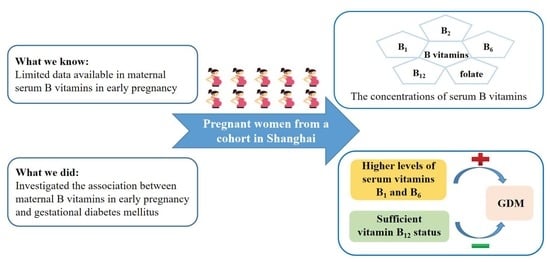
The Association between Maternal B Vitamins in Early Pregnancy and Gestational Diabetes Mellitus: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Diagnosis of GDM
2.4. Biochemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Correlations between Serum B Vitamins and Glucose Levels
3.3. Associations between Serum B Vitamins and GDM Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. Global Diabetes Data Report 2000–2045. Available online: https://diabetesatlas.org/data/en/world/ (accessed on 30 September 2022).
- Gao, C.; Sun, X.; Lu, L.; Liu, F.; Yuan, J. Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J. Diabetes Investig. 2019, 10, 154–162. [Google Scholar] [CrossRef]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational diabetes mellitus and adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2022, 377, e67946. [Google Scholar] [CrossRef]
- Lowe, W.J.; Scholtens, D.M.; Lowe, L.P.; Kuang, A.; Nodzenski, M.; Talbot, O.; Catalano, P.M.; Linder, B.; Brickman, W.J.; Clayton, P.; et al. Association of Gestational Diabetes with Maternal Disorders of Glucose Metabolism and Childhood Adiposity. JAMA 2018, 320, 1005–1016. [Google Scholar] [CrossRef] [Green Version]
- Venkataraman, H.; Ram, U.; Craik, S.; Arungunasekaran, A.; Seshadri, S.; Saravanan, P. Increased fetal adiposity prior to diagnosis of gestational diabetes in South Asians: More evidence for the ‘thin-fat’ baby. Diabetologia 2017, 60, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Kanasaki, K.; Kumagai, A. The impact of micronutrient deficiency on pregnancy complications and development origin of health and disease. J. Obstet. Gynaecol. Res. 2021, 47, 1965–1972. [Google Scholar] [CrossRef]
- Santander, B.S.; Gimenez, C.M.; Ballestin, B.J.; Luesma, B.M. Is Supplementation with Micronutrients Still Necessary during Pregnancy? A Review. Nutrients 2021, 13, 3134. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Huang, L.; Zhong, C.; Chen, R.; Zhou, X.; Chen, X.; Li, X.; Cui, W.; Xiong, T.; et al. High-Dose Folic Acid Supplement Use from Prepregnancy Through Midpregnancy Is Associated with Increased Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study. Diabetes Care 2019, 42, e113–e115. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, S.V.; Prabhakar, B.; Kulkarni, Y.A. Water Soluble Vitamins and their Role in Diabetes and its Complications. Curr. Diabetes Rev. 2020, 16, 649–656. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Lu, L.; Yang, K.; Reis, J.; He, K. Intakes of Folate, Vitamin B6, and Vitamin B12 in Relation to Diabetes Incidence Among American Young Adults: A 30-Year Follow-up Study. Diabetes Care 2020, 43, 2426–2434. [Google Scholar] [CrossRef]
- Finer, S.; Saravanan, P.; Hitman, G.; Yajnik, C. The role of the one-carbon cycle in the developmental origins of Type 2 diabetes and obesity. Diabet. Med. 2014, 31, 263–272. [Google Scholar] [CrossRef]
- Tamura, T.; Picciano, M.F. Folate and human reproduction. Am. J. Clin. Nutr. 2006, 83, 993–1016. [Google Scholar] [CrossRef] [Green Version]
- Chitayat, D.; Matsui, D.; Amitai, Y.; Kennedy, D.; Vohra, S.; Rieder, M.; Koren, G. Folic acid supplementation for pregnant women and those planning pregnancy: 2015 update. J. Clin. Pharmacol. 2016, 56, 170–175. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Chen, H.; Jiang, Y.; Wang, Y.; Wang, D.; Li, M.; Dou, Y.; Sun, X.; Huang, G.; et al. Association of Maternal Folate and Vitamin B12 in Early Pregnancy with Gestational Diabetes Mellitus: A Prospective Cohort Study. Diabetes Care 2021, 44, 217–223. [Google Scholar] [CrossRef]
- Saravanan, P.; Sukumar, N.; Adaikalakoteswari, A.; Goljan, I.; Venkataraman, H.; Gopinath, A.; Bagias, C.; Yajnik, C.S.; Stallard, N.; Ghebremichael-Weldeselassie, Y.; et al. Association of maternal vitamin B12 and folate levels in early pregnancy with gestational diabetes: A prospective UK cohort study (PRiDE study). Diabetologia 2021, 64, 2170–2182. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.S.; Pang, W.W.; Cai, S.; Lee, Y.S.; Chan, J.; Shek, L.; Yap, F.; Tan, K.H.; Godfrey, K.M.; van Dam, R.M.; et al. High folate and low vitamin B12 status during pregnancy is associated with gestational diabetes mellitus. Clin. Nutr. 2018, 37, 940–947. [Google Scholar] [CrossRef]
- Green, C.R.; Wallace, M.; Divakaruni, A.S.; Phillips, S.A.; Murphy, A.N.; Ciaraldi, T.P.; Metallo, C.M. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol. 2016, 12, 15–21. [Google Scholar] [CrossRef] [Green Version]
- De Almeida-Pititto, B.; Dualib, P.M.; Jordao, M.C.; Izar, H.F.M.; Jones, S.R.; Blaha, M.J.; Toth, P.P.; Santos, R.D.; Bensenor, I.M.; Ferreira, S.; et al. Branched-chain amino acids predict incident diabetes in the Brazilian Longitudinal Study of Adult Health—ELSA-Brasil. Diabetes Res. Clin. Pract. 2021, 174, 108747. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, E.; Verni, F. Vitamin B6 and Diabetes: Relationship and Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 3669. [Google Scholar] [CrossRef] [PubMed]
- Eshak, E.S.; Arafa, A.E. Thiamine deficiency and cardiovascular disorders. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Ahmed, A.M.; Siddiqui, J.A.; Panhwar, G.; Shaikh, F.; Ariff, M. Thiamine Level in Type I and Type II Diabetes Mellitus Patients: A Comparative Study Focusing on Hematological and Biochemical Evaluations. Cureus 2020, 12, e8027. [Google Scholar] [CrossRef]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.J.; Christian, P. Micronutrient deficiencies in pregnancy worldwide: Health effects and prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B. Cooperative meta-analysis group of China obesity task force. Predictive values of body mass index and waist circumference to risk factors of related disease in Chinese adult population. Zhonghua Liu Xing Bing Xue Za Zhi 2002, 1, 5–10. [Google Scholar]
- Bassett, D.J. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1396. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.; Hod, M.; Kitzmiler, J.L.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behere, R.V.; Deshmukh, A.S.; Otiv, S.; Gupte, M.D.; Yajnik, C.S. Maternal Vitamin B12 Status During Pregnancy and Its Association with Outcomes of Pregnancy and Health of the Offspring: A Systematic Review and Implications for Policy in India. Front. Endocrinol. 2021, 12, 619176. [Google Scholar] [CrossRef]
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and laboratory studies: A reference table for clinicians. Obstet. Gynecol. 2009, 114, 1326–1331. [Google Scholar] [CrossRef]
- Chinese Nutrition Society. Chinese Dietary Guidelines; People’s Medical Publishing House Press: Beijing, China, 2022. [Google Scholar]
- Liu, C.; Meng, Q.; Zu, C.; Li, R.; Yang, S.; He, P.; Li, H.; Zhang, Y.; Zhou, C.; Liu, M.; et al. U-Shaped association between dietary thiamine intake and new-onset diabetes: A nationwide cohort study. QJM 2022, hcac159. [Google Scholar] [CrossRef]
- Zhou, S.S.; Li, D.; Zhou, Y.M.; Sun, W.P.; Liu, Q.G. B-vitamin consumption and the prevalence of diabetes and obesity among the US adults: Population based ecological study. BMC Public Health 2010, 10, 746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornalley, P.J.; Babaei-Jadidi, R.; Al, A.H.; Rabbani, N.; Antonysunil, A.; Larkin, J.; Ahmed, A.; Rayman, G.; Bodmer, C.W. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia 2007, 50, 2164–2170. [Google Scholar] [CrossRef] [Green Version]
- Di Salvo, M.L.; Contestabile, R.; Safo, M.K. Vitamin B(6) salvage enzymes: Mechanism, structure and regulation. Biochim. Biophys. Acta 2011, 1814, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Dalto, D.B.; Matte, J.J. Pyridoxine (Vitamin B(6)) and the Glutathione Peroxidase System; a Link between One-Carbon Metabolism and Antioxidation. Nutrients 2017, 9, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecriaux, C. Interest of vitamin b6 for treatment of nausea and/or vomiting during pregnancy. Gynecol. Obstet. Fertil. Senol. 2020, 48, 840–843. [Google Scholar] [PubMed]
- Kim, H.; Toyofuku, Y.; Lynn, F.C.; Chak, E.; Uchida, T.; Mizukami, H.; Fujitani, Y.; Kawamori, R.; Miyatsuka, T.; Kosaka, Y.; et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat. Med. 2010, 16, 804–808. [Google Scholar] [CrossRef]
- Fields, A.M.; Welle, K.; Ho, E.S.; Mesaros, C.; Susiarjo, M. Vitamin B6 deficiency disrupts serotonin signaling in pancreatic islets and induces gestational diabetes in mice. Commun. Biol. 2021, 4, 421. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Failla, M.; Hoogwerf, B.; Maryniuk, M.; Wylie-Rosett, J. Selected vitamins and minerals in diabetes. Diabetes Care 1994, 17, 464–479. [Google Scholar] [CrossRef]
- Khare, A.; Lopez, M.; Gogtay, J. Homocysteine, B vitamins, and cardiovascular disease. N. Engl. J. Med. 2006, 206, 209–211. [Google Scholar]
- Shipton, M.J.; Thachil, J. Vitamin B12 deficiency—A 21st century perspective. Clin. Med. 2015, 15, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Sukumar, N.; Rafnsson, S.B.; Kandala, N.B.; Bhopal, R.; Yajnik, C.S.; Saravanan, P. Prevalence of vitamin B-12 insufficiency during pregnancy and its effect on offspring birth weight: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 1232–1251. [Google Scholar] [CrossRef] [Green Version]
- Sukumar, N.; Venkataraman, H.; Wilson, S.; Goljan, I.; Selvamoni, S.; Patel, V.; Saravanan, P. Vitamin B12 Status among Pregnant Women in the UK and Its Association with Obesity and Gestational Diabetes. Nutrients 2016, 8, 768. [Google Scholar] [CrossRef]
- Krishnaveni, G.V.; Hill, J.C.; Veena, S.R.; Bhat, D.S.; Wills, A.K.; Karat, C.L.; Yajnik, C.S.; Fall, C.H. Low plasma vitamin B12 in pregnancy is associated with gestational ‘diabesity’ and later diabetes. Diabetologia 2009, 52, 2350–2358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Qu, Y.Y.; Liu, L.; Qiao, Y.N.; Geng, H.R.; Lin, Y.; Xu, W.; Cao, J.; Zhao, J.Y. Homocysteine inhibits pro-insulin receptor cleavage and causes insulin resistance via protein cysteine-homocysteinylation. Cell Rep. 2021, 37, 109821. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sinha, J.K.; Putcha, U.K.; Raghunath, M. Severe but Not Moderate Vitamin B12 Deficiency Impairs Lipid Profile, Induces Adiposity, and Leads to Adverse Gestational Outcome in Female C57BL/6 Mice. Front. Nutr. 2016, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Giesbertz, P.; Daniel, H. Branched-chain amino acids as biomarkers in diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hou, Y.; Meng, D.; Yang, L.; Meng, X.; Liu, F. Vitamin B12 and Folate Levels During Pregnancy and Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 670289. [Google Scholar] [CrossRef]



| Characteristics | All | GDM (n = 121) | Non-GDM (n = 944) | p-Value |
|---|---|---|---|---|
| Age | 30.8 ± 3.7 | 32.2 ± 3.8 | 30.6 ± 3.6 | <0.001 |
| Education background (n (%)) | 0.381 | |||
| ≤Senior high school | 68 (6.4) | 12 (9.9) | 56 (5.9) | |
| College | 693 (65.1) | 76 (62.8) | 617 (65.4) | |
| ≥Postgraduate degree | 304 (28.5) | 33 (27.3) | 271 (28.7) | |
| Smoking exposure (n (%)) | 131 (12.3) | 19 (15.7) | 112 (11.9) | 0.226 |
| Alcohol drinking (n (%)) | 108 (10.1) | 9 (7.4) | 99 (10.5) | 0.296 |
| Pre-conceptional body mass index (kg/m2) (n (%)) | <0.001 | |||
| <18.5 | 159 (14.9) | 9 (7.4) | 150 (15.9) | |
| 18.5–24 | 726 (68.2) | 76 (62.8) | 650 (68.9) | |
| >24 | 180 (16.9) | 36 (29.8) | 144 (15.3) | |
| First-degree family history of diabetes (n (%)) | <0.001 | |||
| Yes | 136 (12.8) | 24 (19.8) | 112 (11.9) | |
| No | 906 (85.1) | 90 (74.4) | 816 (86.4) | |
| Unclear | 23(2.2) | 7 (5.8) | 16 (1.7) | |
| Primiparity (n (%)) | 874 (82.1) | 91 (75.2) | 783 (82.9) | 0.037 |
| Gestational weight gain at OGTT visits (kg) | 7.9 ± 3.8 | 8.2 ± 3.6 | 7.9 ± 3.8 | 0.478 |
| Physical activity level ≥ 600 MET (n (%)) | 346 (32.5) | 45 (37.2) | 301(31.9) | 0.241 |
| B-vitamin supplements (n (%)) | 0.671 | |||
| Folate supplements | 276 (25.9) | 28 (23.1) | 248 (26.3) | |
| Multivitamin supplements | 728 (68.4) | 87 (71.9) | 641 (67.9) | |
| No | 61(5.7) | 6 (5.0) | 55 (5.8) | |
| Biochemical characteristics | ||||
| B1 (pmol/L) | 86.5 (75.1–98.5) | 95.9 (78.5–110.2) | 86.1 (74.7–97.5) | <0.001 |
| B2 (pmol/L) | 13.5 (12.3–14.9) | 13.2 (11.9–14.7) | 13.5 (12.3–14.9) | 0.406 |
| B6 (pmol/L) | 27.2 (24.2–35.7) | 28.9 (24.8–37.4) | 26.9 (24.1–35.4) | 0.069 |
| Folate (nmol/L) | 11.8 (10.1–13.9) | 11.7 (10.3–14.0) | 11.8 (10.1–13.9) | 0.952 |
| Folate insufficiency at <5.9 nmol/L | 14 (1.3) | 2 (1.7) | 12 (1.3) | 0.729 |
| B12 (pmol/L) | 174.8(132.6–210.3) | 160.0 (124.7–194.8) | 176.1 (134.5–211.1) | 0.038 |
| B12 insufficiency at <150 pmol/L | 350 (32.9) | 54 (44.6) | 296 (31.4) | 0.003 |
| Homocysteine (umol/L) | 6.6 (6.0–7.5) | 6.7 (5.9–7.7) | 6.6 (6.0–7.5) | 0.828 |
| OGTT | Vitamin B1 | Vitamin B2 | Vitamin B6 | Folate | Vitamin B12 |
|---|---|---|---|---|---|
| Fasting | 0.062 * | −0.038 | 0.048 | −0.010 | 0.051 |
| 1 h | 0.123 * | 0.003 | 0.011 | 0.025 | −0.027 |
| 2 h | 0.111 * | 0.030 | 0.036 | 0.012 | 0.010 |
| Vitamin B1 | Vitamin B2 | Vitamin B6 | Vitamin B12 | Folate | |
|---|---|---|---|---|---|
| Vitamin B1 | 1.000 | ||||
| Vitamin B2 | −0.008 | 1.000 | |||
| Vitamin B6 | 0.063 * | −0.037 | 1.000 | ||
| Vitamin B12 | 0.038 | −0.055 | −0.003 | 1.000 | |
| Folate | −0.052 | 0.032 | −0.022 | −0.087 * | 1.000 |
| Variables | GDM/Total (%) | Model 1 † | Model 2 ‡ | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | ||
| Vitamin B1 | |||||||
| Q1 | 23/276 (8.33) | reference | reference | ||||
| Q2 | 21/266 (7.89) | 0.91 | 0.50–1.69 | 0.763 | 0.93 | 0.49–1.77 | 0.831 |
| Q3 | 30/266 (11.28) | 1.35 | 0.76–2.39 | 0.305 | 1.49 | 0.82–2.69 | 0.188 |
| Q4 | 47/266 (17.67) | 2.28 | 1.34–3.87 | 0.002 | 2.20 | 1.27–3.82 | 0.005 |
| p-Trend | <0.001 | ||||||
| Vitamin B2 | |||||||
| Q1 | 34/268 (12.69) | reference | reference | ||||
| Q2 | 30/266 (11.28) | 0.87 | 0.52–1.48 | 0.617 | 0.88 | 0.51–1.50 | 0.631 |
| Q3 | 29/267 (10.86) | 0.84 | 0.49–1.42 | 0.513 | 0.91 | 0.53–1.57 | 0.738 |
| Q4 | 28/264 (10.61) | 0.82 | 0.48–1.39 | 0.455 | 0.73 | 0.42–1.28 | 0.276 |
| p-Trend | 0.435 | 0.310 | |||||
| Vitamin B6 | |||||||
| Q1 | 22/267 (8.24) | reference | reference | ||||
| Q2 | 27/266 (10.15) | 1.26 | 0.70–2.27 | 0.446 | 1.27 | 0.69–2.33 | 0.437 |
| Q3 | 36/266 (13.53) | 1.74 | 1.00–3.05 | 0.052 | 1.93 | 1.08–3.43 | 0.026 |
| Q4 | 36/266 (13.53) | 1.74 | 1.00–3.05 | 0.052 | 1.84 | 1.03–3.29 | 0.040 |
| p-Trend | 0.069 | 0.054 | |||||
| Folate | |||||||
| Q1 | 27/267(10.11) | reference | reference | ||||
| Q2 | 38/269 (14.13) | 1.46 | 0.86–2.47 | 0.156 | 1.55 | 0.90–2.68 | 0.112 |
| Q3 | 22/263 (8.37) | 0.81 | 0.45–1.46 | 0.488 | 0.90 | 0.49–1.66 | 0.745 |
| Q4 | 34/266 (12.78) | 1.30 | 0.76–2.23 | 0.334 | 1.41 | 0.81–2.45 | 0.225 |
| p-Trend | 0.697 | 0.499 | |||||
| Vitamin B12 | |||||||
| Q1 | 37/267(13.86) | reference | reference | ||||
| Q2 | 34/266 (12.78) | 0.91 | 0.55–1.50 | 0.715 | 0.90 | 0.54–1.51 | 0.697 |
| Q3 | 26/266 (9.77) | 0.67 | 0.40–1.15 | 0.146 | 0.71 | 0.41–1.23 | 0.219 |
| Q4 | 24/266 (9.02) | 0.62 | 0.36–1.06 | 0.082 | 0.63 | 0.36–1.11 | 0.110 |
| p-Trend | 0.050 | 0.079 | |||||
| <150 pmol/L | 54/350 (15.43) | reference | reference | ||||
| ≥150 pmol/L | 67/715 (9.37) | 0.57 | 0.39–0.83 | 0.004 | 0.57 | 0.38–0.84 | 0.005 |
| Folate/B12 | |||||||
| Q1 | 28/267 (10.49) | reference | reference | ||||
| Q2 | 23/266 (8.65) | 0.81 | 0.45–1.44 | 0.471 | 0.81 | 0.45–1.47 | 0.497 |
| Q3 | 35/265 (13.21) | 1.30 | 0.77–2.20 | 0.332 | 1.23 | 0.71–2.12 | 0.464 |
| Q4 | 35/267 (13.11) | 1.29 | 0.76–2.19 | 0.349 | 1.37 | 0.79–2.36 | 0.258 |
| p-Trend | 0.176 | 0.121 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Zhou, T.; Ma, X.; Lin, Y.; Ding, Y. The Association between Maternal B Vitamins in Early Pregnancy and Gestational Diabetes Mellitus: A Prospective Cohort Study. Nutrients 2022, 14, 5016. https://doi.org/10.3390/nu14235016
Wang N, Zhou T, Ma X, Lin Y, Ding Y. The Association between Maternal B Vitamins in Early Pregnancy and Gestational Diabetes Mellitus: A Prospective Cohort Study. Nutrients. 2022; 14(23):5016. https://doi.org/10.3390/nu14235016
Chicago/Turabian StyleWang, Na, Tianchun Zhou, Xiaoxia Ma, Yuping Lin, and Yan Ding. 2022. "The Association between Maternal B Vitamins in Early Pregnancy and Gestational Diabetes Mellitus: A Prospective Cohort Study" Nutrients 14, no. 23: 5016. https://doi.org/10.3390/nu14235016
APA StyleWang, N., Zhou, T., Ma, X., Lin, Y., & Ding, Y. (2022). The Association between Maternal B Vitamins in Early Pregnancy and Gestational Diabetes Mellitus: A Prospective Cohort Study. Nutrients, 14(23), 5016. https://doi.org/10.3390/nu14235016