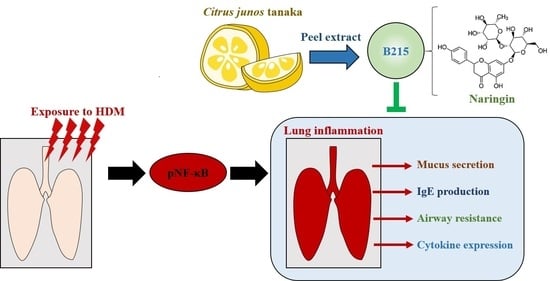
Citrus junos Tanaka Peel Extract Ameliorates HDM-Induced Lung Inflammation and Immune Responses In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Analysis of Citrus junos Tanaka Peel Extract (B215)
2.1.1. Preparation of B215
2.1.2. HPLC Analysis
2.2. Animals and Experimental Design
2.3. Histological Analysis
2.4. Plasma Analysis
2.5. Analysis of Airway Resistance to Methacholine Administration
2.6. Counting Immune Cells in BALF
2.7. Western Blotting
2.8. Gene Expression Analysis
2.9. Statistical Analysis
3. Results
3.1. Establishment of B215 Process
3.2. Administration of B215 Alleviated HDM-Induced Lung Inflammation In Vivo
3.3. Pulmonary Immune Responses Were Controlled by Administration of B215 on HDM-Induced Lung Inflammation
4. Discussion
Limits of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogliani, P.; Calzetta, L.; Matera, M.G.; Laitano, R.; Ritondo, B.L.; Hanania, N.A.; Cazzola, M. Severe Asthma and Biological Therapy: When, Which, and for Whom. Pulm. Ther. 2020, 6, 47–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, D.G.; Tully, J.E.; Nolin, J.D.; Janssen-Heininger, Y.M.; Irvin, C.G. Animal models of allergic airways disease: Where are we and where to next? J. Cell Biochem. 2014, 115, 2055–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tully, J.E.; Hoffman, S.M.; Lahue, K.G.; Nolin, J.D.; Anathy, V.; Lundblad, L.K.; Daphtary, N.; Aliyeva, M.; Black, K.E.; Dixon, A.E.; et al. Epithelial NF-kappaB orchestrates house dust mite-induced airway inflammation, hyperresponsiveness, and fibrotic remodeling. J. Immunol. 2013, 191, 5811–5821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzetta, L.; Matera, M.G.; Coppola, A.; Rogliani, P. Prospects for severe asthma treatment. Curr. Opin. Pharmacol. 2021, 56, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Hirota, R.; Roger, N.N.; Nakamura, H.; Song, H.S.; Sawamura, M.; Suganuma, N. Anti-inflammatory effects of limonene from yuzu (Citrus junos Tanaka) essential oil on eosinophils. J. Food Sci. 2010, 75, H87–H92. [Google Scholar] [CrossRef]
- Bharti, S.; Rani, N.; Krishnamurthy, B.; Arya, D.S. Preclinical evidence for the pharmacological actions of naringin: A review. Planta Med. 2014, 80, 437–451. [Google Scholar] [CrossRef] [Green Version]
- Abe, H.; Ishioka, M.; Fujita, Y.; Umeno, A.; Yasunaga, M.; Sato, A.; Ohnishi, S.; Suzuki, S.; Ishida, N.; Shichiri, M.; et al. Yuzu (Citrus junos Tanaka) Peel Attenuates Dextran Sulfate Sodium-induced Murine Experimental Colitis. J. Oleo Sci. 2018, 67, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Park, S.Y.; Kim, Y.; Jeon, S.; Cha, M.S.; Kim, Y.J.; Yoon, H.G. Beneficial Effects of a Combination of Curcuma longa L. and Citrus junos against Beta-Amyloid Peptide-Induced Neurodegeneration in Mice. J. Med. Food 2022, 25, 12–23. [Google Scholar] [CrossRef]
- Kim, S.H.; Hur, H.J.; Yang, H.J.; Kim, H.J.; Kim, M.J.; Park, J.H.; Sung, M.J.; Kim, M.S.; Kwon, D.Y.; Hwang, J.T. Citrus junos Tanaka Peel Extract Exerts Antidiabetic Effects via AMPK and PPAR-gamma both In Vitro and In Vivo in Mice Fed a High-Fat Diet. Evid. Based Complement Alternat. Med. 2013, 2013, 921012. [Google Scholar] [CrossRef] [Green Version]
- Shin, E.J.; Park, J.H.; Sung, M.J.; Chung, M.Y.; Hwang, J.T. Citrus junos Tanaka peel ameliorates hepatic lipid accumulation in HepG2 cells and in mice fed a high-cholesterol diet. BMC Complement Altern. Med. 2016, 16, 499. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, J.H.; Ku, H.J.; Kim, S.H.; Lim, Y.J.; Park, J.W.; Lee, J.H. Naringin protects acrolein-induced pulmonary injuries through modulating apoptotic signaling and inflammation signaling pathways in mice. J. Nutr. Biochem. 2018, 59, 10–16. [Google Scholar] [CrossRef]
- Huang, W.Y.; Heo, W.; Jeong, I.; Kim, M.J.; Han, B.K.; Shin, E.C.; Kim, Y.J. Ameliorative Effect of Citrus junos Tanaka Waste (By-Product) Water Extract on Particulate Matter 10-Induced Lung Damage. Nutrients 2022, 14, 2270. [Google Scholar] [CrossRef]
- Lee, D.H.; Woo, J.K.; Heo, W.; Huang, W.Y.; Kim, Y.; Chung, S.; Lee, G.H.; Park, J.W.; Han, B.K.; Shin, E.C.; et al. Citrus junos Tanaka Peel Extract and Its Bioactive Naringin Reduce Fine Dust-Induced Respiratory Injury Markers in BALB/c Male Mice. Nutrients 2022, 14, 1101. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, G.H.; Kim, H.K.; Chae, H.J. Platycodi Radix and its active compounds ameliorate against house dust mite-induced allergic airway inflammation and ER stress and ROS by enhancing anti-oxidation. Food Chem. Toxicol. 2019, 123, 412–423. [Google Scholar] [CrossRef]
- Murakami, Y.; Ishii, T.; Nunokawa, H.; Kurata, K.; Narita, T.; Yamashita, N. TLR9-IL-2 axis exacerbates allergic asthma by preventing IL-17A hyperproduction. Sci. Rep. 2020, 10, 18110. [Google Scholar] [CrossRef]
- Kim, S.R.; Park, H.J.; Lee, K.B.; Kim, H.J.; Jeong, J.S.; Cho, S.H.; Lee, Y.C. Epithelial PI3K-delta Promotes House Dust Mite-Induced Allergic Asthma in NLRP3 Inflammasome-Dependent and -Independent Manners. Allergy Asthma Immunol. Res. 2020, 12, 338–358. [Google Scholar] [CrossRef]
- Lee, H.Y.; Chae, H.J.; Park, S.Y.; Kim, J.H. Porcine placenta hydrolysates enhance osteoblast differentiation through their antioxidant activity and effects on ER stress. BMC Complement. Altern. Med. 2016, 16, 291. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Hu, Q.; Zang, X.; Zhou, C.; Liu, D.; Liu, G.; Hong, L. Analysis of Transcripts of Uncertain Coding Potential Using RNA Sequencing During the Preattachment Phase in Goat Endometrium. DNA Cell Biol. 2021, 40, 998–1008. [Google Scholar] [CrossRef]
- Zhao, Q.; Hu, Y.; Deng, S.; Yu, P.; Chen, B.; Wang, Z.; Han, X. Cytidine-phosphate-guanosine oligodeoxynucleotides in combination with CD40 ligand decrease periodontal inflammation and alveolar bone loss in a TLR9-independent manner. J. Appl. Oral Sci. 2018, 26, e20170451. [Google Scholar] [CrossRef]
- Piyadasa, H.; Altieri, A.; Basu, S.; Schwartz, J.; Halayko, A.J.; Mookherjee, N. Biosignature for airway inflammation in a house dust mite-challenged murine model of allergic asthma. Biol. Open 2016, 5, 112–121. [Google Scholar] [CrossRef]
- Aghasafari, P.; George, U.; Pidaparti, R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.D. Lung mucus: A clinician’s view. Eur. Respir. J. 1997, 10, 1914–1917. [Google Scholar] [CrossRef] [Green Version]
- Chapman, D.G.; Irvin, C.G. Mechanisms of airway hyper-responsiveness in asthma: The past, present and yet to come. Clin. Exp. Allergy 2015, 45, 706–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Alba, J.; Raemdonck, K.; Dekkak, A.; Collins, M.; Wong, S.; Nials, A.T.; Knowles, R.G.; Belvisi, M.G.; Birrell, M.A. House dust mite induces direct airway inflammation in vivo: Implications for future disease therapy? Eur. Respir. J. 2010, 35, 1377–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, M.; Li, G.; Qi, M.; Jiang, W.; Zhou, R. Inhibition of the Inflammasome Activity of NLRP3 Attenuates HDM-Induced Allergic Asthma. Front. Immunol. 2021, 12, 718779. [Google Scholar] [CrossRef] [PubMed]
- Woo, L.N.; Guo, W.Y.; Wang, X.; Young, A.; Salehi, S.; Hin, A.; Zhang, Y.; Scott, J.A.; Chow, C.W. A 4-Week Model of House Dust Mite (HDM) Induced Allergic Airways Inflammation with Airway Remodeling. Sci. Rep. 2018, 8, 6925. [Google Scholar] [CrossRef] [Green Version]
- Alam, R.; Gorska, M.M. Mitogen-activated protein kinase signalling and ERK1/2 bistability in asthma. Clin. Exp. Allergy 2011, 41, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Alharbi, K.S.; Fuloria, N.K.; Fuloria, S.; Rahman, S.B.; Al-Malki, W.H.; Javed Shaikh, M.A.; Thangavelu, L.; Singh, S.K.; Rama Raju Allam, V.S.; Jha, N.K.; et al. Nuclear factor-kappa B and its role in inflammatory lung disease. Chem. Biol. Interact. 2021, 345, 109568. [Google Scholar] [CrossRef]
- Hoshino, A.; Tsuji, T.; Matsuzaki, J.; Jinushi, T.; Ashino, S.; Teramura, T.; Chamoto, K.; Tanaka, Y.; Asakura, Y.; Sakurai, T.; et al. STAT6-mediated signaling in Th2-dependent allergic asthma: Critical role for the development of eosinophilia, airway hyper-responsiveness and mucus hypersecretion, distinct from its role in Th2 differentiation. Int. Immunol. 2004, 16, 1497–1505. [Google Scholar] [CrossRef]
- Yoo, E.J.; Ojiaku, C.A.; Sunder, K.; Panettieri, R.A., Jr. Phosphoinositide 3-Kinase in Asthma: Novel Roles and Therapeutic Approaches. Am. J. Respir. Cell Mol. Biol. 2017, 56, 700–707. [Google Scholar] [CrossRef]
- Gagliardo, R.; Chanez, P.; Profita, M.; Bonanno, A.; Albano, G.D.; Montalbano, A.M.; Pompeo, F.; Gagliardo, C.; Merendino, A.M.; Gjomarkaj, M. IkappaB kinase-driven nuclear factor-kappaB activation in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2011, 128, 635–645.e2. [Google Scholar] [CrossRef]
- Gregory, L.G.; Lloyd, C.M. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011, 32, 402–411. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, K.; Mitra, S.; Gavrilin, M.A.; Wewers, M.D. House Dust Mite Allergens and the Induction of Monocyte Interleukin 1beta Production That Triggers an IkappaBzeta-Dependent Granulocyte Macrophage Colony-Stimulating Factor Release from Human Lung Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2015, 53, 400–411. [Google Scholar] [CrossRef] [Green Version]
- Vroling, A.B.; Duinsbergen, D.; Fokkens, W.J.; van Drunen, C.M. Allergen induced gene expression of airway epithelial cells shows a possible role for TNF-alpha. Allergy 2007, 62, 1310–1319. [Google Scholar] [CrossRef]
- Côté, A.; Godbout, K.; Boulet, L.-P. The management of severe asthma in 2020. Biochem. Pharmacol. 2020, 179, 114112. [Google Scholar] [CrossRef]
- Schatz, M.; Rosenwasser, L. The allergic asthma phenotype. J. Allergy Clin. Immunol. Pract. 2014, 2, 645–648. [Google Scholar] [CrossRef]
- Hou, X.L.; Tong, Q.; Wang, W.Q.; Shi, C.Y.; Xiong, W.; Chen, J.; Liu, X.; Fang, J.G. Suppression of Inflammatory Responses by Dihydromyricetin, a Flavonoid from Ampelopsis grossedentata, via Inhibiting the Activation of NF-kappaB and MAPK Signaling Pathways. J. Nat. Prod. 2015, 78, 1689–1696. [Google Scholar] [CrossRef]
- Liu, Q.; Lv, H.; Wen, Z.; Ci, X.; Peng, L. Isoliquiritigenin Activates Nuclear Factor Erythroid-2 Related Factor 2 to Suppress the NOD-Like Receptor Protein 3 Inflammasome and Inhibits the NF-kappaB Pathway in Macrophages and in Acute Lung Injury. Front. Immunol. 2017, 8, 1518. [Google Scholar] [CrossRef]
- Xu, W.; Lu, H.; Yuan, Y.; Deng, Z.; Zheng, L.; Li, H. The Antioxidant and Anti-Inflammatory Effects of Flavonoids from Propolis via Nrf2 and NF-kappaB Pathways. Foods 2022, 11, 2439. [Google Scholar] [CrossRef]
- Ha, S.K.; Park, H.Y.; Eom, H.; Kim, Y.; Choi, I. Narirutin fraction from citrus peels attenuates LPS-stimulated inflammatory response through inhibition of NF-kappaB and MAPKs activation. Food Chem. Toxicol. 2012, 50, 3498–3504. [Google Scholar] [CrossRef] [PubMed]
- Funaguchi, N.; Ohno, Y.; La, B.L.; Asai, T.; Yuhgetsu, H.; Sawada, M.; Takemura, G.; Minatoguchi, S.; Fujiwara, T.; Fujiwara, H. Narirutin inhibits airway inflammation in an allergic mouse model. Clin. Exp. Pharmacol. Physiol. 2007, 34, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Scheidereit, C. The IkappaB kinase complex in NF-kappaB regulation and beyond. EMBO Rep. 2014, 15, 46–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guihua, X.; Shuyin, L.; Jinliang, G.; Wang, S. Naringin Protects Ovalbumin-Induced Airway Inflammation in a Mouse Model of Asthma. Inflammation 2016, 39, 891–899. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, B.K.; Lee, Y.C. Antiasthmatic effects of hesperidin, a potential Th2 cytokine antagonist, in a mouse model of allergic asthma. Mediat. Inflamm. 2011, 2011, 485402. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Dai, J.; Liu, H.; Li, R.R.; Sun, P.L.; Du, Q.; Pang, L.L.; Chen, Z.; Yin, K.S. Naringenin inhibits allergen-induced airway inflammation and airway responsiveness and inhibits NF-kappaB activity in a murine model of asthma. Can. J. Physiol. Pharmacol. 2009, 87, 729–735. [Google Scholar] [CrossRef]
- Mahmutovic Persson, I.; Menzel, M.; Ramu, S.; Cerps, S.; Akbarshahi, H.; Uller, L. IL-1beta mediates lung neutrophilia and IL-33 expression in a mouse model of viral-induced asthma exacerbation. Respir. Res. 2018, 19, 16. [Google Scholar] [CrossRef] [Green Version]
- Namakanova, O.A.; Gorshkova, E.A.; Zvartsev, R.V.; Nedospasov, S.A.; Drutskaya, M.S.; Gubernatorova, E.O. Therapeutic Potential of Combining IL-6 and TNF Blockade in a Mouse Model of Allergic Asthma. Int. J. Mol. Sci. 2022, 23, 3521. [Google Scholar] [CrossRef]







| Forward (5′ → 3′) | Reverse (5′ → 3′) | |
|---|---|---|
| Gapdh [17] | ATCACCATCTTCCAGGAG | ATGGACTGTGGTCATGAG |
| Il1b [18] | AGTTGACGGACCCCAAAAG | AGCTGGATGCTCTCATCAGG |
| Il6 [18] | CCAGGTAGCTATGGTACTCCA | GCTACCAAACTGGCTATAATC |
| Tnfa [19] | CACAGAAGCATGATCCGCGACGT | CGGCAGAGAGGAGGTTGACTTTCT |
| AST (U/L) | ALT (U/L) | TG (mg/dL) | Cholesterol (mg/dL) | |
|---|---|---|---|---|
| CTR (n = 8) | 64.10 ± 56.72 | 253.65 ± 79.58 | 93.13 ± 26.14 | 103.75 ± 11.11 |
| HDM (n = 8) | 57.86 ± 24.37 | 211.80 ± 41.18 | 67.53 ± 19.01 | 90.50 ± 10.06 |
| HDM+50B215 (n = 8) | 48.33 ± 16.93 | 151.53 ± 99.03 | 94.64 ± 18.13 | 102.00 ± 8.72 |
| HDM+100B215 (n = 8) | 44.04 ± 16.03 | 124.83 ± 59.32 | 104.74 ± 20.83 * | 102.38 ± 11.88 |
| HDM+200B215 (n = 8) | 72.21 ± 29.27 | 241.68 ± 77.48 | 93.45 ± 19.52 | 104.38 ± 11.19 |
| Neutrophil | Eosinophil | Macrophage | Lymphocyte | Basophil | |
|---|---|---|---|---|---|
| CTR (n = 8) | 55,175 ± 8053 | 22,660 ± 8831 | 213,955 ± 84,259 | 437,068 ± 72,351 | 9345 ± 4441 |
| HDM (n = 8) | 136,915 ± 43,542 # | 177,660 ± 57,119 # | 416,829 ± 93,790 # | 719,788 ± 187,911 # | 58,694 ± 51,335 # |
| HDM+50B215 (n = 8) | 63,950 ± 36,434 * | 67,800 ± 30,746 * | 343,780 ± 136,016 | 990,873 ± 137,140 * | 9814 ± 3381 * |
| HDM+100B215 (n = 8) | 63,791 ± 45,781 * | 52,811 ± 22,536 * | 259,221 ± 83,073 * | 978,280 ± 77,775 * | 5580 ± 3303 * |
| HDM+200B215 (n = 8) | 50,826 ± 43,614 * | 56,610 ± 31,207 * | 204,960 ± 119,757 * | 746,438 ± 152,338 | 9998 ± 7595 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, D.; Kim, H.-J.; Lee, J.; Lee, Y.-M.; Park, J.-W.; Yang, S.; Lee, G.-H.; Chung, M.J.; Chae, H.-J. Citrus junos Tanaka Peel Extract Ameliorates HDM-Induced Lung Inflammation and Immune Responses In Vivo. Nutrients 2022, 14, 5024. https://doi.org/10.3390/nu14235024
Shim D, Kim H-J, Lee J, Lee Y-M, Park J-W, Yang S, Lee G-H, Chung MJ, Chae H-J. Citrus junos Tanaka Peel Extract Ameliorates HDM-Induced Lung Inflammation and Immune Responses In Vivo. Nutrients. 2022; 14(23):5024. https://doi.org/10.3390/nu14235024
Chicago/Turabian StyleShim, Dahee, Hwa-Jin Kim, Jungu Lee, You-Min Lee, Jae-Woong Park, Siyoung Yang, Gyeong-Hweon Lee, Myoung Ja Chung, and Han-Jung Chae. 2022. "Citrus junos Tanaka Peel Extract Ameliorates HDM-Induced Lung Inflammation and Immune Responses In Vivo" Nutrients 14, no. 23: 5024. https://doi.org/10.3390/nu14235024
APA StyleShim, D., Kim, H. -J., Lee, J., Lee, Y. -M., Park, J. -W., Yang, S., Lee, G. -H., Chung, M. J., & Chae, H. -J. (2022). Citrus junos Tanaka Peel Extract Ameliorates HDM-Induced Lung Inflammation and Immune Responses In Vivo. Nutrients, 14(23), 5024. https://doi.org/10.3390/nu14235024