Physiological Benefits and Performance of Sea Water Ingestion for Athletes in Endurance Events: A Systematic Review
Abstract
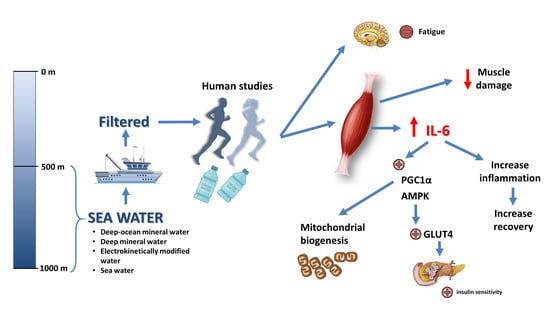
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Criteria Used for Selection
2.3. Reliability and Extraction of Data
2.4. Evaluation of the Validity and Reliability of the Evidence
3. Results
3.1. Selected Studies
3.1.1. Deep-Ocean Mineral Water
3.1.2. Deep Mineral Water
3.1.3. Sea Water
4. Discussion
Limitations and Strength
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afshari, L.; Mohammadi, S.; Shakerian, S.; Amani, R. The effect of oral carbohydrate solutions on the performance of swimmers. Ann. Appl. Sport Sci. 2014, 2, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Hanna, E.G.; Tait, P.W. Limitations to Thermoregulation and Acclimatization Challenge Human Adaptation to Global Warming. Int. J. Environ. Res. Public Health 2015, 12, 8034–8074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, L.E. Rehydration during Endurance Exercise: Challenges, Research, Options, Methods. Nutrients 2021, 13, 887. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; D’Anci, K.E.; Rosenberg, I.H. Water, hydration, and health. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef]
- Sawka, M.N.; Noakes, T.D. Does dehydration impair exercise performance? Med. Sci. Sports Exerc. 2007, 39, 1209–1217. [Google Scholar] [CrossRef]
- Goulet, E.D.B.; Hoffman, M.D. Impact of Ad Libitum Versus Programmed Drinking on Endurance Performance: A Systematic Review with Meta-Analysis. Sports Med. 2019, 49, 221–232. [Google Scholar] [CrossRef]
- Gonzalez-Alonso, J.; Mora-Rodriguez, R.; Below, P.R.; Coyle, E.F. Dehydration markedly impairs cardiovascular function in hyperthermic endurance athletes during exercise. J. Appl. Physiol. 1997, 82, 1229–1236. [Google Scholar] [CrossRef] [Green Version]
- Hew-Butler, T.; Rosner, M.H.; Fowkes-Godek, S.; Dugas, J.P.; Hoffman, M.D.; Lewis, D.P.; Maughan, R.J.; Miller, K.C.; Montain, S.J.; Rehrer, N.J.; et al. Statement of the Third International Exercise-Associated Hyponatremia Consensus Development Conference, Carlsbad, California, 2015. Clin. J. Sport Med. 2015, 25, 303–320. [Google Scholar] [CrossRef]
- Lau, W.Y.; Kato, H.; Nosaka, K. Effect of oral rehydration solution versus spring water intake during exercise in the heat on muscle cramp susceptibility of young men. J. Int. Soc. Sports Nutr. 2021, 18, 22. [Google Scholar] [CrossRef]
- Keen, D.A.; Constantopoulos, E.; Konhilas, J.P. The impact of post-exercise hydration with deep-ocean mineral water on rehydration and exercise performance. J. Int. Soc. Sports Nutr. 2016, 13, 17. [Google Scholar] [CrossRef]
- Stasiule, L.; Capkauskiene, S.; Vizbaraite, D.; Stasiulis, A. Deep mineral water accelerates recovery after dehydrating aerobic exercise: A randomized, double-blind, placebo-controlled crossover study. J. Int. Soc. Sports Nutr. 2014, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.W.; Tsai, Y.S.; Jean, W.H.; Chen, C.Y.; Ivy, J.L.; Huang, C.Y.; Kuo, C.H. Deep ocean mineral water accelerates recovery from physical fatigue. J. Int. Soc. Sports Nutr. 2013, 10, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, B. Hydration and physical performance. J. Am. Coll. Nutr. 2007, 26, 542S–548S. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M. Minerals and exercise immunology. In Nutrition and Exercise Immunology; Nieman, D.C., Pedersen, B.K., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 137–154. [Google Scholar]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef]
- Castizo-Olier, J.; Carrasco-Marginet, M.; Roy, A.; Chaverri, D.; Iglesias, X.; Pérez-Chirinos, C.; Rodríguez, F.; Irurtia, A. Bioelectrical impedance vector analysis (BIVA) and body mass changes in an ultra-endurance triathlon event. J. Sport Sci. Med. 2018, 17, 571. [Google Scholar]
- Mueller, S.M.; Anliker, E.; Knechtle, P.; Knechtle, B.; Toigo, M. Changes in body composition in triathletes during an Ironman race. Eur. J. Appl. Physiol. 2013, 113, 2343–2352. [Google Scholar] [CrossRef]
- Chlibkova, D.; Rosemann, T.; Posch, L.; Matousek, R.; Knechtle, B. Pre- and Post-Race Hydration Status in Hyponatremic and Non-Hyponatremic Ultra-Endurance Athletes. Chin. J. Physiol. 2016, 59, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Seal, A.D.; Anastasiou, C.A.; Skenderi, K.P.; Echegaray, M.; Yiannakouris, N.; Tsekouras, Y.E.; Matalas, A.L.; Yannakoulia, M.; Pechlivani, F.; Kavouras, S.A. Incidence of Hyponatremia During a Continuous 246-km Ultramarathon Running Race. Front. Nutr. 2019, 6, 161. [Google Scholar] [CrossRef]
- Maughan, R.J.; Merson, S.J.; Broad, N.P.; Shirreffs, S.M. Fluid and electrolyte intake and loss in elite soccer players during training. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 333–346. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Kenefick, R.W. Dehydration: Physiology, assessment, and performance effects. Compr. Physiol. 2011, 4, 257–285. [Google Scholar]
- Luk, H.Y.; Levitt, D.E.; Lee, E.C.; Ganio, M.S.; McDermott, B.P.; Kupchak, B.R.; McFarlin, B.K.; Hill, D.W.; Armstrong, L.E.; Vingren, J.L. Pro- and anti-inflammatory cytokine responses to a 164-km road cycle ride in a hot environment. Eur. J. Appl. Physiol. 2016, 116, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Aghili, S.A. The Effect of Immediate Consumption of High Carbohydrate and Caffeinated Drinks on Speed, Coordination and Cognitive Function in Professional Futsal Players. Int. J. Sport Stud. Health 2022, 5, e130662. [Google Scholar]
- Gamage, J.P.; De Silva, A. Nutrient intake and dietary practices of elite volleyball athletes during the competition day. Ann. Appl. Sport Sci. 2014, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol. 1999, 515 Pt 1, 287–291. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K. Chapter 7: Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual; Aromataris, E., Munn, Z., Eds.; The Joanna Briggs Institute: Adelaide, Australia, 2017. [Google Scholar]
- Haynes, A.; Kersbergen, I.; Sutin, A.; Daly, M.; Robinson, E. A systematic review of the relationship between weight status perceptions and weight loss attempts, strategies, behaviours and outcomes. Obes. Rev. 2018, 19, 347–363. [Google Scholar] [CrossRef] [Green Version]
- Molina-Garcia, P.; Migueles, J.H.; Cadenas-Sanchez, C.; Esteban-Cornejo, I.; Mora-Gonzalez, J.; Rodriguez-Ayllon, M.; Plaza-Florido, A.; Vanrenterghem, J.; Ortega, F.B. A systematic review on biomechanical characteristics of walking in children and adolescents with overweight/obesity: Possible implications for the development of musculoskeletal disorders. Obes. Rev. 2019, 20, 1033–1044. [Google Scholar] [CrossRef]
- Van Ekris, E.; Altenburg, T.M.; Singh, A.S.; Proper, K.I.; Heymans, M.W.; Chinapaw, M.J.M. An evidence-update on the prospective relationship between childhood sedentary behaviour and biomedical health indicators: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 712–714. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.Y.; Chen, C.Y.; Liao, Y.H.; Tsai, Y.S.; Huang, C.Y.; Chaunchaiyakul, R.; Higgins, M.F.; Kuo, C.H. Deep Ocean Mineral Supplementation Enhances the Cerebral Hemodynamic Response during Exercise and Decreases Inflammation Postexercise in Men at Two Age Levels. Front. Physiol. 2017, 8, 1016. [Google Scholar] [CrossRef] [Green Version]
- Perez-Turpin, J.A.; Trottini, M.; Chinchilla-Mira, J.J.; Cyganik, W. Effects of seawater ingestion on lactate response to exercise in runners. Biol. Sport 2017, 34, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, P.R.; Keen, D.A.; Constantopoulos, E.; Weninger, S.N.; Hines, E.; Koppinger, M.P.; Khalpey, Z.I.; Konhilas, J.P. Fluid type influences acute hydration and muscle performance recovery in human subjects. J. Int. Soc. Sports Nutr. 2019, 16, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, M.F.; Rudkin, B.; Kuo, C.H. Oral Ingestion of Deep Ocean Minerals Increases High-Intensity Intermittent Running Capacity in Soccer Players after Short-Term Post-Exercise Recovery: A Double-Blind, Placebo-Controlled Crossover Trial. Mar. Drugs 2019, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Acevedo, O.; Aragon-Vela, J.; De la Cruz Marquez, J.C.; Marin, M.M.; Casuso, R.A.; Huertas, J.R. Seawater Hydration Modulates IL-6 and Apelin Production during Triathlon Events: A Crossover Randomized Study. Int. J. Environ. Res. Public Health 2022, 19, 9581. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lee, C.-L. Comparison of the Improvement Effect of Deep Ocean Water with Different Mineral Composition on the High Fat Diet-Induced Blood Lipid and Nonalcoholic Fatty Liver Disease in a Mouse Model. Nutrients 2021, 13, 1732. [Google Scholar] [CrossRef] [PubMed]
- Nani, M.; Zura, S.; Majid, F.; Jaafar, A.; Mahdzir, A.; Musa, M. Potential health benefits of deep sea water: A review. Evid. Based Complement. Altern. Med. 2016, 2016, 6520475. [Google Scholar]
- Ha, B.G.; Moon, D.-S.; Kim, H.J.; Shon, Y.H. Magnesium and calcium-enriched deep-sea water promotes mitochondrial biogenesis by AMPK-activated signals pathway in 3T3-L1 preadipocytes. Biomed. Pharmacother. 2016, 83, 477–484. [Google Scholar] [CrossRef]
- Ha, B.G.; Park, J.-E.; Cho, H.-J.; Shon, Y.H. Stimulatory effects of balanced deep sea water on mitochondrial biogenesis and function. PLoS ONE 2015, 10, e0129972. [Google Scholar] [CrossRef] [Green Version]
- Killilea, D.W.; Ames, B.N. Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts. Proc. Natl. Acad. Sci. USA 2008, 105, 5768–5773. [Google Scholar] [CrossRef] [Green Version]
- Wright, D.C.; Geiger, P.C.; Han, D.-H.; Jones, T.E.; Holloszy, J.O. Calcium induces increases in peroxisome proliferator-activated receptor γ coactivator-1α and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J. Biol. Chem. 2007, 282, 18793–18799. [Google Scholar] [CrossRef] [Green Version]
- Judge, L.W.; Bellar, D.M.; Popp, J.K.; Craig, B.W.; Schoeff, M.A.; Hoover, D.L.; Fox, B.; Kistler, B.M.; Al-Nawaiseh, A.M. Hydration to Maximize Performance and Recovery: Knowledge, Attitudes, and Behaviors Among Collegiate Track and Field Throwers. J. Hum. Kinet. 2021, 79, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Shirreffs, S.M. Development of individual hydration strategies for athletes. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 457–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pound, C.M.; Blair, B.; Boctor, D.L.; Casey, L.M.; Critch, J.N.; Farrell, C.; Gowrishankar, M.; Kim, J.H.; Roth, D.; Sant’Anna, A.M. Energy and sports drinks in children and adolescents. Paediatr. Child Health 2017, 22, 406–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratama, R.Y.; Muliarta, I.M.; Sundari, L.P.R.; Sutjana, I.D.P.; Dewi, N.N.A.; Griadi, I.P.A. Provision of Coconut Water as Good as Packed Coconut Water and Isotonic Beverages on Hydration Status in Football Athlete. J. Phys. Educ. Health Sport 2022, 9, 18–26. [Google Scholar]
- MacDonald, C.; Wojtaszewski, J.F.; Pedersen, B.K.; Kiens, B.; Richter, E.A. Interleukin-6 release from human skeletal muscle during exercise: Relation to AMPK activity. J. Appl. Physiol. 2003, 95, 2273–2277. [Google Scholar] [CrossRef]
- Pedersen, B.K. Muscles and their myokines. J. Exp. Biol. 2011, 214, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Petersen, A.; Pedersen, B. The role of IL-6 in mediating the anti inflammatory. J. Physiol. Pharmacol. 2006, 57, 43–51. [Google Scholar]
- Jorgensen, S. How is AMPK activity regulated in skeletal muscles during exercise. Front. Biosci. 2018, 13, 5589–5604. [Google Scholar] [CrossRef] [Green Version]
- Munoz-Canoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]


| Database | Search Strategy | Limits | Filters |
|---|---|---|---|
| Web of Science | (ALL (deep sea water AND endurance exercise OR deep-sea water AND sweating OR deep-sea water AND sweating OR deep sea mineral water AND endurance exercise OR deep sea mineral water AND sweating OR deep sea mineral water AND hydration)) | Publication date, 2000–2022, English language, Article, Search strategy (Topic) | 54 items filtered |
| PubMed | (Deep sea water OR deep sea mineral water) AND (endurance exercise OR sweating OR hydration) | Publication date, 1 January 2000–31 July 2022, Humans, Adults: 18–50 years, English language, Search strategy (All Fields) | 97 items filtered |
| Scopus | TITLE-ABS-KEY (deep AND sea AND water AND hydration) OR (deep AND sea AND water AND hydration AND endurance AND exercise) OR (deep AND sea AND water AND hydration AND endurance AND exercise AND barrier) OR (deep AND sea AND water AND hydration AND endurance AND exercise) AND LANGUAGE (English) AND (PUBYEAR > 2000) | Publication date, 2000–2022, English language, Article or review, Search strategy (TITLE-ABS-KEY) | 407 items filtered |
| Criteriums According to Kind of Study | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Percentage Reached | Quality Level |
| Hou et al., 2013 [12] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100% | HQ |
| Stasiule et al., 2014 [11] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 85% | HQ |
| Keen et al., 2016 [10] | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 75% | HQ | |||||
| Wei et al., 2017 [32] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 92% | HQ |
| Pérez-Turpin et al., 2017 [33] | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 77% | HQ |
| Harris et al., 2019 [34] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100% | HQ |
| Higgins et al., 2019 [35] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 92% | HQ |
| González Acevedo et al., 2022 [36] | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 76% | HQ |
| First Author, Year of Publication | Country | Type of Study | Aim | Sample | Study Design | Results | Composition of the Seawater |
|---|---|---|---|---|---|---|---|
| Hou et al., 2013 [12] | Taiwan | Randomized, double-blind, placebo-controlled crossover study | DSW on recovery time | 12 healthy, male volunteers. 24 ± 0.8 years. | The study was to evaluate the effect of DOM on the recovery time at 30 °C. |
| Following the two filtration procedures, molecules larger than 1.5 kilodalton were removed. As a means of masking the taste difference between DOM and placebo, the same amounts of sucrose, artificial flavors, citrate, citrus juice, calcium lactate, potassium chloride, vitamin C, and mixed amino acids were added to both. |
| Stasiule et al., 2014 [11] | Lithuania | Randomized, double-blind, placebo-controlled crossover study | The effect of DMW on the recovery | 9 healthy, physically active, women. 24.0 ± 3.7 years. | Aerobic capacity (VO2 max) and peak lower-body muscle capacity were the measures selected for assessing the degree of recovery. VO2 max was measured using the ramp exercise test. |
| Na (76 mg L−1), K (19 mg L−1), Ca (220 mg L−1), Mg (73 mg L−1), Cl (46 mg L−1), SO4 (874 mg L−1), F (0.3 mg L−1), Cu (0.0054 mg L−1), Fe (1.2326 mg L−1), Mn (0.0163 mg L−1), Cr (0.0025 mg L−1) P (0.5434 mg L−1), B (0.4175 mg L−1), and Zn (0.0124 mg L−1) |
| Keen et al., 2016 [10] | Arizona | Well-conditioned student-athletes study | DSW on rehydration | Eight student-athletes. Three experimental groups. 23.0 ± 1.2 years. | Subjects were exposed to an exercise-challenge under warm conditions. Body mass measurements were taken prior to exercise and then at 15 min intervals throughout the exercise trial and during rehydration. At each interval also, stimulated saliva was collected, and salivary osmolality was measured. |
| Na (85 mg L−1), K (4 mg L−1), Ca (1.4 mg L−1), Mg (4.3 mg L−1), Cl (150 mg L−1), B (0.65 mg L−1), Bromide (540 µg L−1), and Cr (2.2 µg L−1) |
| Wei et al., 2016 [32] | Taiwan | Double-blind placebo-controlled crossover study | DOM supplementation on the cerebral hemodynamic response | 9 middle-aged men and 12 young men. 46.8 ± 1.4, 21.2 ± 0.4 years, respectively | The counter-balanced trials of DOM and placebo were separated by a 2-week washout period. DOM and placebo were orally supplemented in drinks before, during, and after cycling exercise. Cerebral hemodynamic response was measured during cycling at 75% VO2 max using near-infrared spectroscopy. |
| After this two-filtration procedure, molecules larger than 1.5 kilodalton were removed. For the purpose of masking the taste difference between DOM and placebo, the same amount of erythritol (3%) was added to each drink. |
| Harris et al., 2019 [34] | Arizona | Counterbalanced, crossover study | DOM supplementation on muscle performance recovery | 17 participants. 20–25 years. | A dehydrating exercise protocol under heat stress until achieving 3% body mass loss. Participants rehydrated with either DOM water (Deep), mountain spring water (Spring), or a carbohydrate-based sports drink (Sports) at a volume equal to the volume of fluid loss. |
| Na (85 mg L−1), K (4 mg L−1), Ca (1.4 mg L−1), Mg (4.3 mg L−1), Cl (150 mg L−1), B (0.65 mg L−1), Bromide (540 µg L−1), and Cr (2.2 µg L−1) |
| González Acevedo et al., 2022 [36] | Spain | CRS | SW and recovery | 15 triathletes. 38.8 ± 5.6 years | Fifteen trained male triathletes randomly performed 3 triathlons, one of them consuming SW, the other one consuming tap water-hydration at libitum, and the last one with isocaloric placebo |
| Electrolytes were supplied in the amount of 27.297 mg L−1 Na, 0.465 mg L−1 K, 19.5 mg L−1 Mg, and 1.377 mg L−1 Ca. |
| First Author, Year of Publication | Country | Type of Study | Aim | Sample | Study Design | Results | Composition of the Seawater |
|---|---|---|---|---|---|---|---|
| Hou et al., 2013 [12] | Taiwan | Randomized, double-blind, placebo-controlled crossover study | DSW on performance | 12 healthy, male volunteers. 24 ± 0.8 years | The study was to evaluate the effect of DOM on the exercise performed at 30 °C. |
| Following the two filtration procedures, molecules larger than 1.5 kilodalton were removed. As a means of masking the taste difference between DOM and placebo, the same amounts of sucrose, artificial flavors, citrate, citrus juice, calcium lactate, potassium chloride, vitamin C, and mixed amino acids were added to both. |
| Keen et al., 2016 [10] | Arizona | Well-conditioned student-athletes randomized study | DSW on physical performance | Eight student-athletes. Three experimental groups. 23.0 ± 1.2 years. | Subjects were exposed to an exercise-challenge under warm conditions. Body mass measurements were taken prior to exercise and then at 15 min intervals throughout the exercise trial and during rehydration. At each interval also, stimulated saliva was collected and salivary osmolality was measured. |
| Na (85 mg L−1), K (4 mg L−1), Ca (1.4 mg L−1), Mg (4.3 mg L−1), Cl (150 mg L−1), B (0.65 mg L−1), bromide (540 µg L−1), and Cr (2.2 µg L−1) |
| Pérez-Turpin et al., 2017 [33] | Spain | Crossover, double-blind randomized trial | SW ingestion on running performance | 12 experienced male runners. 32 ± 6.4 years | Five minutes before the exercise protocol started, the subjects consumed SW or pure water in an equivalent amount of 50 mL. Subjects were asked to run on a motorized treadmill at 40% VO2 max at a room temperature of 30 °C until attaining a 3% decrease in body mass. |
| Electrolytes were supplied in the amount of 27.297 mg L−1 Na, 0.465 mg L−1 K, 19.5 mg L−1 Mg, and 1.377 mg L−1 Ca. |
| Higgins et al., 2019 [35] | Taiwan | Crossover, and counterbalanced study | DOM supplementation on high-intensity intermittent running capacity | 9 healthy, nonelite male soccer players. 22.0 ± 1.0 years | Two experimental trials were completed on a motorised treadmill, separated by 7 d, undertaken at ambient room temperature, and commenced at similar times of day. Incremental increases of 1 km h−1 were applied every three minutes until volitional exhaustion. |
| Na (54.64 mg L−1), K (56.64 mg L−1), Ca (0.22 mg L−1), Mg (170.7 mg L−1), B (0.54 mg L−1), and Rb (0.02 mg L−1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragón-Vela, J.; González-Acevedo, O.; Plaza-Diaz, J.; Casuso, R.A.; Huertas, J.R. Physiological Benefits and Performance of Sea Water Ingestion for Athletes in Endurance Events: A Systematic Review. Nutrients 2022, 14, 4609. https://doi.org/10.3390/nu14214609
Aragón-Vela J, González-Acevedo O, Plaza-Diaz J, Casuso RA, Huertas JR. Physiological Benefits and Performance of Sea Water Ingestion for Athletes in Endurance Events: A Systematic Review. Nutrients. 2022; 14(21):4609. https://doi.org/10.3390/nu14214609
Chicago/Turabian StyleAragón-Vela, Jerónimo, Olivia González-Acevedo, Julio Plaza-Diaz, Rafael A. Casuso, and Jesús R. Huertas. 2022. "Physiological Benefits and Performance of Sea Water Ingestion for Athletes in Endurance Events: A Systematic Review" Nutrients 14, no. 21: 4609. https://doi.org/10.3390/nu14214609
APA StyleAragón-Vela, J., González-Acevedo, O., Plaza-Diaz, J., Casuso, R. A., & Huertas, J. R. (2022). Physiological Benefits and Performance of Sea Water Ingestion for Athletes in Endurance Events: A Systematic Review. Nutrients, 14(21), 4609. https://doi.org/10.3390/nu14214609