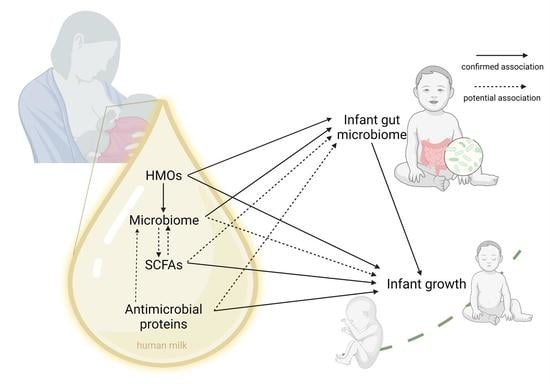
Human Milk Microbiome and Microbiome-Related Products: Potential Modulators of Infant Growth
Abstract
:1. Introduction
2. Review Methodology
3. The Infant Gut Microbiome Can Influence Infant Growth
4. Development of Infant Gut Microbiota and Breastfeeding
5. The Human Milk Microbiome
5.1. Human Milk Microbes Colonise the Infant Gut
5.2. The Potential Role of the Human Milk Microbiota in Infant Growth
5.3. The Milk Microbiome as a Potential Contributor to the Intergenerational Transmission of Body Composition
6. Human Milk Oligosaccharides (HMOs)
HMOs Are Associated with Infant Growth
7. Short-Chain Fatty Acids
Human Milk SCFAs and Infant Growth
8. Antimicrobial Proteins—Lactoferrin and Lysozyme
9. Interactions of Microbiome and Microbiome-Related Components within the Lactating Mammary Gland
10. Summary and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheung, Y.; Yip, P.; Karlberg, J. Fetal Growth, Early Postnatal Growth and Motor Development in Pakistani Infants. Int. J. Epidemiol. 2001, 30, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Belfort, M.B.; Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Kleinman, K.P.; Oken, E.; Gillman, M.W. Infant Growth and Child Cognition at 3 Years of Age. Pediatrics 2008, 122, e689–e695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, K.; Loos, R. Rapid Infancy Weight Gain and Subsequent Obesity: Systematic Reviews and Hopeful Suggestions. Acta Paediatr. 2006, 95, 904–908. [Google Scholar] [CrossRef]
- Belfort, M.B.; Rifas-Shiman, S.L.; Sullivan, T.; Collins, C.T.; McPhee, A.J.; Ryan, P.; Kleinman, K.P.; Gillman, M.W.; Gibson, R.A.; Makrides, M. Infant Growth Before and After Term: Effects on Neurodevelopment in Preterm Infants. Pediatrics 2011, 128, e899–e906. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.J.; Sawaya, A.L.; Verreschi, I.; Tucker, K.L.; Roberts, S.B. Why Are Nutritionally Stunted Children at Increased Risk of Obesity? Studies of Metabolic Rate and Fat Oxidation in Shantytown Children from São Paulo, Brazil. Am. J. Clin. Nutr. 2000, 72, 702–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gittner, L.S.; Ludington-Hoe, S.M.; Haller, H.S. Utilising Infant Growth to Predict Obesity Status at 5 Years: Infant Obesity. J. Paediatr. Child Health 2013, 49, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Dietz, W.H. Childhood Weight Affects Adult Morbidity and Mortality. J. Nutr. 1998, 128, 411S–414S. [Google Scholar] [CrossRef] [Green Version]
- Gillman, M.W. Risk of Overweight Among Adolescents Who Were Breastfed as Infants. JAMA 2001, 285, 2461. [Google Scholar] [CrossRef] [Green Version]
- Dietz, W.H. Breastfeeding May Help Prevent Childhood Overweight. JAMA 2001, 285, 2506. [Google Scholar] [CrossRef]
- Dewey, K.G. Is Breastfeeding Protective Against Child Obesity? J. Hum. Lact. 2003, 19, 9–18. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef] [Green Version]
- Cho, I.; Yamanishi, S.; Cox, L.; Methé, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in Early Life Alter the Murine Colonic Microbiome and Adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Differding, M.K.; Benjamin-Neelon, S.E.; Østbye, T.; Hoyo, C.; Mueller, N.T. Association of Prenatal Antibiotics with Measures of Infant Adiposity and the Gut Microbiome. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 18. [Google Scholar] [CrossRef]
- Azad, M.B.; Bridgman, S.L.; Becker, A.B.; Kozyrskyj, A.L. Infant Antibiotic Exposure and the Development of Childhood Overweight and Central Adiposity. Int. J. Obes. 2014, 38, 1290–1298. [Google Scholar] [CrossRef]
- Scheepers, L.E.J.M.; Penders, J.; Mbakwa, C.A.; Thijs, C.; Mommers, M.; Arts, I.C.W. The Intestinal Microbiota Composition and Weight Development in Children: The KOALA Birth Cohort Study. Int. J. Obes. 2015, 39, 16–25. [Google Scholar] [CrossRef]
- Vael, C.; Verhulst, S.L.; Nelen, V.; Goossens, H.; Desager, K.N. Intestinal Microflora and Body Mass Index during the First Three Years of Life: An Observational Study. Gut. Pathog. 2011, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Stanislawski, M.A.; Dabelea, D.; Wagner, B.D.; Iszatt, N.; Dahl, C.; Sontag, M.K.; Knight, R.; Lozupone, C.A.; Eggesbø, M. Gut Microbiota in the First 2 Years of Life and the Association with Body Mass Index at Age 12 in a Norwegian Birth Cohort. mBio 2018, 9, e01751-18. [Google Scholar] [CrossRef] [Green Version]
- Korpela, K.; Renko, M.; Vänni, P.; Paalanne, N.; Salo, J.; Tejesvi, M.V.; Koivusaari, P.; Ojaniemi, M.; Pokka, T.; Kaukola, T.; et al. Microbiome of the First Stool and Overweight at Age 3 Years: A Prospective Cohort Study. Pediatr. Obes. 2020, 15, e12680. [Google Scholar] [CrossRef]
- White, R.A.; Bjørnholt, J.V.; Baird, D.D.; Midtvedt, T.; Harris, J.R.; Pagano, M.; Hide, W.; Rudi, K.; Moen, B.; Iszatt, N.; et al. Novel Developmental Analyses Identify Longitudinal Patterns of Early Gut Microbiota That Affect Infant Growth. PLoS Comput. Biol. 2013, 9, e1003042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalliomäki, M.; Carmen Collado, M.; Salminen, S.; Isolauri, E. Early Differences in Fecal Microbiota Composition in Children May Predict Overweight. Am. J. Clin. Nutr. 2008, 87, 534–538. [Google Scholar] [CrossRef] [Green Version]
- Forbes, J.D.; Azad, M.B.; Vehling, L.; Tun, H.M.; Konya, T.B.; Guttman, D.S.; Field, C.J.; Lefebvre, D.; Sears, M.R.; Becker, A.B.; et al. Association of Exposure to Formula in the Hospital and Subsequent Infant Feeding Practices With Gut Microbiota and Risk of Overweight in the First Year of Life. JAMA Pediatr. 2018, 172, e181161. [Google Scholar] [CrossRef]
- Reyna, M.E.; Petersen, C.; Dai, D.L.Y.; Dai, R.; Becker, A.B.; Azad, M.B.; Miliku, K.; Lefebvre, D.L.; Moraes, T.J.; Mandhane, P.J.; et al. Longitudinal Body Mass Index Trajectories at Preschool Age: Children with Rapid Growth Have Differential Composition of the Gut Microbiota in the First Year of Life. Int. J. Obes 2022, 46, 1351–1358. [Google Scholar] [CrossRef]
- Jiménez, E.; Fernández, L.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Nueno-Palop, C.; Narbad, A.; Olivares, M.; Xaus, J.; Rodríguez, J.M. Isolation of Commensal Bacteria from Umbilical Cord Blood of Healthy Neonates Born by Cesarean Section. Curr. Microbiol. 2005, 51, 270–274. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, K.M.; Gerlach, M.J.; Adam, T.; Heimesaat, M.M.; Rossi, L.; Surette, M.G.; Sloboda, D.M.; Braun, T. Fetal Meconium Does Not Have a Detectable Microbiota before Birth. Nat. Microbiol. 2021, 6, 865–873. [Google Scholar] [CrossRef]
- Kuperman, A.; Zimmerman, A.; Hamadia, S.; Ziv, O.; Gurevich, V.; Fichtman, B.; Gavert, N.; Straussman, R.; Rechnitzer, H.; Barzilay, M.; et al. Deep Microbial Analysis of Multiple Placentas Shows No Evidence for a Placental Microbiome. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 159–169. [Google Scholar] [CrossRef]
- Bergström, A.; Skov, T.H.; Bahl, M.I.; Roager, H.M.; Christensen, L.B.; Ejlerskov, K.T.; Mølgaard, C.; Michaelsen, K.F.; Licht, T.R. Establishment of Intestinal Microbiota during Early Life: A Longitudinal, Explorative Study of a Large Cohort of Danish Infants. Appl. Env. Microbiol. 2014, 80, 2889–2900. [Google Scholar] [CrossRef] [Green Version]
- Nagpal, R.; Tsuji, H.; Takahashi, T.; Nomoto, K.; Kawashima, K.; Nagata, S.; Yamashiro, Y. Ontogenesis of the Gut Microbiota Composition in Healthy, Full-Term, Vaginally Born and Breast-Fed Infants over the First 3 Years of Life: A Quantitative Bird’s-Eye View. Front. Microbiol. 2017, 8, 1388. [Google Scholar] [CrossRef]
- Hollister, E.B.; Riehle, K.; Luna, R.A.; Weidler, E.M.; Rubio-Gonzales, M.; Mistretta, T.-A.; Raza, S.; Doddapaneni, H.V.; Metcalf, G.A.; Muzny, D.M.; et al. Structure and Function of the Healthy Pre-Adolescent Pediatric Gut Microbiome. Microbiome 2015, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Ou, Y.; Belzer, C.; Smidt, H.; de Weerth, C. Development of the Gut Microbiota in Healthy Children in the First Ten Years of Life: Associations with Internalizing and Externalizing Behavior. Gut. Microbes. 2022, 14, 2038853. [Google Scholar] [CrossRef]
- Roswall, J.; Olsson, L.M.; Kovatcheva-Datchary, P.; Nilsson, S.; Tremaroli, V.; Simon, M.-C.; Kiilerich, P.; Akrami, R.; Krämer, M.; Uhlén, M.; et al. Developmental Trajectory of the Healthy Human Gut Microbiota during the First 5 Years of Life. Cell Host Microbe 2021, 29, 765–776.e3. [Google Scholar] [CrossRef]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A.; et al. Temporal Development of the Gut Microbiome in Early Childhood from the TEDDY Study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef] [Green Version]
- Chernikova, D.A.; Madan, J.C.; Housman, M.L.; Zain-ul-abideen, M.; Lundgren, S.N.; Morrison, H.G.; Sogin, M.L.; Williams, S.M.; Moore, J.H.; Karagas, M.R.; et al. The Premature Infant Gut Microbiome during the First 6 Weeks of Life Differs Based on Gestational Maturity at Birth. Pediatr. Res. 2018, 84, 71–79. [Google Scholar] [CrossRef]
- Lundgren, S.N.; Madan, J.C.; Emond, J.A.; Morrison, H.G.; Christensen, B.C.; Karagas, M.R.; Hoen, A.G. Maternal Diet during Pregnancy Is Related with the Infant Stool Microbiome in a Delivery Mode-Dependent Manner. Microbiome 2018, 6, 109. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, C.M.; Mazzoni, C.; Hogstrom, L.; Bryant, A.; Bergerat, A.; Cher, A.; Pochan, S.; Herman, P.; Carrigan, M.; Sharp, K.; et al. Delivery Mode Affects Stability of Early Infant Gut Microbiota. Cell Rep. Med. 2020, 1, 100156. [Google Scholar] [CrossRef]
- Gasparrini, A.J.; Crofts, T.S.; Gibson, M.K.; Tarr, P.I.; Warner, B.B.; Dantas, G. Antibiotic Perturbation of the Preterm Infant Gut Microbiome and Resistome. Gut. Microbes 2016, 7, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Judge, M.; Xu, W.; Diallo, A.; Janton, S.; Brownell, E.A.; Maas, K.; Graf, J. Influence of Feeding Type on Gut Microbiome Development in Hospitalized Preterm Infants. Nurs. Res. 2017, 66, 123–133. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshioka, H.; Iseki, K.; Fujita, K. Development and Differences of Intestinal Flora in the Neonatal Period in Breast-Fed and Bottle-Fed Infants. Pediatrics 1983, 72, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yi, W.; Liu, B.; Dai, Y.; Jiang, T.; Chen, S.; Wang, J.; Feng, B.; Qiao, W.; Liu, Y.; et al. MFGM Components Promote Gut Bifidobacterium Growth in Infant and in Vitro. Eur. J. Nutr. 2022, 61, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.T.; Li, F.; Lee-Sarwar, K.A.; Tun, H.M.; Brown, B.P.; Pannaraj, P.S.; Bender, J.M.; Azad, M.B.; Thompson, A.L.; Weiss, S.T.; et al. Meta-Analysis of Effects of Exclusive Breastfeeding on Infant Gut Microbiota across Populations. Nat. Commun. 2018, 9, 4169. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of Gut Microbiota in Exclusively Breast-Fed and Formula-Fed Babies: A Study of 91 Term Infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef]
- Stinson, L.F.; Sindi, A.S.M.; Cheema, A.S.; Lai, C.T.; Mühlhäusler, B.S.; Wlodek, M.E.; Payne, M.S.; Geddes, D.T. The Human Milk Microbiome: Who, What, When, Where, Why, and How? Nutr. Rev. 2021, 79, 529–543. [Google Scholar] [CrossRef]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Rochat, F.; Chassard, C. Vertical Mother-Neonate Transfer of Maternal Gut Bacteria via Breastfeeding: Mother-Neonate Bacterial Transfer. Env. Microbiol. 2014, 16, 2891–2904. [Google Scholar] [CrossRef]
- Fernández, L.; Langa, S.; Martín, V.; Maldonado, A.; Jiménez, E.; Martín, R.; Rodríguez, J.M. The Human Milk Microbiota: Origin and Potential Roles in Health and Disease. Pharmacol. Res. 2013, 69, 1–10. [Google Scholar] [CrossRef]
- McGuire, M.K.; McGuire, M.A. Got Bacteria? The Astounding, yet Not-so-Surprising, Microbiome of Human Milk. Curr. Opin. Biotechnol. 2017, 44, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Fitzstevens, J.L.; Smith, K.C.; Hagadorn, J.I.; Caimano, M.J.; Matson, A.P.; Brownell, E.A. Systematic Review of the Human Milk Microbiota. Nutr. Clin. Pr. 2017, 32, 354–364. [Google Scholar] [CrossRef]
- Gomez-Gallego, C.; Garcia-Mantrana, I.; Salminen, S.; Collado, M.C. The Human Milk Microbiome and Factors Influencing Its Composition and Activity. Semin. Fetal Neonatal Med. 2016, 21, 400–405. [Google Scholar] [CrossRef]
- Moeller, A.H.; Suzuki, T.A.; Phifer-Rixey, M.; Nachman, M.W. Transmission Modes of the Mammalian Gut Microbiota. Science 2018, 362, 453–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asnicar, F.; Manara, S.; Zolfo, M.; Truong, D.T.; Scholz, M.; Armanini, F.; Ferretti, P.; Gorfer, V.; Pedrotti, A.; Tett, A.; et al. Studying Vertical Microbiome Transmission from Mothers to Infants by Strain-Level Metagenomic Profiling. mSystems 2017, 2, e00164-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solís, G.; de los Reyes-Gavilan, C.G.; Fernández, N.; Margolles, A.; Gueimonde, M. Establishment and Development of Lactic Acid Bacteria and Bifidobacteria Microbiota in Breast-Milk and the Infant Gut. Anaerobe 2010, 16, 307–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, K.; Curley, D.; O’Callaghan, T.F.; O’Shea, C.-A.; Dempsey, E.M.; O’Toole, P.W.; Ross, R.P.; Ryan, C.A.; Stanton, C. The Composition of Human Milk and Infant Faecal Microbiota Over the First Three Months of Life: A Pilot Study. Sci. Rep. 2017, 7, 40597. [Google Scholar] [CrossRef] [Green Version]
- Milani, C.; Mancabelli, L.; Lugli, G.A.; Duranti, S.; Turroni, F.; Ferrario, C.; Mangifesta, M.; Viappiani, A.; Ferretti, P.; Gorfer, V.; et al. Exploring Vertical Transmission of Bifidobacteria from Mother to Child. Appl. Env. Microbiol. 2015, 81, 7078–7087. [Google Scholar] [CrossRef] [Green Version]
- Kordy, K.; Gaufin, T.; Mwangi, M.; Li, F.; Cerini, C.; Lee, D.J.; Adisetiyo, H.; Woodward, C.; Pannaraj, P.S.; Tobin, N.H.; et al. Contributions to Human Breast Milk Microbiome and Enteromammary Transfer of Bifidobacterium Breve. PLoS ONE 2020, 15, e0219633. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.C.; Olm, M.R.; Diamond, S.; Crits-Christoph, A.; Firek, B.A.; Baker, R.; Morowitz, M.J.; Banfield, J.F. Infant Gut Strain Persistence Is Associated with Maternal Origin, Phylogeny, and Traits Including Surface Adhesion and Iron Acquisition. Cell Rep. Med. 2021, 2, 100393. [Google Scholar] [CrossRef]
- Duranti, S.; Lugli, G.A.; Mancabelli, L.; Armanini, F.; Turroni, F.; James, K.; Ferretti, P.; Gorfer, V.; Ferrario, C.; Milani, C.; et al. Maternal Inheritance of Bifidobacterial Communities and Bifidophages in Infants through Vertical Transmission. Microbiome 2017, 5, 66. [Google Scholar] [CrossRef]
- Cheema, A.S.; Gridneva, Z.; Furst, A.J.; Roman, A.S.; Trevenen, M.L.; Turlach, B.A.; Lai, C.T.; Stinson, L.F.; Bode, L.; Payne, M.S.; et al. Human Milk Oligosaccharides and Bacterial Profile Modulate Infant Body Composition during Exclusive Breastfeeding. IJMS 2022, 23, 2865. [Google Scholar] [CrossRef]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct Composition of Gut Microbiota during Pregnancy in Overweight and Normal-Weight Women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [CrossRef]
- Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E. Maternal Weight and Excessive Weight Gain during Pregnancy Modify the Immunomodulatory Potential of Breast Milk. Pediatr. Res. 2012, 72, 77–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, L. Human Milk Oligosaccharides: Every Baby Needs a Sugar Mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, L. The Functional Biology of Human Milk Oligosaccharides. Early Hum. Dev. 2015, 91, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, A.M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Human Milk Glycobiome and Its Impact on the Infant Gastrointestinal Microbiota. Proc. Natl. Acad. Sci. USA. 2011, 108, 4653–4658. [Google Scholar] [CrossRef] [Green Version]
- Soyyılmaz, B.; Mikš, M.H.; Röhrig, C.H.; Matwiejuk, M.; Meszaros-Matwiejuk, A.; Vigsnæs, L.K. The Mean of Milk: A Review of Human Milk Oligosaccharide Concentrations throughout Lactation. Nutrients 2021, 13, 2737. [Google Scholar] [CrossRef]
- Thum, C.; Wall, C.R.; Weiss, G.A.; Wang, W.; Szeto, I.M.-Y.; Day, L. Changes in HMO Concentrations throughout Lactation: Influencing Factors, Health Effects and Opportunities. Nutrients 2021, 13, 2272. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic Review of the Concentrations of Oligosaccharides in Human Milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, P.; Warren, C.D.; Altaye, M.; Morrow, A.L.; Ruiz-Palacios, G.; Pickering, L.K.; Newburg, D.S. Fucosylated Human Milk Oligosaccharides Vary between Individuals and over the Course of Lactation. Glycobiology 2001, 11, 365–372. [Google Scholar] [CrossRef]
- Coppa, G.; Pierani, P.; Zampini, L.; Carloni, I.; Carlucci, A.; Gabrielli, O. Oligosaccharides in Human Milk during Different Phases of Lactation. Acta Paediatr. 2007, 88, 89–94. [Google Scholar] [CrossRef]
- Han, S.M.; Derraik, J.G.B.; Binia, A.; Sprenger, N.; Vickers, M.H.; Cutfield, W.S. Maternal and Infant Factors Influencing Human Milk Oligosaccharide Composition: Beyond Maternal Genetics. J. Nutr. 2021, 151, 1383–1393. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, H.; Li, K.; Zheng, C.; Ju, M.; Lyu, Y.; Zhao, R.; Wang, W.; Zhang, W.; Xu, Y.; et al. Dynamic Changes in Human Milk Oligosaccharides in Chinese Population: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2912. [Google Scholar] [CrossRef] [PubMed]
- Seferovic, M.D.; Mohammad, M.; Pace, R.M.; Engevik, M.; Versalovic, J.; Bode, L.; Haymond, M.; Aagaard, K.M. Maternal Diet Alters Human Milk Oligosaccharide Composition with Implications for the Milk Metagenome. Sci Rep 2020, 10, 22092. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef] [Green Version]
- Quin, C.; Vicaretti, S.D.; Mohtarudin, N.A.; Garner, A.M.; Vollman, D.M.; Gibson, D.L.; Zandberg, W.F. Influence of Sulfonated and Diet-Derived Human Milk Oligosaccharides on the Infant Microbiome and Immune Markers. J. Biol. Chem. 2020, 295, 4035–4048. [Google Scholar] [CrossRef]
- Meyer, K.M.; Mohammad, M.; Bode, L.; Chu, D.M.; Ma, J.; Haymond, M.; Aagaard, K. 20: Maternal Diet Structures the Breast Milk Microbiome in Association with Human Milk Oligosaccharides and Gut-Associated Bacteria. Am. J. Obstet. Gynecol. 2017, 216, S15. [Google Scholar] [CrossRef] [Green Version]
- Selma-Royo, M.; González, S.; Gueimonde, M.; Chang, M.; Fürst, A.; Martínez-Costa, C.; Bode, L.; Collado, M.C. Maternal Diet Is Associated with Human Milk Oligosaccharide Profile. Mol. Nutr. Food Res 2022, 66, 2200058. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.W.; Lind, M.V.; Laursen, R.P.; Yonemitsu, C.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F.; Bode, L. Human Milk Oligosaccharide Composition Is Associated With Excessive Weight Gain During Exclusive Breastfeeding—An Explorative Study. Front. Pediatr. 2019, 7, 297. [Google Scholar] [CrossRef] [Green Version]
- Saben, J.L.; Sims, C.R.; Abraham, A.; Bode, L.; Andres, A. Human Milk Oligosaccharide Concentrations and Infant Intakes Are Associated with Maternal Overweight and Obesity and Predict Infant Growth. Nutrients 2021, 13, 446. [Google Scholar] [CrossRef]
- Samuel, T.M.; Binia, A.; de Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Costeira, M.J.; Marchini, G.; Martínez-Costa, C.; et al. Impact of Maternal Characteristics on Human Milk Oligosaccharide Composition over the First 4 Months of Lactation in a Cohort of Healthy European Mothers. Sci. Rep. 2019, 9, 11767. [Google Scholar] [CrossRef] [Green Version]
- Natividad, J.M.; Marsaux, B.; Rodenas, C.L.G.; Rytz, A.; Vandevijver, G.; Marzorati, M.; Van den Abbeele, P.; Calatayud, M.; Rochat, F. Human Milk Oligosaccharides and Lactose Differentially Affect Infant Gut Microbiota and Intestinal Barrier In Vitro. Nutrients 2022, 14, 2546. [Google Scholar] [CrossRef]
- Asakuma, S.; Hatakeyama, E.; Urashima, T.; Yoshida, E.; Katayama, T.; Yamamoto, K.; Kumagai, H.; Ashida, H.; Hirose, J.; Kitaoka, M. Physiology of Consumption of Human Milk Oligosaccharides by Infant Gut-Associated Bifidobacteria. J. Biol. Chem. 2011, 286, 34583–34592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masi, A.C.; Stewart, C.J. Untangling Human Milk Oligosaccharides and Infant Gut Microbiome. iScience 2022, 25, 103542. [Google Scholar] [CrossRef]
- Egan, M.; O’Connell Motherway, M.; Kilcoyne, M.; Kane, M.; Joshi, L.; Ventura, M.; van Sinderen, D. Cross-feeding by Bifidobacterium breve UCC2003 during co-cultivation with Bifidobacterium bifidum PRL2010 in a mucin-based medium. BMC Microbiol. 2014, 14, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, P.K.; Plows, J.F.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Ryoo, J.H.; Bode, L.; Goran, M.I. Human Milk Oligosaccharides and Hispanic Infant Weight Gain in the First 6 Months. Obesity 2020, 28, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Alderete, T.L.; Autran, C.; Brekke, B.E.; Knight, R.; Bode, L.; Goran, M.I.; Fields, D.A. Associations between Human Milk Oligosaccharides and Infant Body Composition in the First 6 Mo of Life. Am. J. Clin. Nutr. 2015, 102, 1381–1388. [Google Scholar] [CrossRef] [Green Version]
- Menzel, P.; Vogel, M.; Austin, S.; Sprenger, N.; Grafe, N.; Hilbert, C.; Jurkutat, A.; Kiess, W.; Binia, A. Concentrations of Oligosaccharides in Human Milk and Child Growth. BMC Pediatr. 2021, 21, 481. [Google Scholar] [CrossRef]
- Lagström, H.; Rautava, S.; Ollila, H.; Kaljonen, A.; Turta, O.; Mäkelä, J.; Yonemitsu, C.; Gupta, J.; Bode, L. Associations between Human Milk Oligosaccharides and Growth in Infancy and Early Childhood. Am. J. Clin. Nutr. 2020, 111, 769–778. [Google Scholar] [CrossRef]
- Binia, A.; Lavalle, L.; Chen, C.; Austin, S.; Agosti, M.; Al-Jashi, I.; Pereira, A.B.; Costeira, M.J.; Silva, M.G.; Marchini, G.; et al. Human Milk Oligosaccharides, Infant Growth, and Adiposity over the First 4 Months of Lactation. Pediatr. Res. 2021, 90, 684–693. [Google Scholar] [CrossRef]
- Davis, J.C.C.; Lewis, Z.T.; Krishnan, S.; Bernstein, R.M.; Moore, S.E.; Prentice, A.M.; Mills, D.A.; Lebrilla, C.B.; Zivkovic, A.M. Growth and Morbidity of Gambian Infants Are Influenced by Maternal Milk Oligosaccharides and Infant Gut Microbiota. Sci. Rep. 2017, 7, 40466. [Google Scholar] [CrossRef] [Green Version]
- Rozé, J.-C.; Hartweg, M.; Simon, L.; Billard, H.; Chen, Y.; Austin, S.; Boscher, C.; Moyon, T.; Darmaun, D.; Garcia Rodenas, C.L.; et al. Human Milk Oligosaccharides in Breast Milk and 2-Year Outcome in Preterm Infants: An Exploratory Analysis. Clin. Nutr. 2022, 41, 1896–1905. [Google Scholar] [CrossRef]
- Samuel, T.M.; Hartweg, M.; Lebumfacil, J.D.; Buluran, K.B.; Lawenko, R.B.; Estorninos, E.M.; Binia, A.; Sprenger, N. Dynamics of Human Milk Oligosaccharides in Early Lactation and Relation with Growth and Appetitive Traits of Filipino Breastfed Infants. Sci. Rep. 2022, 12, 17304. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Berger, B.; Carnielli, V.; Ksiazyk, J.; Lagström, H.; Sanchez Luna, M.; Migacheva, N.; Mosselmans, J.-M.; Picaud, J.-C.; Possner, M.; et al. Human Milk Oligosaccharides: 2′-Fucosyllactose (2′-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. Nutrients 2018, 10, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 121, pp. 91–119. ISBN 978-0-12-800100-4. [Google Scholar]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-Chain Fatty Acids in Control of Body Weight and Insulin Sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut Microbial Metabolites in Obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.M.; Reimann, F.; Blottiere, H.M. SCFAs Strongly Stimulate PYY Production in Human Enteroendocrine Cells. Sci. Rep. 2018, 8, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of Targeted Delivery of Propionate to the Human Colon on Appetite Regulation, Body Weight Maintenance and Adiposity in Overweight Adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef] [Green Version]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The Short-Chain Fatty Acid Acetate Reduces Appetite via a Central Homeostatic Mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [Green Version]
- Chambers, E.S.; Byrne, C.S.; Aspey, K.; Chen, Y.; Khan, S.; Morrison, D.J.; Frost, G. Acute Oral Sodium Propionate Supplementation Raises Resting Energy Expenditure and Lipid Oxidation in Fasted Humans. Diabetes Obes. Metab. 2018, 20, 1034–1039. [Google Scholar] [CrossRef]
- Canfora, E.E.; van der Beek, C.M.; Jocken, J.W.E.; Goossens, G.H.; Holst, J.J.; Olde Damink, S.W.M.; Lenaerts, K.; Dejong, C.H.C.; Blaak, E.E. Colonic Infusions of Short-Chain Fatty Acid Mixtures Promote Energy Metabolism in Overweight/Obese Men: A Randomized Crossover Trial. Sci. Rep. 2017, 7, 2360. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, P.; Flint, H.J. Diversity, Metabolism and Microbial Ecology of Butyrate-Producing Bacteria from the Human Large Intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic Distribution of Three Pathways for Propionate Production within the Human Gut Microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [Green Version]
- Duncan, S.H.; Holtrop, G.; Lobley, G.E.; Calder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of Acetate to Butyrate Formation by Human Faecal Bacteria. Br. J. Nutr. 2004, 91, 915–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henson, M.A.; Phalak, P. Suboptimal Community Growth Mediated through Metabolite Crossfeeding Promotes Species Diversity in the Gut Microbiota. PLoS Comput. Biol. 2018, 14, e1006558. [Google Scholar] [CrossRef]
- Prentice, P.M.; Schoemaker, M.H.; Vervoort, J.; Hettinga, K.; Lambers, T.T.; van Tol, E.A.F.; Acerini, C.L.; Olga, L.; Petry, C.J.; Hughes, I.A.; et al. Human Milk Short-Chain Fatty Acid Composition Is Associated with Adiposity Outcomes in Infants. J. Nutr. 2019, 149, 716–722. [Google Scholar] [CrossRef]
- Paparo, L.; Nocerino, R.; Ciaglia, E.; Di Scala, C.; De Caro, C.; Russo, R.; Trinchese, G.; Aitoro, R.; Amoroso, A.; Bruno, C.; et al. Butyrate as a Bioactive Human Milk Protective Component against Food Allergy. Allergy 2021, 76, 1398–1415. [Google Scholar] [CrossRef]
- Stinson, L.F.; Gay, M.C.L.; Koleva, P.T.; Eggesbø, M.; Johnson, C.C.; Wegienka, G.; du Toit, E.; Shimojo, N.; Munblit, D.; Campbell, D.E.; et al. Human Milk From Atopic Mothers Has Lower Levels of Short Chain Fatty Acids. Front. Immunol. 2020, 11, 1427. [Google Scholar] [CrossRef]
- Lönnerdal, B. Nutritional and Physiologic Significance of Human Milk Proteins. Am. J. Clin. Nutr. 2003, 77, 1537S–1543S. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, H.; Lonnerdal, B. Isolation and Function of a Receptor for Human Lactoferrin in Human Fetal Intestinal Brush-Border Membranes. Am. J. Physiol.—Gastrointest. Liver Physiol. 1991, 261, G841–G846. [Google Scholar] [CrossRef]
- Lönnerdal, B.; Iyer, S. Lactoferrin: Molecular Structure and Biological Function. Annu. Rev. Nutr. 1995, 15, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Jahani, S.; Shakiba, A.; Jahani, L. The Antimicrobial Effect of Lactoferrin on Gram-Negative and Gram-Positive Bacteria. Int. J. Infect. 2015, 2, e27594. [Google Scholar] [CrossRef] [Green Version]
- Farnaud, S.; Evans, R.W. Lactoferrin—A Multifunctional Protein with Antimicrobial Properties. Mol. Immunol. 2003, 40, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.R.; Brewer, M.; Gauthier, J.J. Bactericidal Activity of Human Lactoferrin: Sensitivity of a Variety of Microorganisms. Infect. Immun. 1980, 28, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral Properties of Lactoferrin—A Natural Immunity Molecule. Molecules 2011, 16, 6992–7018. [Google Scholar] [CrossRef] [Green Version]
- Jenssen, H. Anti Herpes Simplex Virus Activity of Lactoferrin/Lactoferricin—An Example of Antiviral Activity of Antimicrobial Protein/Peptide. Cell. Mol. Life Sci. 2005, 62, 3002–3013. [Google Scholar] [CrossRef]
- Ikeda, M.; Nozaki, A.; Sugiyama, K.; Tanaka, T.; Naganuma, A.; Tanaka, K.; Sekihara, H.; Shimotohno, K.; Saito, M.; Kato, N. Characterization of Antiviral Activity of Lactoferrin against Hepatitis C Virus Infection in Human Cultured Cells. Virus Res. 2000, 66, 51–63. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Carter, D.A. The Antifungal Activity of Lactoferrin and Its Derived Peptides: Mechanisms of Action and Synergy with Drugs against Fungal Pathogens. Front. Microbiol. 2017, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Soukka, T.; Tenovuo, J.; Lenander-Lumikari, M. Fungicidal Effect of Human Lactoferrin against Candida Albicans. FEMS Microbiol. Lett. 1992, 90, 223–228. [Google Scholar] [CrossRef]
- Kirkpatrick, C.H.; Green, I.; Rich, R.R.; Schade, A.L. Inhibition of Growth of Candida Albicans by Iron-Unsaturated Lactoferrin: Relation to Host-Defense Mechanisms in Chronic Mucocutaneous Candidiasis. J. Infect. Dis. 1971, 124, 539–544. [Google Scholar] [CrossRef]
- Aguilar-Diaz, H.; Canizalez-Roman, A.; Nepomuceno-Mejia, T.; Gallardo-Vera, F.; Hornelas-Orozco, Y.; Nazmi, K.; Bolscher, J.G.M.; Carrero, J.C.; Leon-Sicairos, C.; Leon-Sicairos, N. Parasiticidal Effect of Synthetic Bovine Lactoferrin Peptides on the Enteric Parasite Giardia Intestinalis. Biochem. Cell Biol. 2017, 95, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Soto, F.; León-Sicairos, N.; Nazmi, K.; Bolscher, J.G.; de la Garza, M. Microbicidal Effect of the Lactoferrin Peptides Lactoferricin17–30, Lactoferrampin265–284, and Lactoferrin Chimera on the Parasite Entamoeba Histolytica. Biometals 2010, 23, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, G.A. Iron Chelators as Therapeutic Agents against Pneumocystis Carinii. Antimicrob. Agents Chemother. 1994, 38, 997–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liepke, C.; Adermann, K.; Raida, M.; Mägert, H.-J.; Forssmann, W.-G.; Zucht, H.-D. Human Milk Provides Peptides Highly Stimulating the Growth of Bifidobacteria: Human Milk Peptides as Bifidus Factors. Eur. J. Biochem. 2002, 269, 712–718. [Google Scholar] [CrossRef]
- Kim, W.-S.; Ohashi, M.; Tanaka, T.; Kumura, H.; Kim, G.-Y.; Kwon, I.-K.; Goh, J.-S.; Shimazaki, K. Growth-Promoting Effects of Lactoferrin on L. Acidophilus and Bifidobacterium Spp. Biometals 2004, 17, 279–283. [Google Scholar] [CrossRef]
- Oda, H.; Wakabayashi, H.; Yamauchi, K.; Abe, F. Lactoferrin and Bifidobacteria. Biometals 2014, 27, 915–922. [Google Scholar] [CrossRef]
- Ellison, R.T.; Giehl, T.J. Killing of Gram-Negative Bacteria by Lactoferrin and Lysozyme. J. Clin. Invest. 1991, 88, 1080–1091. [Google Scholar] [CrossRef] [Green Version]
- Chipman, D.M.; Sharon, N. Mechanism of Lysozyme Action: Lysozyme Is the First Enzyme for Which the Relation between Structure and Function Has Become Clear. Science 1969, 165, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Maga, E.A.; Desai, P.T.; Weimer, B.C.; Dao, N.; Kültz, D.; Murray, J.D. Consumption of Lysozyme-Rich Milk Can Alter Microbial Fecal Populations. Appl. Env. Microbiol. 2012, 78, 6153–6160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marra, A.; Hanson, M.A.; Kondo, S.; Erkosar, B.; Lemaitre, B. Drosophila Antimicrobial Peptides and Lysozymes Regulate Gut Microbiota Composition and Abundance. mBio 2021, 12, e00824-21. [Google Scholar] [CrossRef] [PubMed]
- Blais, A.; Fan, C.; Voisin, T.; Aattouri, N.; Dubarry, M.; Blachier, F.; Tomé, D. Effects of Lactoferrin on Intestinal Epithelial Cell Growth and Differentiation: An in Vivo and in Vitro Study. Biometals 2014, 27, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Naot, D.; Grey, A.; Reid, I.R.; Cornish, J. Lactoferrin—A Novel Bone Growth Factor. Clin. Med. Res. 2005, 3, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Nichols, B.L.; Mckee, K.S.; Henry, J.F.; Putman, M. Human Lactoferrin Stimulates Thymidine Incorporation into DNA of Rat Crypt Cells. Pediatr. Res. 1987, 21, 563–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernell, O.; Lönnerdal, B. Iron Status of Infants Fed Low-Iron Formula: No Effect of Added Bovine Lactoferrin or Nucleotides. Am. J. Clin. Nutr. 2002, 76, 858–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochoa, T.J.; Chea-Woo, E.; Campos, M.; Pecho, I.; Prada, A.; McMahon, R.J.; Cleary, T.G. Impact of Lactoferrin Supplementation on Growth and Prevalence of Giardia Colonization in Children. Clin. Infect. Dis. 2008, 46, 1881–1883. [Google Scholar] [CrossRef] [Green Version]
- Bol’shakova, A.M.; Shcherbakova, E.G.; Ivanova, S.D.; Medvedeva, M.M.; Zhuravleva, T.P. Lysozyme in the feeding of premature infants with mixed pathology. Antibiotiki 1984, 29, 784–790. [Google Scholar]
- Braun, O.H.; Sandkühler, H. Relationships Between Lysozyme Concentration of Human Milk, Bacteriologic Content, and Weight Gain of Premature Infants. J. Pediatric Gastroenterol. Nutr. 1985, 4, 583–586. [Google Scholar] [CrossRef]
- Gridneva, Z.; Lai, C.T.; Rea, A.; Tie, W.J.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Human Milk Immunomodulatory Proteins Are Related to Development of Infant Body Composition during the First Year of Lactation. Pediatr. Res. 2021, 89, 911–921. [Google Scholar] [CrossRef]
- Aakko, J.; Kumar, H.; Rautava, S.; Wise, A.; Autran, C.; Bode, L.; Isolauri, E.; Salminen, S. Human Milk Oligosaccharide Categories Define the Microbiota Composition in Human Colostrum. Benef. Microbes 2017, 8, 563–567. [Google Scholar] [CrossRef]
- Moossavi, S.; Atakora, F.; Miliku, K.; Sepehri, S.; Robertson, B.; Duan, Q.L.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Moraes, T.J.; et al. Integrated Analysis of Human Milk Microbiota With Oligosaccharides and Fatty Acids in the CHILD Cohort. Front. Nutr. 2019, 6, 58. [Google Scholar] [CrossRef]

| Anthropometrics | |
|---|---|
| Weight | Positive 3′SL * [59,78,87,89], LNnT [84], 2′FL * [59,77,87], LNFP II [78], 3FL * [59,78,87], DSLNT [84], DFLac * [59,77,87], LSTb ** [78], DFLNH * [59], DSLNH ** [78], DFLNT [59], 6′GL [91] |
| Negative LNnT [77,87], 6′SL ** [59], LNFP II [84], LNT [83], LNFP I [85], LSTb [87], LSTc [89], DFLNH [77], MFLNH III [91] | |
| Length | Positive 3′SL * [59,86], LNnT [86], 2′FL [87,90], 3FL ** [59], DFLNH * [59], A-tetra [90], DFLNT [59], LNnDFH [91] |
| Negative 3′SL [88], LNnT [59,86,87,88], LNT [86], 3FL [91], LNFP I [86], LSTb [87], MFLNH III [91], LNFP V [86], FLNH [86] | |
| Weight for length | Positive 3′SL [88], LNFP II [78], LNT [78], 3FL ** [78], LSTb ** [78], LSTc [88], LDFT [83], IFLNH 1 [83] |
| Negative LNFP II [83], LNT [83], LSTa [83], DFLNHc [83] | |
| Head circumference | Positive 3′SL [77,83], 6′SL [90], LNFP III [88], DFLac [77], MFLNH III [88], LDFT [91], A-tetra [88], LNDFH I [91], LNnDFH [91] |
| Negative 6′SL [83], LNFP III [89], LNT [83], LNFP I [89], DFLNH [77], MFLNH III [91], LNnFP [86], DFLNHa [89], LNFP V [86] | |
| Body mass index | Positive DFLac ** [59], LSTb ** [59], DFLNT ** [59] |
| Negative LNnT [87], 2′FL [86], 6′SL [77], LNT [86], LNnFP [86], LNFP V [86] | |
| Body composition | |
| Fat mass | Positive 3′SL ** [78], 2′FL * [59], 6′SL ** [78], LNFP III ** [78], LNFP II * [78,85], DSLNT * [78,85], LSTb ** [78], FDSLNH [85], DSLNH ** [78], DFLNT [59] |
| Negative LNnT [77,85], 6′SL ** [59], LNFP III ** [59] LNFP I [85], DFLNH [77] | |
| Fat-free mass | Positive 3′SL * [59,90], 3FL ** [59], DFLac ** [59], DFLNH * [59], DFLNT ** [59] |
| Negative LNT [90], LNFP I [85], LSTc [90] | |
| Fat mass/fat-free mass ratio | Negative LNFP III ** [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Palmer, D.J.; Geddes, D.; Lai, C.T.; Stinson, L. Human Milk Microbiome and Microbiome-Related Products: Potential Modulators of Infant Growth. Nutrients 2022, 14, 5148. https://doi.org/10.3390/nu14235148
Ma J, Palmer DJ, Geddes D, Lai CT, Stinson L. Human Milk Microbiome and Microbiome-Related Products: Potential Modulators of Infant Growth. Nutrients. 2022; 14(23):5148. https://doi.org/10.3390/nu14235148
Chicago/Turabian StyleMa, Jie, Debra J. Palmer, Donna Geddes, Ching Tat Lai, and Lisa Stinson. 2022. "Human Milk Microbiome and Microbiome-Related Products: Potential Modulators of Infant Growth" Nutrients 14, no. 23: 5148. https://doi.org/10.3390/nu14235148
APA StyleMa, J., Palmer, D. J., Geddes, D., Lai, C. T., & Stinson, L. (2022). Human Milk Microbiome and Microbiome-Related Products: Potential Modulators of Infant Growth. Nutrients, 14(23), 5148. https://doi.org/10.3390/nu14235148