keller, B.B. Zinc as a countermeasure for cadmium toxicity. Acta Pharmacol. Sin. 2021, 42, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Babaknejad, N.; Bahrami, S.; Moshtaghie, A.A.; Nayeri, H.; Rajabi, P.; Iranpour, F.G. Cadmium Testicular Toxicity in Male Wistar Rats: Protective Roles of Zinc and Magnesium. Biol. Trace Elem. Res. 2018, 185, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Su, Y.; Zheng, Y.; Fu, B.M.; Tang, L.; Qin, Y.-X. Zinc regulates vascular endothelial cell activity through zinc-sensing receptor ZnR/GPR39. Am. J. Physiol. Physiol. 2018, 314, C404–C414. [Google Scholar] [CrossRef]
- Mishima, A.; Kaji, T.; Yamamoto, C.; Sakamoto, M.; Kozuka, H. Zinc-induced tolerance to cadmium cytotoxicity without metallothionein induction in cultured bovine aortic endothelial cells. Toxicol. Lett. 1995, 75, 85–92. [Google Scholar] [CrossRef]
- Kaji, T.; Mishima, A.; Koyanagi, E.; Yamamoto, C.; Sakamoto, M.; Kozuka, H. Possible mechanism for zinc protection against cadmium cytotoxicity in cultured vascular endothelial cells. Toxicology 1992, 76, 257–270. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [Green Version]
- Black, P.H.; Garbutt, L.D. Stress, inflammation and cardiovascular disease. J. Psychosom. Res. 2002, 52, 1–23. [Google Scholar] [CrossRef]
- Mężyńska, M.; Brzóska, M.M.; Rogalska, J.; Piłat-Marcinkiewicz, B. Extract from Aronia melanocarpa L. Berries Prevents Cadmium-Induced Oxidative Stress in the Liver: A Study in A Rat Model of Low-Level and Moderate Lifetime Human Exposure to this Toxic Metal. Nutrients 2019, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Torabimehr, F.; Kazemi, N.; Hosseini, S.A. Effects of Resistance and Endurance Training on HIF-1α and VEGF in Heart Tissues of Pregnant Rats with Cadmium Toxicity. Gene Cell Tissue 2019, in press. [Google Scholar] [CrossRef]
- Xu, F.; Liu, S.; Li, S. Effects of Selenium and Cadmium on Changes in the Gene Expression of Immune Cytokines in Chicken Splenic Lymphocytes. Biol. Trace Element Res. 2015, 165, 214–221. [Google Scholar] [CrossRef]
- Odewumi, C.; Latinwo, L.M.; Sinclair, A.; Badisa, V.L.; Abdullah, A.; Badisa, R.B. Effect of cadmium on the expression levels of interleukin-1α and interleukin-10 cytokines in human lung cells. Mol. Med. Rep. 2015, 12, 6422–6426. [Google Scholar] [CrossRef] [PubMed]
- Kim, K. Blood cadmium concentration and lipid profile in Korean adults. Environ. Res. 2012, 112, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Azimi-Nezhad, M.; Shabestari, M.M.; Azad, F.J.; Farkhondeh, T.; Bafandeh, F. Effect of chronic exposure to cadmium on serum lipid, lipoprotein and oxidative stress indices in male rats. Interdiscip. Toxicol. 2015, 8, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Lu, H.; Wang, X.Q.; Vaziri, N.D.; Zhou, X.J. Upregulation of Endothelial and Inducible Nitric Oxide Synthase Expression by Reactive Oxygen Species. Am. J. Hypertens. 2008, 21, 28–34. [Google Scholar] [CrossRef]
- Al-Naemi, H.A.; Das, S.C. Cadmium-induced endothelial dysfunction mediated by asymmetric dimethylarginine. Environ. Sci. Pollut. Res. Int. 2020, 27, 16246–16253. [Google Scholar] [CrossRef]
- Mousa, S.A. Expression of adhesion molecules during cadmium hepatotoxicity. Life Sci. 2004, 75, 93–105. [Google Scholar] [CrossRef]
- Fagerberg, B.; Bergström, G.; Borén, J.; Barregard, L. Cadmium exposure, intercellular adhesion molecule-1 and peripheral artery disease: A cohort and an experimental study. BMJ Open 2013, 3, e002489. [Google Scholar] [CrossRef]
- Sundaresan, S.; John, S.; Paneerselvam, G.; Andiapppan, R.; Christopher, G.; Selvam, G.S. Gallic acid attenuates cadmium mediated cardiac hypertrophic remodelling through upregulation of Nrf2 and PECAM-1signalling in rats. Environ. Toxicol. Pharmacol. 2021, 87, 103701. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Cao, Y.; Gao, J.; Fu, B.; Ren, J.; Ba, L.; Song, C.; Qi, H.; Huang, W.; Guan, X.; et al. Allicin improves the function of cardiac microvascular endothelial cells by increasing PECAM-1 in rats with cardiac hypertrophy. Phytomedicine 2018, 51, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar] [PubMed]
- Wang, C.-C.; Si, L.-F.; Guo, S.-N.; Zheng, J.-L. Negative effects of acute cadmium on stress defense, immunity, and metal homeostasis in liver of zebrafish: The protective role of environmental zinc pre-exposure. Chemosphere 2019, 222, 91–97. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Wathurapatha, W.S.; Ishara, M.H.; Jayawardana, R.; Galappatthy, P.; Katulanda, P.; Constantine, G. Effects of Zinc supplementation on serum lipids: A systematic review and meta-analysis. Nutr. Metab. 2015, 12, 1–16. [Google Scholar] [CrossRef]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Park, E.; Kim, J.; Kim, B.; Park, E.Y. Association between environmental exposure to cadmium and risk of suspected non-alcoholic fatty liver disease. Chemosphere 2021, 246, 128947. [Google Scholar] [CrossRef]
- Domingo-Relloso, A.; Riffo-Campos, A.L.; Haack, K.; Rentero-Garrido, P.; Ladd-Acosta, C.; Fallin, D.M.; Tang, W.Y.; Herreros-Martinez, M.; Gonzalez, J.R.; Bozack, A.K.; et al. Cadmium, smoking, and human blood DNA methylation profiles in adults from the strong heart study. Environ. Health Perspect. 2020, 128, 1–14. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, H.R.; Lee, M.; Kim, N.H.; Wang, K.M.; Lee, S.; Park, O.; Hong, E.J.; Youn, J.W.; Kim, Y.Y. Smoking-related DNA methylation is differentially associated with cadmium concentration in blood. Biochem. Genet. 2020, 58, 617–630. [Google Scholar] [CrossRef]
- Martins, A.C.; Urbano, M.R.; Lopes, A.C.B.A.; Carvalho, M.F.H.; Buzzo, M.L.; Docea, A.O.; Mesas, A.E.; Aschner, M.; Silva, A.M.R.; Silbergeld, E.K.; et al. Blood cadmium levels and sources of exposure in an adult urban population in southern Brazil. Environ. Res. 2020, 187, 109618. [Google Scholar] [CrossRef]
- Liao, K.W.; Pan, W.H.; Liou, S.H.; Sun, C.W.; Huang, P.C.; Wang, S.L. Levels and temporal variations of urinary lead, cadmium, cobalt, and copper exposure in the general population of Taiwan. Environ. Sci. Pollut. Res. Int. 2019, 26, 6048–6064. [Google Scholar] [CrossRef] [PubMed]
- Ghoochani, M.; Dehghani, M.H.; Rastkari, N.; Nodehi, R.N.; Yunesian, M.; Mesdaghinia, A.; Houshiarrad, A.; Saraei, M. Association among sources exposure of cadmium in the adult non-smoking general population of Tehran. Biol. Trace. Elem. Res. 2019, 191, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Tratnik, J.S.; Falnoga, I.; Mazej, D.; Kocman, D.; Fajon, V.; Jagodic, M.; Stajnko, A.; Trdin, A.; Šlejkovec, Z.; Jeran, Z.; et al. Results of the first national human biomonitoring in Slovenia: Trace elements in men and lactating women, predictors of exposure and reference values. Int. J. Hyg. Environ. Health 2019, 222, 563–582. [Google Scholar] [CrossRef]
- Li, H.; Fagerberg, B.; Sallsten, G.; Borné, Y.; Hedblad, B.; Engström, G.; Barregard, L.; Andersson, E.M. Smoking-induced risk of future cardiovascular disease is partly mediated by cadmium in tobacco: Malmö Diet and Cancer Cohort Study. Environ. Health 2019, 18, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, G.; Wang, Z.; Liang, Y.; Chen, B.; He, P.; Nordberg, M.; Nordberg, G.F.; Ding, X.; Jin, T. The association between dietary cadmium exposure and renal dysfunction—The benchmark dose estimation of reference levels: The ChinaCad study. J. Appl. Toxicol. 2018, 38, 1365–1373. [Google Scholar] [CrossRef]
- Mortada, W.I.; Hassanien, M.M.; Donia, A.F.; Shokeir, A.A. Application of cloud point extraction for cadmium in biological samples of occupationally exposed workers: Relation between cadmium exposure and renal lesion. Biol. Trace Elem. Res. 2015, 168, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Pawlas, N.; Strömberg, U.; Carlberg, B.; Cerna, M.; Harari, F.; Harari, R.; Horvat, M.; Hruba, F.; Koppova, K.; Krskova, A.; et al. Cadmium, mercury and lead in the blood of urban women in Croatia, the Czech Republic, Poland, Slovakia, Slovenia, Sweden, China, Ecuador and Morocco. Int. J. Occup. Med. Environ. Health 2013, 26, 58–72. [Google Scholar] [CrossRef]
| Experimental Group | Cd Intake (mg/kg b.w./24 h) | Zn Intake (mg/kg b.w./24 h) |
|---|---|---|
| Control | 0 | 0 |
| Zn30 | 0 | 1.26–3.67 |
| Zn60 | 0 | 2.40–6.41 |
| Cd5 | 0.222–0.731 | 0 |
| Cd5 + Zn30 | 0.243–0.745 | 1.33–3.57 |
| Cd5 + Zn60 | 0.260–0.740 | 2.41–6.67 |
| Cd50 | 1.850–4.340 | 0 |
| Cd50 + Zn30 | 2.000–4.370 | 1.41–3.98 |
| Cd50 + Zn60 | 2.000–4.440 | 2.70–7.14 |
| Experimental Group | TAS (nmol/mg Protein) | TOS (nmol/mg Protein) | OSI |
|---|---|---|---|
| Control | 87.5 78.1–108.0 | 11.78 9.82–13.60 | 0.126 0.122–0.142 |
| Zn30 | 102.2 98.5–111.2 | 4.63 a* 2.70–6.36 | 0.041 a† 0.019–0.089 |
| Zn60 | 85.4 74.1–108.6 | 4.31 a* 3.18–5.34 | 0.051 a† 0.031–0.058 |
| Cd5 | 52.8 a* b‡ c* 30.5–60.5 | 14.98 b* c* 11.31–17.05 | 0.328 a† b‡ c‡ 0.294–0.373 |
| Cd5 + Zn30 | 80.1 60.4–98.4 | 5.32 4.35–8.50 | 0.074 d* 0.056–0.091 |
| Cd5 + Zn60 | 45.3 a† b‡ c† 30.4–50.2 | 3.46 a† d† 3.04–5.13 | 0.079 d* 0.069–0.159 |
| Cd50 | 48.1 a† b‡ 38.6–67.1 | 33.43 a† b‡ c‡ e* f‡ 7.38–46.14 | 0.669 a‡ b‡ c‡ e† f* 0.598–0.775 |
| Cd50 + Zn30 | 56.0 b† 45.1–98.7 | 4.83 a* d* g† 0.93–5.78 | 0.067 d* g† 0.0570–0.102 |
| Cd50 + Zn60 | 54.6 b‡ 36.4–64.3 | 4.57 a* g† 1.54–6.84 | 0.095 g* 0.074–0.110 |
| Experimental Group | TC (mg/g Tissue) | TG (mg/g Tissue) | eNOS (ng/mg Protein) |
|---|---|---|---|
| Control | 1.048 1.012–1.134 | 1.919 1.519–2.047 | 9.809 6.482–15.94 |
| Zn30 | 1.054 1.016–1.215 | 1.906 1.792–2.059 | 9.840 8.210–12.01 |
| Zn60 | 1.088 0.969–1.165 | 1.779 1.558–2.067 | 9.579 7.463–12.84 |
| Cd5 | 1.550 a‡ b† c† 1.496–1.731 | 3.092 a† b* c‡ 2.363–3.882 | 11.50 7.147–16.55 |
| Cd5 + Zn30 | 1.296 1.221–1.579 | 2.064 1.941–2.217 | 9.267 5.199–13.85 |
| Cd5 + Zn60 | 1.1914 1.037–1.215 | 2.243 1.858–2.743 | 10.17 4.073–14.99 |
| Cd50 | 1.689 a‡ b† c‡ 1.507–1.721 | 2.784 a† b* c† 2.370–3.143 | 24.43 a* e* f* 16.50–38.53 |
| Cd50 + Zn30 | 1.076 d† g† 0.908–1.338 | 1.770 d‡ g‡ 1.626–1.994 | 7.029 g‡ 3.393–10.10 |
| Cd50 + Zn60 | 1.198 g* 0.808–1.353 | 1.932 d† g† 1.546–2.042 | 6.851 g‡ 5.588–14.23 |
| Experimental Group | Vascular Tissue | Serum | |||
|---|---|---|---|---|---|
| IL-1β (pg/mg Protein) | IL-10 (pg/mg Protein) | IL-1β (pg/mL) | IL-10 (pg/mL) | CRP (μg/mL) | |
| Control | 12.69 9.04–16.51 | 22.05 18.72–22.87 | 25.37 18.08–33.02 | 109.7 93.2–137.2 | 67.55 53.81–90.84 |
| Zn30 | 12.30 8.77–16.38 | 27.24 20.83–29.44 | 24.60 17.54–33.13 | 136.2 104.1–147.2 | 56.29 41.89–64.18 |
| Zn60 | 12.90 10.48–15.72 | 24.70 22.88–26.31 | 25.80 20.97–31.43 | 123.5 114.4–131.5 | 86.21 44.13–116.8 |
| Cd5 | 288.1 a* b* c* 237.0–422.1 | 26.11 22.06–41.97 | 144.0 a* b* c* 118.5–211.0 | 31.33 a† b‡ c‡ 26.47–50.37 | 275.0 a‡ b‡ c† 236.9–349.9 |
| Cd5 + Zn30 | 466.9 a† b† c† 306.9–578.9 | 48.63 a‡ 34.89–57.27 | 233.4 a† b† c† 153.5–289.5 | 60.79 d* 43.61–71.59 | 108.8 95.95–171.1 |
| Cd5 + Zn60 | 395.4 a† b† c† 314.9–664.7 | 34.97 26.57–50.56 | 197.7 a† b† c† 157.5–332.3 | 43.72 b* 33.21–63.20 | 154.0 133.1–215.3 |
| Cd50 | 761.4 a‡ b‡ c‡ 568.4–898.3 | 20.34 14.54–24.60 | 423.0 a‡ b‡ c‡ 315.8–499.1 | 28.18 a‡ b‡ c‡ 17.45–32.88 | 484.8 a‡ b‡ c‡ e† 430.5–605.3 |
| Cd50 + Zn30 | 221.1 g* 174. 7–313.3 | 47.30 a‡ e‡ g‡ 36.23–62.08 | 100.5 g* 79.4–142.4 | 39.42 b† c* 30.19–51.73 | 179.5 b* g† 146.2–190.5 |
| Cd50 + Zn60 | 222.8 g* 202.4–461.3 | 53.34 a‡ b* c* g‡ 39.45–88.57 | 101.3 g* 92.0–209.7 | 44.45 b* 32.88–73.81 | 237.0 a† b‡ c* 205.4–217.4 |
| Experimental Group | VEGF (pg/mL) | |
|---|---|---|
| Median | Minimum and Maximum | |
| Control | 0.612 | 0.294–1.229 |
| Zn30 | 0.258 | 0.055–0.453 |
| Zn60 | 0.385 | 0.067–0.730 |
| Cd5 | 1.744 b* c* | 0.723–9.146 |
| Cd5 + Zn30 | 0.562 | 0.083–0.928 |
| Cd5 + Zn60 | 0.400 d* | 0.095–0.955 |
| Cd50 | 1.398 b* c* f* | 1.131–3.606 |
| Cd50 + Zn30 | 0.409 g* | 0.153–0.760 |
| Cd50 + Zn60 | 0.140 d† g† | 0.063–0.951 |
| Experimental Group | PECAM-1 (%) | ICAM-1 (%) | L-Selectin (%) |
|---|---|---|---|
| Control | 4.65 3.40–5.10 | 2.85 1.20–3.20 | 3.41 2.50–4.33 |
| Zn30 | 1.60 0.90–2.90 | 0.81 0.32–1.50 | 0.75 0.56–1.22 |
| Zn60 | 1.78 0.90–3.00 | 0.68 0.33–2.49 | 1.48 0.80–2.79 |
| Cd5 | 0.55 a† 0.40–1.00 | 0.19 a† 0.09–0.46 | 0.42 a* 0.39–0.54 |
| Cd5 + Zn30 | 0.50 a† 0.40–0.70 | 0.20 a* 0.13–0.27 | 0.23 a† c* 0.15–0.30 |
| Cd5 + Zn60 | 0.40 a‡ b* c* 0.20–1.00 | 0.13 a‡ b* 0.05–0.22 | 0.08 a‡ b† c‡ 0.04–0.17 |
| Cd50 | 1.05 a* 0.70–1.80 | 0.27 a* 0.18–0.49 | 0.75 a* f† 0.48–0.91 |
| Cd50 + Zn30 | 0.40 a‡ b† c† g* 0.20–0.50 | 0.16 a† 0.10–0.26 | 0.17 a‡ c† 0.09–0.22 |
| Cd50 + Zn60 | 0.30 a‡ b† c† g* 0.20–0.50 | 0.10 a‡ b‡ 0.05–0.13 | 0.12 a‡ b† c‡ g* 0.08–0.15 |
| Experimental Group | ICAM-1 (%) | L-Selectin (%) | ||
|---|---|---|---|---|
| Median | Minimum and Maximum | Median | Minimum and Maximum | |
| Control | 0.114 | 0.038–0.300 | 28.4 | 23.7–31.4 |
| Zn30 | 0.052 | 0.039–0.075 | 19.5 | 15.5–25.5 |
| Zn60 | 0.051 | 0.039–0.059 | 34.8 | 28.0–41.9 |
| Cd5 | 0.783 | 0.423–1.475 | 27.6 | 20.1–41.4 |
| Cd5 + Zn30 | 2.295 a‡ b‡ c‡ | 1.910–2.790 | 6.67 a* c† d* | 4.20–8.97 |
| Cd5 + Zn60 | 1.490 a† b† c† | 1.240–1.960 | 3.91 a† c‡ d† | 3.08–5.00 |
| Cd50 | 0.329 e* | 0.064–0.441 | 22.1 | 12.6–28.3 |
| Cd50 + Zn30 | 2.255 a‡ b‡ c‡ g* | 1.520–2.940 | 7.04 a* c† g* | 6.36–9.00 |
| Cd50 + Zn60 | 1.000 | 0.600–1.220 | 2.06 a‡ b* c‡ d‡ g‡ | 0.78–3.17 |
| Parameter | Regression Analysis | TAS | TOS | OSI | TC | TG | eNOS | IL-1β | IL-10 | PECAM-1 | ICAM-1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TOS | β p | NS | |||||||||
| R2 | |||||||||||
| OSI | β p | –0.412 ‡ | 0.957 ‡ | ||||||||
| R2 | 0.158 | 0.914 | |||||||||
| TC | β p | –0.423 ‡ | 0.665 ‡ | 0.757 ‡ | |||||||
| R2 | 0.167 | 0.435 | 0.566 | ||||||||
| TG | β p | –0.461 ‡ | 0.572 ‡ | 0.687 ‡ | 0.742 ‡ | ||||||
| R2 | 0.201 | 0.318 | 0.464 | 0.544 | |||||||
| eNOS | β p | NS | 0.773 ‡ | 0.751 ‡ | 0.510 ‡ | 0.480 ‡ | |||||
| R2 | 0.592 | 0.558 | 0.250 | 0.220 | |||||||
| IL-1β | β p | –0.625 ‡ | 0.566 ‡ | 0.664 ‡ | 0.672 ‡ | 0.513 ‡ | 0.468 ‡ | ||||
| R2 | 0.381 | 0.311 | 0.432 | 0.444 | 0.253 | 0.208 | |||||
| IL-10 | β p | NS | –0.420 ‡ | –0.374 † | NS | –0.242 * | –0.380 ‡ | NS | |||
| R2 | 0.164 | 0.127 | 0.045 | 0.131 | |||||||
| PECAM-1 | β p | 0.526 ‡ | NS | NS | –0.285 * | –0.224 # | NS | –0.481 ‡ | –0.504 ‡ | ||
| R2 | 0.266 | 0.068 | 0.036 | 0.220 | 0.243 | ||||||
| ICAM-1 | β p | 0.528 ‡ | NS | NS | –0.338 † | –0.261 * | NS | –0.536 ‡ | –0.420 ‡ | 0.965 ‡ | |
| R2 | 0.268 | 0.101 | 0.055 | 0.277 | 0.164 | 0.931 | |||||
| L-selectin | β p | 0.470 ‡ | NS | NS | –0.270 * | NS | NS | –0.490 ‡ | –0.489 ‡ | 0.966 ‡ | 0.954 ‡ |
| R2 | 0.210 | 0.060 | 0.229 | 0.228 | 0.931 | 0.909 |
| Serum | Regression Analysis | Vascular Tissue of the Abdominal Aorta | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TAS | TOS | OSI | TC | TG | eNOS | IL-1β | IL-10 | PECAM-1 | ICAM-1 | L-Selectin | ||
| CRP | β p | –0.586 ‡ | 0.812 ‡ | 0.893 ‡ | 0.719 ‡ | 0.597 ‡ | 0.652 ‡ | 0.749 ‡ | NS | –0.355 † | –0.431 ‡ | –0.334 † |
| R2 | 0.334 | 0.654 | 0.794 | 0.510 | 0.347 | 0.416 | 0.555 | 0.113 | 0.174 | 0.099 | ||
| IL-1β | β p | –0.573 ‡ | 0.637 ‡ | 0.722 ‡ | 0.688 ‡ | 0.526 ‡ | 0.535 ‡ | 0.991 ‡ | NS | –0.406 ‡ | –0.474 ‡ | –0.418 ‡ |
| R2 | 0.319 | 0.397 | 0.514 | 0.466 | 0.267 | 0.276 | 0.982 | 0.153 | 0.213 | 0.163 | ||
| IL-10 | β p | 0.759 ‡ | –0.368 † | –0.497 ‡ | –0.561 ‡ | –0.495 ‡ | –0.240 * | –0.760 ‡ | –0.760 ‡ | 0.614 ‡ | 0.621 ‡ | 0.591 ‡ |
| R2 | 0.569 | 0.123 | 0.236 | 0.305 | 0.234 | 0.044 | 0.571 | 0.571 | 0.368 | 0.377 | 0.340 | |
| VEGF | β p | –0.245 * | 0.476 ‡ | 0.481 ‡ | 0.581 ‡ | 0.547 ‡ | 0.317 † | 0.243 * | NS | NS | NS | NS |
| R2 | 0.046 | 0.215 | 0.220 | 0.328 | 0.289 | 0.088 | 0.046 | |||||
| Biological Material | Regression Analysis | Cd in the Blood | Zn in the Serum | ||
|---|---|---|---|---|---|
| β p | R2 | β p | R2 | ||
| Vascular Tissue of the Abdominal Aorta | TAS | –0.428 ‡ | 0.171 | 0.375 † | 0.128 |
| TOS | 0.443 ‡ | 0.185 | –0.262 * | 0.056 | |
| OSI | 0.488 ‡ | 0.227 | –0.301 ‡ | 0.078 | |
| TC | 0.240 * | 0.044 | –0.328 † | 0.095 | |
| TG | NS | –0.287 * | 0.069 | ||
| eNOS | 0.338 † | 0.101 | NS | ||
| IL-1β | 0.515 ‡ | 0.254 | –0.310 † | 0.083 | |
| IL-10 | 0.289 * | 0,069 | NS | ||
| Endothelial Cells of the Abdominal Aorta | PECAM-1 | –0.381 † | 0.133 | 0.403 ‡ | 0.150 |
| ICAM-1 | –0.408 ‡ | 0.154 | 0.410 ‡ | 0.156 | |
| L-selectin | –0.370 † | 0.124 | 0.350 † | 0.110 | |
| Blood Leukocytes | ICAM-1 | NS | NS | ||
| L-selectin | –0.362 † | 0.118 | NS | ||
| Serum | IL-1β | 0.495 ‡ | 0.234 | –0.280 * | 0.065 |
| IL-10 | –0.613 ‡ | 0.367 | 0.462 ‡ | 0.202 | |
| CRP | 0.688 ‡ | 0.465 | –0.431 ‡ | 0.174 | |
| VEGF | NS | NS | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzóska, M.M.; Kozłowska, M.; Rogalska, J. The Beneficial Impact of Zinc Supplementation on the Vascular Tissue of the Abdominal Aorta under Repeated Intoxication with Cadmium: A Study in an In Vivo Experimental Model. Nutrients 2022, 14, 4080. https://doi.org/10.3390/nu14194080
Brzóska MM, Kozłowska M, Rogalska J. The Beneficial Impact of Zinc Supplementation on the Vascular Tissue of the Abdominal Aorta under Repeated Intoxication with Cadmium: A Study in an In Vivo Experimental Model. Nutrients. 2022; 14(19):4080. https://doi.org/10.3390/nu14194080
Chicago/Turabian StyleBrzóska, Małgorzata M., Magdalena Kozłowska, and Joanna Rogalska. 2022. "The Beneficial Impact of Zinc Supplementation on the Vascular Tissue of the Abdominal Aorta under Repeated Intoxication with Cadmium: A Study in an In Vivo Experimental Model" Nutrients 14, no. 19: 4080. https://doi.org/10.3390/nu14194080
APA StyleBrzóska, M. M., Kozłowska, M., & Rogalska, J. (2022). The Beneficial Impact of Zinc Supplementation on the Vascular Tissue of the Abdominal Aorta under Repeated Intoxication with Cadmium: A Study in an In Vivo Experimental Model. Nutrients, 14(19), 4080. https://doi.org/10.3390/nu14194080