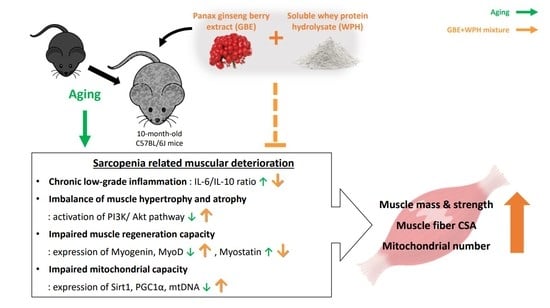
The Panax ginseng Berry Extract and Soluble Whey Protein Hydrolysate Mixture Ameliorates Sarcopenia-Related Muscular Deterioration in Aged Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of GBE and WPH
2.2. Mice and Design of Experiment
2.3. Assessment of Serum Cytokine Levels
2.4. Histological Analysis
2.5. Western Blotting
2.6. Quantitative Real Time-PCR (qRT-PCR)
2.7. Statistics
3. Results
3.1. The GBE + WPH Mixture Alleviates the Chronic Low-Grade Inflammation in Aged Mice
3.2. The GBE + WPH Mixture Suppresses Muscle Weakness and Increases Muscle Mass in Aged Mice
3.3. The GBE + WPH Mixture Activates PI3K/Akt Pathway in Skeletal Muscle of Aged Mice
3.4. The GBE + WPH Mixture Improves Muscle Regeneration and Mitochondrial Biogenesis in Skeletal Muscle of Aged Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Doherty, T.J. Invited review: Aging and sarcopenia. J. Appl. Physiol. 2003, 95, 1717–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marty, E.; Liu, Y.; Samuel, A.; Or, O.; Lane, J. A review of sarcopenia: Enhancing awareness of an increasingly prevalent disease. Bone 2017, 105, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, J.; Geerlings, M.A.; Reijnierse, E.M.; Phassouliotis, C.; Lim, W.K.; Maier, A.B. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta-analysis. Exp. Gerontol. 2020, 131, 110801. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Leung, K.-S.; Chow, S.K.-H.; Cheung, W.-H. Inflammation and age-associated skeletal muscle deterioration (sarcopaenia). J. Orthop. Transl. 2017, 10, 94–101. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, B.; Liang, C.; Li, Y.; Song, Y.-H. Cytokine signaling in skeletal muscle wasting. Trends Endocrinol. Metab. 2016, 27, 335–347. [Google Scholar] [CrossRef]
- Ko, F.; Abadir, P.; Marx, R.; Westbrook, R.; Cooke, C.; Yang, H.; Walston, J. Impaired mitochondrial degradation by autophagy in the skeletal muscle of the aged female interleukin 10 null mouse. Exp. Gerontol. 2016, 73, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Rong, Y.-D.; Bian, A.-L.; Hu, H.-Y.; Ma, Y.; Zhou, X.-Z. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018, 18, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Su, J.; Xie, Y.; Yin, M.T.; Huang, Y.; Xu, L.; Zhou, Q.; Zhu, B. Plasma IL-6/IL-10 ratio and IL-8, LDH, and HBDH level predict the severity and the risk of death in AIDS patients with Pneumocystis pneumonia. J. Immunol. Res. 2016, 2016, 10. [Google Scholar] [CrossRef] [Green Version]
- Sapan, H.B.; Paturusi, I.; Jusuf, I.; Patellongi, I.; Massi, M.N.; Pusponegoro, A.D.; Arief, S.K.; Labeda, I.; Islam, A.A.; Rendy, L. Pattern of cytokine (IL-6 and IL-10) level as inflammation and anti-inflammation mediator of multiple organ dysfunction syndrome (MODS) in polytrauma. Int. J. Burn. Trauma 2016, 6, 37. [Google Scholar]
- Ziaaldini, M.M.; Marzetti, E.; Picca, A.; Murlasits, Z. Biochemical pathways of sarcopenia and their modulation by physical exercise: A narrative review. Front. Med. 2017, 4, 167. [Google Scholar] [CrossRef]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Models Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Distefano, G.; Goodpaster, B.H. Effects of exercise and aging on skeletal muscle. Cold Spring Harb. Perspect. Med. 2018, 8, a029785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coen, P.M.; Musci, R.V.; Hinkley, J.M.; Miller, B.F. Mitochondria as a target for mitigating sarcopenia. Front. Physiol. 2019, 9, 1883. [Google Scholar] [CrossRef] [Green Version]
- Yamakawa, H.; Kusumoto, D.; Hashimoto, H.; Yuasa, S. Stem cell aging in skeletal muscle regeneration and disease. Int. J. Mol. Sci. 2020, 21, 1830. [Google Scholar] [CrossRef] [Green Version]
- Narici, M.V.; Maffulli, N. Sarcopenia: Characteristics, mechanisms and functional significance. Br. Med. Bull. 2010, 95, 139–159. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.-Z.; No, M.-H.; Heo, J.-W.; Park, D.-H.; Kang, J.-H.; Kim, S.H.; Kwak, H.-B. Role of exercise in age-related sarcopenia. J. Exerc. Rehabil. 2018, 14, 551. [Google Scholar] [CrossRef]
- Han, M.J.; Shin, J.E.; Park, S.J.; Choung, S.-Y. Synergetic effect of soluble whey protein hydrolysate and Panax ginseng berry extract on muscle atrophy in hindlimb-immobilized C57BL/6 mice. J. Ginseng Res. 2021. [Google Scholar] [CrossRef]
- Afzali, A.M.; Müntefering, T.; Wiendl, H.; Meuth, S.G.; Ruck, T. Skeletal muscle cells actively shape (auto) immune responses. Autoimmun. Rev. 2018, 17, 518–529. [Google Scholar] [CrossRef]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Domingues-Faria, C.; Vasson, M.-P.; Goncalves-Mendes, N.; Boirie, Y.; Walrand, S. Skeletal muscle regeneration and impact of aging and nutrition. Ageing Res. Rev. 2016, 26, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Brace, C.S.; Rensing, N.; Cliften, P.; Wozniak, D.F.; Herzog, E.D.; Yamada, K.A.; Imai, S.-I. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013, 18, 416–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.E.; Park, S.J.; Ahn, S.I.; Choung, S.-Y. Soluble whey protein hydrolysate ameliorates muscle atrophy induced by immobilization via regulating the PI3K/Akt pathway in C57BL/6 mice. Nutrients 2020, 12, 3362. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, K.; Schjerling, P.; Pedersen, B.K. Physical activity and plasma interleukin-6 in humans–effect of intensity of exercise. Eur. J. Appl. Physiol. 2000, 83, 512–515. [Google Scholar] [CrossRef]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol. 1999, 515, 287–291. [Google Scholar] [CrossRef]
- Dagdeviren, S.; Young Jung, D.; Friedline, R.H.; Noh, H.L.; Kim, J.H.; Patel, P.R.; Tsitsilianos, N.; Inashima, K.; Tran, D.A.; Hu, X. IL-10 prevents aging-associated inflammation and insulin resistance in skeletal muscle. FASEB J. 2017, 31, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Del Campo, A.; Contreras-Hernández, I.; Castro-Sepúlveda, M.; Campos, C.A.; Figueroa, R.; Tevy, M.F.; Eisner, V.; Casas, M.; Jaimovich, E. Muscle function decline and mitochondria changes in middle age precede sarcopenia in mice. Aging 2018, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Talbot, J.; Maves, L. Skeletal muscle fiber type: Using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 518–534. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Jiang, F.; Wei, K.; Jiang, Z. Exercise activates the PI3K-AKT signal pathway by decreasing the expression of 5α-reductase type 1 in PCOS rats. Sci. Rep. 2018, 8, 7982. [Google Scholar] [CrossRef]
- Zhu, S.; Tian, Z.; Torigoe, D.; Zhao, J.; Xie, P.; Sugizaki, T.; Sato, M.; Horiguchi, H.; Terada, K.; Kadomatsu, T. Aging-and obesity-related peri-muscular adipose tissue accelerates muscle atrophy. PLoS ONE 2019, 14, e0221366. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.; Vernus, B.; Chelh, I.; Cassar-Malek, I.; Gabillard, J.-C.; Sassi, A.H.; Seiliez, I.; Picard, B.; Bonnieu, A. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell. Mol. Life Sci. 2014, 71, 4361–4371. [Google Scholar] [CrossRef] [PubMed]
- Dillon, L.M.; Rebelo, A.P.; Moraes, C.T. The role of PGC-1 coactivators in aging skeletal muscle and heart. IUBMB Life 2012, 64, 231–241. [Google Scholar] [CrossRef] [PubMed]




| Gene | Sequences | |
|---|---|---|
| 16S rRNA | F: CCGCAAGGGAAAGATGAAAGAC | R: TCGTTTGGTTTCGGGGTTTC |
| Hexokinase 2 | F: GCCAGCCTCTCCTGATTTTAGTGT | R: GGGAACACAAAAGACCTCTTCTGG |
| Myostatin | F: ACTGGACCTCTCGATAGAACACT | R: ACTTAGTGCTGTGTGTGTGGAGAT |
| Myogenin | F: TGGTCCCAACCCAGGAGATCATTT | R: ACATATCCTCCACCGTGATGCTGT |
| MyoD | F: CAACTGCTCTGATGGCATGATGG | R: TGTTCTGCATCGCTTGAGGATGTC |
| Sirt1 | F: CAAGATGCTGTTGCAAAGGAACC | R: CAAGATGCTGTTGCAAAGGAACC |
| PGC1α | F: AAGTGTGGAACTCTCTGGAACTG | R: GGGTTATCTTGGTTGGCTTTATG |
| β-actin | F: ATATCGCTGCGCTGGTCGTC | R: AGGATGGCGTGAGGGAGAGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.-J.; Park, S.-J.; Lee, S.-J.; Choung, S.-Y. The Panax ginseng Berry Extract and Soluble Whey Protein Hydrolysate Mixture Ameliorates Sarcopenia-Related Muscular Deterioration in Aged Mice. Nutrients 2022, 14, 799. https://doi.org/10.3390/nu14040799
Han M-J, Park S-J, Lee S-J, Choung S-Y. The Panax ginseng Berry Extract and Soluble Whey Protein Hydrolysate Mixture Ameliorates Sarcopenia-Related Muscular Deterioration in Aged Mice. Nutrients. 2022; 14(4):799. https://doi.org/10.3390/nu14040799
Chicago/Turabian StyleHan, Min-Ji, Seok-Jun Park, Sang-Jun Lee, and Se-Young Choung. 2022. "The Panax ginseng Berry Extract and Soluble Whey Protein Hydrolysate Mixture Ameliorates Sarcopenia-Related Muscular Deterioration in Aged Mice" Nutrients 14, no. 4: 799. https://doi.org/10.3390/nu14040799
APA StyleHan, M. -J., Park, S. -J., Lee, S. -J., & Choung, S. -Y. (2022). The Panax ginseng Berry Extract and Soluble Whey Protein Hydrolysate Mixture Ameliorates Sarcopenia-Related Muscular Deterioration in Aged Mice. Nutrients, 14(4), 799. https://doi.org/10.3390/nu14040799