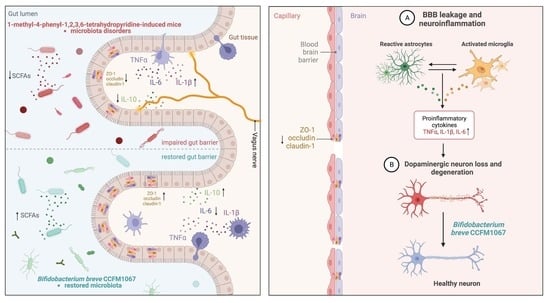
Neuroprotective Effects of Bifidobacterium breve CCFM1067 in MPTP-Induced Mouse Models of Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Bifidobacterium breve CCFM1067 Preparation
2.3. Behavioral Tests for Motor Functions
2.4. Neurochemical and Biochemicall Analyses
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Immunohistochemistry
2.7. Gut Microbial and Bioinformatics Analysis
2.8. Statistical Analysis
3. Results
3.1. B. breve CCFM1067 Improves MPTP-Induced Motor Impairments
3.2. B. breve CCFM1067 Alleviates MPTP-Induced Neuropathological Alterations
3.3. B. breve CCFM1067 Increases Antioxidant Levels and Reduces MPTP-Induced Neuroinflammation
3.4. B. breve CCFM1067 Treatment Helps Improve Blood–Brain and Intestinal Barrier Damage
3.5. B. breve CCFM1067 Ameliorated the Dysbiosis of the Mouse Gut Microbiota of MPTP-Induced
3.6. Functional Predictions Suggested That B. breve CCFM1067 May Modify Functional Modules of the Gut Microbiota
3.7. Correlations Support the Involvement of the MGBA in the MPTP-Treated Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α-SYN | α-synuclein |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| CAT | Catalase |
| CNS | Central nervous system |
| DA | Dopamine |
| DAB | 3,3′-diaminobenzidine |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| ENS | Enteric nervous system |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GDNF | Glial cell line-derived neurotrophic factor |
| GFAP | Glial fibrillary acidic protein |
| GSH | Glutathione |
| HPLC | High Performance Liquid Chromatography |
| HVA | Homovanillic acid |
| Iba1 | Ionized calcium binding adapter molecule 1 |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IHC | Immunohistochemistry |
| LDA | Linear discriminant analysis |
| L-DOPA | Levodopa |
| LPS | Lipopolysaccharide |
| MGBA | Microbiota–gut–brain axis |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MRS | De Man Rogosa Sharpe |
| NBT | Narrow-beam test |
| OUT | Operational taxonomic unit |
| OFT | Open field test |
| PCA | Principal component analysis |
| PCoA | Principal coordinate analysis |
| PD | Parkinson’s disease |
| perMANOVA | Permutational multivariate analysis of variance |
| PICRUSt | Phylogenetic Investigation of Communities by Reconstruction of Unobserved States |
| PT | Pole test |
| QIIME | Quantitative Insights into Microbial Ecology |
| qRT-PCR | Quantitative real-time polymerase chain reaction assay |
| ROS | Reactive oxygen species |
| RTR | Rotarod test |
| SCFAs | Short-chain fatty acids |
| SEM | Standard error of mean |
| SN | Substantia nigra |
| SOD | Superoxide dismutase |
| TNF-α | Tumor necrosis factor-α |
| TH | Tyrosine hydroxylase |
| TrkB | Tyrosine Kinase receptor B |
| ZO-1 | Zonula occludens-1 |
| 5-HT | 5-hydroxytryptamine |
| 5-HIAA | 5-hydroxyindoleacetic acid |
References
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Vélez, G.E.; Zoghbi, H.Y. Parkinson’s Disease Genetics and Pathophysiology. Annu. Rev. Neurosci. 2021, 44, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Ozansoy, M.; Başak, A.N. The Central Theme of Parkinson’s Disease: α-Synuclein. Mol. Neurobiol. 2013, 47, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.B.; O’Callaghan, J.P. Biomarkers of Parkinson’s Disease: Present and Future. Metabolism 2015, 64, S40–S46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasano, A.; Visanji, N.P.; Liu, L.W.C.; Lang, A.E.; Pfeiffer, R.F. Gastrointestinal Dysfunction in Parkinson’s Disease. Lancet Neurol. 2015, 14, 625–639. [Google Scholar] [CrossRef]
- Liddle, R.A. Parkinson’s Disease from the Gut. Brain Res. 2018, 1693, 201–206. [Google Scholar] [CrossRef]
- Liao, J.-F.; Cheng, Y.-F.; You, S.-T.; Kuo, W.-C.; Huang, C.-W.; Chiou, J.-J.; Hsu, C.-C.; Hsieh-Li, H.-M.; Wang, S.; Tsai, Y.-C. Lactobacillus Plantarum PS128 Alleviates Neurodegenerative Progression in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Mouse Models of Parkinson’s Disease. Brain Behav. Immun. 2020, 90, 26–46. [Google Scholar] [CrossRef]
- Lei, Q.; Wu, T.; Wu, J.; Hu, X.; Guan, Y.; Wang, Y.; Yan, J.; Shi, G. Roles of A-synuclein in Gastrointestinal Microbiome Dysbiosis-related Parkinson’s Disease Progression (Review). Mol. Med. Rep. 2021, 24, 734. [Google Scholar] [CrossRef]
- Unger, M.M.; Spiegel, J.; Dillmann, K.-U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.-H. Short Chain Fatty Acids and Gut Microbiota Differ between Patients with Parkinson’s Disease and Age-Matched Controls. Parkinsonism Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, F.; Liu, Y.; Ma, T.; Jin, H.; Quan, K.; Leng, B.; Zhao, J.; Yuan, X.; Li, Z.; et al. Probiotics Synergized with Conventional Regimen in Managing Parkinson’s Disease. NPJ Parkinsons. Dis. 2022, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-H.; Kuo, C.-W.; Hsieh, K.-H.; Shieh, M.-J.; Peng, C.-W.; Chen, Y.-C.; Chang, Y.-L.; Huang, Y.-Z.; Chen, C.-C.; Chang, P.-K.; et al. Probiotics Alleviate the Progressive Deterioration of Motor Functions in a Mouse Model of Parkinson’s Disease. Brain Sci. 2020, 10, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamtaji, O.R.; Taghizadeh, M.; Daneshvar Kakhaki, R.; Kouchaki, E.; Bahmani, F.; Borzabadi, S.; Oryan, S.; Mafi, A.; Asemi, Z. Clinical and Metabolic Response to Probiotic Administration in People with Parkinson’s Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2019, 38, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.H.; Lim, S.-Y.; Chong, K.K.; Manap, M.A.A.A.; Hor, J.W.; Lim, J.L.; Low, S.C.; Chong, C.W.; Mahadeva, S.; Lang, A.E. Probiotics for Constipation in Parkinson Disease: A Randomized Placebo-Controlled Study. Neurology 2021, 96, e772–e782. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, D.; Ancusa, O.E.; Georgescu, L.A.; Ionita, I.; Reisz, D. Nonmotor Gastrointestinal Disorders in Older Patients with Parkinson’s Disease: Is There Hope? Clin. Interv. Aging 2016, 11, 1601–1608. [Google Scholar] [CrossRef] [Green Version]
- Barichella, M.; Pacchetti, C.; Bolliri, C.; Cassani, E.; Iorio, L.; Pusani, C.; Pinelli, G.; Privitera, G.; Cesari, I.; Faierman, S.A.; et al. Probiotics and Prebiotic Fiber for Constipation Associated with Parkinson Disease: An RCT. Neurology 2016, 87, 1274–1280. [Google Scholar] [CrossRef]
- Cassani, E.; Privitera, G.; Pezzoli, G.; Pusani, C.; Madio, C.; Iorio, L.; Barichella, M. Use of Probiotics for the Treatment of Constipation in Parkinson’s Disease Patients. Minerva Gastroenterol. Dietol. 2011, 57, 117–121. [Google Scholar]
- Krüger, J.F.; Hillesheim, E.; Pereira, A.C.S.N.; Camargo, C.Q.; Rabito, E.I. Probiotics for Dementia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2021, 79, 160–170. [Google Scholar] [CrossRef]
- Ilie, O.-D.; Paduraru, E.; Robea, M.-A.; Balmus, I.-M.; Jijie, R.; Nicoara, M.; Ciobica, A.; Nita, I.-B.; Dobrin, R.; Doroftei, B. The Possible Role of Bifidobacterium Longum BB536 and Lactobacillus Rhamnosus HN001 on Locomotor Activity and Oxidative Stress in a Rotenone-Induced Zebrafish Model of Parkinson’s Disease. Oxidative Med. Cell. Longev. 2021, 2021, 9629102. [Google Scholar] [CrossRef]
- Abdelhamid, M.; Zhou, C.; Jung, C.-G.; Michikawa, M. Probiotic Bifidobacterium Breve MCC1274 Mitigates Alzheimer’s Disease-Related Pathologies in Wild-Type Mice. Nutrients 2022, 14, 2543. [Google Scholar] [CrossRef]
- Cao, J.; Amakye, W.K.; Qi, C.; Liu, X.; Ma, J.; Ren, J. Bifidobacterium Lactis Probio-M8 Regulates Gut Microbiota to Alleviate Alzheimer’s Disease in the APP/PS1 Mouse Model. Eur. J. Nutr. 2021, 60, 3757–3769. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Administration of Bifidobacterium Breve Improves the Brain Function of Aβ(1-42)-Treated Mice via the Modulation of the Gut Microbiome. Nutrients 2021, 13, 1602. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J.-Z. Therapeutic Potential of Bifidobacterium Breve Strain A1 for Preventing Cognitive Impairment in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13510. [Google Scholar] [CrossRef] [Green Version]
- Ishii, T.; Furuoka, H.; Kaya, M.; Kuhara, T. Oral Administration of Probiotic Bifidobacterium Breve Improves Facilitation of Hippocampal Memory Extinction via Restoration of Aberrant Higher Induction of Neuropsin in an MPTP-Induced Mouse Model of Parkinson’s Disease. Biomedicines 2021, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Tian, P.; Zhu, H.; Zou, R.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Lactobacillus Paracasei CCFM1229 and Lactobacillus Rhamnosus CCFM1228 Alleviated Depression- and Anxiety-Related Symptoms of Chronic Stress-Induced Depression in Mice by Regulating Xanthine Oxidase Activity in the Brain. Nutrients 2022, 14, 1294. [Google Scholar] [CrossRef]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the Role of 5-Hydroxytryptophan Synthesis Regulation Alleviates the Symptom of Depression and Related Microbiota Dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Guatteo, E.; Rizzo, F.R.; Federici, M.; Cordella, A.; Ledonne, A.; Latini, L.; Nobili, A.; Viscomi, M.T.; Biamonte, F.; Landrock, K.K.; et al. Functional Alterations of the Dopaminergic and Glutamatergic Systems in Spontaneous α-Synuclein Overexpressing Rats. Exp. Neurol. 2017, 287, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Haavik, J.; Toska, K. Tyrosine Hydroxylase and Parkinson’s Disease. Mol. Neurobiol. 1998, 16, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, I.; Fukunaga, K. Degradation of Tyrosine Hydroxylase by the Ubiquitin-Proteasome System in the Pathogenesis of Parkinson’s Disease and Dopa-Responsive Dystonia. Int. J. Mol. Sci. 2020, 21, 3779. [Google Scholar] [CrossRef]
- Nicholson, S.L.; Brotchie, J.M. 5-Hydroxytryptamine (5-HT, Serotonin) and Parkinson’s Disease—Opportunities for Novel Therapeutics to Reduce the Problems of Levodopa Therapy. Eur. J. Neurol. 2002, 9 (Suppl. S3), 1–6. [Google Scholar] [CrossRef]
- Yan, T.; Mao, Q.; Zhang, X.; Wu, B.; Bi, K.; He, B.; Jia, Y. Schisandra Chinensis Protects against Dopaminergic Neuronal Oxidative Stress, Neuroinflammation and Apoptosis via the BDNF/Nrf2/NF-ΚB Pathway in 6-OHDA-Induced Parkinson’s Disease Mice. Food Funct. 2021, 12, 4079–4091. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as Therapeutic Options for Neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Ali, T.; Zheng, C.; He, K.; Liu, Z.; Shah, F.A.; Li, N.; Yu, Z.-J.; Li, S. Anti-Depressive-like Behaviors of APN KO Mice Involve Trkb/BDNF Signaling Related Neuroinflammatory Changes. Mol. Psychiatry 2022, 27, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakopoulou, V.; Botsakis, K.; Delis, F.; Mourtzi, T.; Tzatzarakis, M.N.; Dimopoulou, A.; Poulia, N.; Antoniou, K.; Stathopoulos, G.T.; Matsokis, N.; et al. Anti-Neuroinflammatory, Protective Effects of the Synthetic Microneurotrophin BNN-20 in the Advanced Dopaminergic Neurodegeneration of “Weaver” Mice. Neuropharmacology 2020, 165, 107919. [Google Scholar] [CrossRef] [PubMed]
- Patel, M. Targeting Oxidative Stress in Central Nervous System Disorders. Trends Pharmacol. Sci. 2016, 37, 768–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, M.H.; Wang, X.; Zhu, X. Mitochondrial Defects and Oxidative Stress in Alzheimer Disease and Parkinson Disease. Free Radic. Biol. Med. 2013, 62, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Picca, A.; Guerra, F.; Calvani, R.; Romano, R.; Coelho-Júnior, H.J.; Bucci, C.; Marzetti, E. Mitochondrial Dysfunction, Protein Misfolding and Neuroinflammation in Parkinson’s Disease: Roads to Biomarker Discovery. Biomolecules 2021, 11, 1508. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Zhao, L.; Zhao, Y.; Yang, G.; Wang, C.; Gao, L.; Niu, C.; Li, S. Lactobacillus Plantarum DP189 Reduces α-SYN Aggravation in MPTP-Induced Parkinson’s Disease Mice via Regulating Oxidative Damage, Inflammation, and Gut Microbiota Disorder. J. Agric. Food Chem. 2022, 70, 1163–1173. [Google Scholar] [CrossRef]
- Smeyne, M.; Smeyne, R.J. Glutathione Metabolism and Parkinson’s Disease. Free Radic. Biol. Med. 2013, 62, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Lu, M.; Mei, M.; Wang, H.; Han, Z.; Chen, M.; Yao, H.; Song, N.; Ding, X.; Ding, J.; et al. Pyridoxine Induces Glutathione Synthesis via PKM2-Mediated Nrf2 Transactivation and Confers Neuroprotection. Nat. Commun. 2020, 11, 941. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Peana, M.; Maes, M.; Dadar, M.; Severin, B. The Glutathione System in Parkinson’s Disease and Its Progression. Neurosci. Biobehav. Rev. 2021, 120, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.; Olanow, C.W. Oxidative Stress and the Pathogenesis of Parkinson’s Disease. Neurology 1996, 47, S161–S170. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Ban, Y.; Khan, S. Antioxidant Activity of Calycosin against α-Synuclein Amyloid Fibrils-Induced Oxidative Stress in Neural-like Cells as a Model of Preventive Care Studies in Parkinson’s Disease. Int. J. Biol. Macromol. 2021, 182, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ortíz de Zárate, A.; Pérez-Torralba, M.; Bonet Isidro, I.; López, C.; Claramunt, R.M.; Martínez-Casanova, D.; Sánchez-Vera, I.; Jiménez-González, J.; Lavandera, J.L. 1,5-Benzodiazepin-2(3H)-Ones: In Vitro Evaluation as Antiparkinsonian Agents. Antioxidants 2021, 10, 1584. [Google Scholar] [CrossRef]
- Cheng, J.; Liao, Y.; Dong, Y.; Hu, H.; Yang, N.; Kong, X.; Li, S.; Li, X.; Guo, J.; Qin, L.; et al. Microglial Autophagy Defect Causes Parkinson Disease-like Symptoms by Accelerating Inflammasome Activation in Mice. Autophagy 2020, 16, 2193–2205. [Google Scholar] [CrossRef]
- Sun, M.-F.; Zhu, Y.-L.; Zhou, Z.-L.; Jia, X.-B.; Xu, Y.-D.; Yang, Q.; Cui, C.; Shen, Y.-Q. Neuroprotective Effects of Fecal Microbiota Transplantation on MPTP-Induced Parkinson’s Disease Mice: Gut Microbiota, Glial Reaction and TLR4/TNF-α Signaling Pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Han, X.; Sun, S.; Sun, Y.; Song, Q.; Zhu, J.; Song, N.; Chen, M.; Sun, T.; Xia, M.; Ding, J.; et al. Small Molecule-Driven NLRP3 Inflammation Inhibition via Interplay between Ubiquitination and Autophagy: Implications for Parkinson Disease. Autophagy 2019, 15, 1860–1881. [Google Scholar] [CrossRef]
- Lahooti, B.; Chhibber, T.; Bagchi, S.; Varahachalam, S.P.; Jayant, R.D. Therapeutic Role of Inflammasome Inhibitors in Neurodegenerative Disorders. Brain Behav. Immun. 2021, 91, 771–783. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Cao, Z.; Liu, Q.; Cheng, Y. Cerebrospinal Fluid Inflammatory Cytokine Aberrations in Alzheimer’s Disease, Parkinson’s Disease and Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Front. Immunol. 2018, 9, 2122. [Google Scholar] [CrossRef] [Green Version]
- Alrafiah, A.; Al-Ofi, E.; Obaid, M.T.; Alsomali, N. Assessment of the Levels of Level of Biomarkers of Bone Matrix Glycoproteins and Inflammatory Cytokines from Saudi Parkinson Patients. BioMed Res. Int. 2019, 2019, 2690205. [Google Scholar] [CrossRef] [PubMed]
- Belarbi, K.; Cuvelier, E.; Bonte, M.-A.; Desplanque, M.; Gressier, B.; Devos, D.; Chartier-Harlin, M.-C. Glycosphingolipids and Neuroinflammation in Parkinson’s Disease. Mol. Neurodegener. 2020, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Matheoud, D.; Cannon, T.; Voisin, A.; Penttinen, A.-M.; Ramet, L.; Fahmy, A.M.; Ducrot, C.; Laplante, A.; Bourque, M.-J.; Zhu, L.; et al. Intestinal Infection Triggers Parkinson’s Disease-like Symptoms in Pink1(-/-) Mice. Nature 2019, 571, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ning, J.; Bao, X.Q.; Shang, M.; Ma, J.; Li, G.; Zhang, D. Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome 2021, 9, 226. [Google Scholar] [CrossRef]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-Analysis of the Parkinson’s Disease Gut Microbiome Suggests Alterations Linked to Intestinal Inflammation. NPJ Parkinsons. Dis. 2021, 7, 27. [Google Scholar] [CrossRef]
- Lubomski, M.; Tan, A.H.; Lim, S.-Y.; Holmes, A.J.; Davis, R.L.; Sue, C.M. Parkinson’s Disease and the Gastrointestinal Microbiome. J. Neurol. 2020, 267, 2507–2523. [Google Scholar] [CrossRef]
- Houser, M.C.; Tansey, M.G. The Gut-Brain Axis: Is Intestinal Inflammation a Silent Driver of Parkinson’s Disease Pathogenesis? NPJ Parkinsons Dis. 2017, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Luo, Y.; Ray Chaudhuri, K.; Reynolds, R.; Tan, E.-K.; Pettersson, S. The Role of Gut Dysbiosis in Parkinson’s Disease: Mechanistic Insights and Therapeutic Options. Brain 2021, 144, 2571–2593. [Google Scholar] [CrossRef]
- Caputi, V.; Giron, M.C. Microbiome-Gut-Brain Axis and Toll-Like Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 1689. [Google Scholar] [CrossRef] [Green Version]
- Varatharaj, A.; Galea, I. The Blood-Brain Barrier in Systemic Inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Haruwaka, K.; Ikegami, A.; Tachibana, Y.; Ohno, N.; Konishi, H.; Hashimoto, A.; Matsumoto, M.; Kato, D.; Ono, R.; Kiyama, H.; et al. Dual Microglia Effects on Blood Brain Barrier Permeability Induced by Systemic Inflammation. Nat. Commun. 2019, 10, 5816. [Google Scholar] [CrossRef]
- Więckowska-Gacek, A.; Mietelska-Porowska, A.; Wydrych, M.; Wojda, U. Western Diet as a Trigger of Alzheimer’s Disease: From Metabolic Syndrome and Systemic Inflammation to Neuroinflammation and Neurodegeneration. Ageing Res. Rev. 2021, 70, 101397. [Google Scholar] [CrossRef] [PubMed]
- Dodiya, H.B.; Forsyth, C.B.; Voigt, R.M.; Engen, P.A.; Patel, J.; Shaikh, M.; Green, S.J.; Naqib, A.; Roy, A.; Kordower, J.H.; et al. Chronic Stress-Induced Gut Dysfunction Exacerbates Parkinson’s Disease Phenotype and Pathology in a Rotenone-Induced Mouse Model of Parkinson’s Disease. Neurobiol. Dis. 2020, 135, 104352. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Du, Z.R.; Wang, X.; Sun, X.R.; Zhao, Q.; Zhao, F.; Wong, W.T.; Wong, K.H.; Dong, X.-L. Polymannuronic Acid Prebiotic plus Lacticaseibacillus Rhamnosus GG Probiotic as a Novel Synbiotic Promoted Their Separate Neuroprotection against Parkinson’s Disease. Food Res. Int. 2022, 155, 111067. [Google Scholar] [CrossRef]
- Xie, W.; Gao, J.; Jiang, R.; Liu, X.; Lai, F.; Tang, Y.; Xiao, H.; Jia, Y.; Bai, Q. Twice Subacute MPTP Administrations Induced Time-Dependent Dopaminergic Neurodegeneration and Inflammation in Midbrain and Ileum, as Well as Gut Microbiota Disorders in PD Mice. Neurotoxicology 2020, 76, 200–212. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Jin, Y.; Yu, J.; Mao, S.; Su, K.-P.; Ling, Z.; Liu, J. Probiotic Clostridium Butyricum Ameliorated Motor Deficits in a Mouse Model of Parkinson’s Disease via Gut Microbiota-GLP-1 Pathway. Brain Behav. Immun. 2021, 91, 703–715. [Google Scholar] [CrossRef]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered Microbiomes Distinguish Alzheimer’s Disease from Amnestic Mild Cognitive Impairment and Health in a Chinese Cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.-D.; Xiao, Q. Alteration of the Fecal Microbiota in Chinese Patients with Parkinson’s Disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar] [CrossRef]
- Chen, T.-J.; Feng, Y.; Liu, T.; Wu, T.-T.; Chen, Y.-J.; Li, X.; Li, Q.; Wu, Y.-C. Fisetin Regulates Gut Microbiota and Exerts Neuroprotective Effect on Mouse Model of Parkinson’s Disease. Front. Neurosci. 2020, 14, 549037. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, J.; Wei, K.; Wei, J.; Tian, P.; Yue, M.; Wang, Y.; Hong, D.; Li, F.; Wang, B.; et al. Neuroprotective Effect of Ceftriaxone on MPTP-Induced Parkinson’s Disease Mouse Model by Regulating Inflammation and Intestinal Microbiota. Oxidative Med. Cell. Longev. 2021, 2021, 9424582. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurized Bacterium Improves Metabolism in Obese and Diabetic Mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Hänninen, A.; Toivonen, R.; Pöysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; De Vos, W.M. Akkermansia Muciniphila Induces Gut Microbiota Remodelling and Controls Islet Autoimmunity in NOD Mice. Gut 2018, 67, 1445–1453. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia Muciniphila Is a Promising Probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Zhang, Y.; Chen, S.; Zeng, Y.; Fu, X.; Chen, T.; Luo, S.; Zhang, X. The Role of the Probiotic Akkermansia Muciniphila in Brain Functions: Insights Underpinning Therapeutic Potential. Crit. Rev. Microbiol. 2022, 11, 1–26. [Google Scholar] [CrossRef]
- Pascale, A.; Marchesi, N.; Govoni, S.; Barbieri, A. Targeting the Microbiota in Pharmacology of Psychiatric Disorders. Pharmacol. Res. 2020, 157, 104856. [Google Scholar] [CrossRef]
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut Microbiota Are Related to Parkinson’s Disease and Clinical Phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef]
- Hung, T.V.; Suzuki, T. Short-Chain Fatty Acids Suppress Inflammatory Reactions in Caco-2 Cells and Mouse Colons. J. Agric. Food Chem. 2018, 66, 108–117. [Google Scholar] [CrossRef]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., 3rd; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut Microbes Promote Colonic Serotonin Production through an Effect of Short-Chain Fatty Acids on Enterochromaffin Cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, T.J.; Gates, E.J.; Ranger, A.L.; Klegeris, A. Short-Chain Fatty Acids (SCFAs) Alone or in Combination Regulate Select Immune Functions of Microglia-like Cells. Mol. Cell. Neurosci. 2020, 105, 103493. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Chu, C.; Yu, L.; Zhai, Q.; Wang, S.; Zhao, J.; Zhang, H.; Chen, W.; Tian, F. Neuroprotective Effects of Bifidobacterium breve CCFM1067 in MPTP-Induced Mouse Models of Parkinson’s Disease. Nutrients 2022, 14, 4678. https://doi.org/10.3390/nu14214678
Li T, Chu C, Yu L, Zhai Q, Wang S, Zhao J, Zhang H, Chen W, Tian F. Neuroprotective Effects of Bifidobacterium breve CCFM1067 in MPTP-Induced Mouse Models of Parkinson’s Disease. Nutrients. 2022; 14(21):4678. https://doi.org/10.3390/nu14214678
Chicago/Turabian StyleLi, Tiantian, Chuanqi Chu, Leilei Yu, Qixiao Zhai, Shunhe Wang, Jianxin Zhao, Hao Zhang, Wei Chen, and Fengwei Tian. 2022. "Neuroprotective Effects of Bifidobacterium breve CCFM1067 in MPTP-Induced Mouse Models of Parkinson’s Disease" Nutrients 14, no. 21: 4678. https://doi.org/10.3390/nu14214678
APA StyleLi, T., Chu, C., Yu, L., Zhai, Q., Wang, S., Zhao, J., Zhang, H., Chen, W., & Tian, F. (2022). Neuroprotective Effects of Bifidobacterium breve CCFM1067 in MPTP-Induced Mouse Models of Parkinson’s Disease. Nutrients, 14(21), 4678. https://doi.org/10.3390/nu14214678