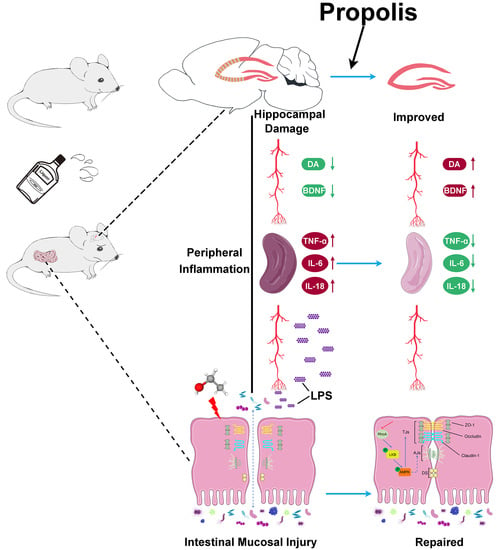
Propolis Ameliorates Alcohol-Induced Depressive Symptoms in C57BL/6J Mice by Regulating Intestinal Mucosal Barrier Function and Inflammatory Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Propolis Preparation
2.2. Animals, Experimental Models and Intervention Strategy
2.3. Sucrose Preference Test (SPT)
2.4. Open-Field Test (OFT)
2.5. Elevated Plus Maze (EPM)
2.6. Forced Swim Test (FST)
2.7. Preparation of Specimens
2.8. Histopathology
2.9. ELISA (Enzyme-Linked Immunosorbent Assays)
2.10. Immunofluorescence
2.11. Western Blotting
2.12. Tracer Experiment
2.13. Statistical Analysis
3. Results
3.1. Body Weight and Daily Food Consumption
3.2. Propolis Ameliorates Alcohol-Induced Behavioral Deficits
3.3. Propolis Treatment Attenuates the Alcohol-Induced Injury of Nerve Cells in the Hippocampal CA3 Region in the Brain
3.4. Propolis Diminishes the Alcohol-Induced Decrease in BDNF and Monoamine Expression in the Serum of Mice
3.5. Effect of Propolis on the Intestinal Mucosal Barrier in Alcohol Exposed Mice
3.6. Propolis Alleviates the Alcohol-Induced Injury of Intestinal Permeability
3.7. Effect of Propolis on the LKB1/AMPK Signaling Pathway
3.8. Propolis Reduces the Alcohol-Induced Upregulated LPS and FABP2 Levels in the Serum of Mice
3.9. Propolis Attenuates Alcohol-Induced Inflammatory Cytokine Release in the Spleen of Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Friedrich, M.J. Depression is the leading cause of disability around the world. JAMA 2017, 317, 1517. [Google Scholar] [CrossRef] [PubMed]
- Cui, R. Editorial (Thematic selection: A systematic review of depression). CN 2015, 13, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. World Health Organization Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017.
- Hammen, C. Risk factors for depression: An autobiographical review. Annu. Rev. Clin. Psychol. 2018, 14, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Boschloo, L.; Vogelzangs, N.; van den Brink, W.; Smit, J.H.; Veltman, D.J.; Beekman, A.T.F.; Penninx, B.W.J.H. Alcohol use disorders and the course of depressive and anxiety disorders. Br. J. Psychiatry 2012, 200, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, L.E.; Fiellin, D.A.; O’Connor, P.G. The prevalence and impact of alcohol problems in major depression: A systematic review. Am. J. Med. 2005, 118, 330–341. [Google Scholar] [CrossRef]
- Boden, J.M.; Fergusson, D.M. Alcohol and depression: Alcohol and depression. Addiction 2011, 106, 906–914. [Google Scholar] [CrossRef]
- Schuckit, M.A. Alcohol-use disorders. Lancet 2009, 373, 492–501. [Google Scholar] [CrossRef]
- Grant, B.F.; Goldstein, R.B.; Saha, T.D.; Chou, S.P.; Jung, J.; Zhang, H.; Pickering, R.P.; Ruan, W.J.; Smith, S.M.; Huang, B.; et al. Epidemiology of DSM-5 alcohol use disorder: Results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry 2015, 72, 757. [Google Scholar] [CrossRef] [Green Version]
- Degenhardt, L.; Charlson, F.; Ferrari, A.; Santomauro, D.; Erskine, H.; Mantilla-Herrara, A.; Whiteford, H.; Leung, J.; Naghavi, M.; Griswold, M.; et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Psychiatry 2018, 5, 987–1012. [Google Scholar] [CrossRef] [Green Version]
- Qamar, N.; Castano, D.; Patt, C.; Chu, T.; Cottrell, J.; Chang, S.L. Meta-analysis of alcohol induced gut dysbiosis and the resulting behavioral impact. Behav. Brain Res. 2019, 376, 112196. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Leclercq, S.; le Roy, T.; Furgiuele, S.; Coste, V.; Bindels, L.B.; Leyrolle, Q.; Neyrinck, A.M.; Quoilin, C.; Amadieu, C.; Petit, G.; et al. Gut microbiota-induced changes in β-hydroxybutyrate metabolism are linked to altered sociability and depression in alcohol use disorder. Cell Rep. 2020, 33, 108238. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.J.; Marshall, S.A.; Thiele, T.E. Assessment of depression-like behavior and anhedonia after repeated cycles of binge-like ethanol drinking in male C57BL/6J mice. Pharmacol. Biochem. Behav. 2018, 168, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Table of Contents. Alc. Drug Abuse Wkly. 2009, 21, 1–8. [CrossRef]
- Foster, J.A.; McVey Neufeld, K.-A. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Chen, Y.-C.; Chen, S.-J.; Lee, C.-H.; Cheng, C.-M. Alcohol addiction, gut microbiota, and alcoholism treatment: A review. Int. J. Mol. Sci. 2020, 21, 6413. [Google Scholar] [CrossRef]
- Leclercq, S.; Matamoros, S.; Cani, P.D.; Neyrinck, A.M.; Jamar, F.; Stärkel, P.; Windey, K.; Tremaroli, V.; Bäckhed, F.; Verbeke, K.; et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. USA 2014, 111, E4485–E4493. [Google Scholar] [CrossRef] [Green Version]
- Koopman, M.; El Aidy, S. Depressed gut? The microbiota-diet-inflammation trialogue in depression. Curr. Opin. Psychiatry 2017, 30, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Setiawan, E.; Wilson, A.A.; Mizrahi, R.; Rusjan, P.M.; Miler, L.; Rajkowska, G.; Suridjan, I.; Kennedy, J.L.; Rekkas, P.V.; Houle, S.; et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 2015, 72, 268. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [Green Version]
- Brunoni, A.R.; Supasitthumrong, T.; Teixeira, A.L.; Vieira, E.L.; Gattaz, W.F.; Benseñor, I.M.; Lotufo, P.A.; Lafer, B.; Berk, M.; Carvalho, A.F.; et al. Differences in the immune-inflammatory profiles of unipolar and bipolar depression. J. Affect. Disord. 2020, 262, 8–15. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.; Corfe, B. The role of diet and nutrition on mental health and wellbeing. Proc. Nutr. Soc. 2017, 76, 425–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adan, R.A.H.; van der Beek, E.M.; Buitelaar, J.K.; Cryan, J.F.; Hebebrand, J.; Higgs, S.; Schellekens, H.; Dickson, S.L. Nutritional psychiatry: Towards improving mental health by what you eat. Eur. Neuropsychopharmacol. 2019, 29, 1321–1332. [Google Scholar] [CrossRef]
- Osés, S.M.; Pascual-Maté, A.; Fernández-Muiño, M.A.; López-Díaz, T.M.; Sancho, M.T. Bioactive properties of honey with propolis. Food Chem. 2016, 196, 1215–1223. [Google Scholar] [CrossRef]
- Franchin, M.; Freires, I.A.; Lazarini, J.G.; Nani, B.D.; da Cunha, M.G.; Colón, D.F.; de Alencar, S.M.; Rosalen, P.L. The use of brazilian propolis for discovery and development of novel anti-inflammatory drugs. Eur. J. Med. Chem. 2018, 153, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, D.; Miryan, M.; Tutunchi, H.; Navashenaq, J.G.; Sadeghi, E.; Ghayour-Mobarhan, M.; Ferns, G.A.; Ostadrahimi, A. A systematic review of preclinical studies on the efficacy of propolis for the treatment of inflammatory bowel disease. Phytother. Res. 2021, 35, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Liu, Y.; Xu, H.; Zhou, Z.; Ma, Y.; Sun, T.; Liu, M.; Zhang, H.; Liang, H. Propolis modulates the gut microbiota and improves the intestinal mucosal barrier function in diabetic rats. Biomed. Pharmacother. 2019, 118, 109393. [Google Scholar] [CrossRef]
- Roquetto, A.R.; Monteiro, N.E.S.; Moura, C.S.; Toreti, V.C.; de Pace, F.; dos Santos, A.; Park, Y.K.; Amaya-Farfan, J. Green propolis modulates gut microbiota, reduces endotoxemia and expression of TLR4 pathway in mice fed a high-fat diet. Food Res. Int. 2015, 76, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Kashiwakura, J.; Yoshihara, M.; Saitoh, K.; Kagohashi, K.; Sasaki, Y.; Kobayashi, F.; Inagaki, I.; Kitai, Y.; Muromoto, R.; Matsuda, T. Propolis suppresses cytokine production in activated basophils and basophil-mediated skin and intestinal allergic inflammation in mice. Allergol. Int. 2021, 70, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, G.; Beta, T.; Dong, J. Inhibitory properties of aqueous ethanol extracts of propolis on alpha-glucosidase. Evid. -Based Complement. Altern. Med. 2015, 2015, 587383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Liu, Y.; Gao, M.; Xue, M.; Wang, Z.; Liang, H. Nicotinamide riboside alleviates alcohol-induced depression-like behaviours in C57BL/6J mice by altering the intestinal microbiota associated with microglial activation and BDNF expression. Food Funct. 2020, 11, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, X.; Gao, M.-Q.; Xue, M.; Xu, H.; Chang, Z.; Jiang, Y.; Liang, H. Aplysin retards pancreatic necrosis and inflammatory responses in NOD Mice by stabilizing intestinal barriers and regulating gut microbial composition. Mediat. Inflamm. 2020, 2020, 1280130. [Google Scholar] [CrossRef]
- Yeomans, M.R.; Caton, S.; Hetherington, M.M. Alcohol and food intake. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 639–644. [Google Scholar] [CrossRef]
- Björkholm, C.; Monteggia, L.M. BDNF—A key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a promising therapeutic agent in Parkinson’s disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H. Reward and aversion. Annu. Rev. Neurosci. 2016, 39, 297–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, T.; Zhao, C.; Li, F.; Gu, Z.; Liu, L.; Zhang, L.; Wang, Y.; He, L.; Liu, Y.; Liu, Q.; et al. Intestinal HIF-1α deletion exacerbates alcoholic liver disease by inducing intestinal dysbiosis and barrier dysfunction. J. Hepatol. 2018, 69, 886–895. [Google Scholar] [CrossRef]
- Antón, M.; Rodríguez-González, A.; Ballesta, A.; González, N.; del Pozo, A.; de Fonseca, F.R.; Gómez-Lus, M.L.; Leza, J.C.; García-Bueno, B.; Caso, J.R.; et al. Alcohol binge disrupts the rat intestinal barrier: The partial protective role of oleoylethanolamide: OEA in ethanol-induced gut dysfunction. Br. J. Pharmacol. 2018, 175, 4464–4479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schouten, M.J.E.; Christ, C.; Dekker, J.J.M.; Riper, H.; Goudriaan, A.E.; Blankers, M. Digital interventions for people with co-occurring depression and problematic alcohol use: A systematic review and meta-analysis. Alcohol Alcohol. 2021, 57, 113–124. [Google Scholar] [CrossRef]
- Menezes da Silveira, C.C.S.; Luz, D.A.; Silva, C.C.S.; Prediger, R.D.S.; Martins, M.D.; Martins, M.A.T.; Fontes-Júnior, E.A.; Maia, C.S.F. Propolis: A useful agent on psychiatric and neurological disorders? A focus on CAPE and pinocembrin components. Med. Res. Rev. 2021, 41, 1195–1215. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.X.; Chen, Y.M.; He, J.; Zeng, T.; Wang, J.H. Effects of morphine and its withdrawal on Y-maze spatial recognition memory in mice. Neuroscience 2007, 147, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The role of neural plasticity in depression: From hippocampus to prefrontal cortex. Neural Plast. 2017, 2017, 6871089. [Google Scholar] [CrossRef] [Green Version]
- Petrik, D.; Lagace, D.C.; Eisch, A.J. The neurogenesis hypothesis of affective and anxiety disorders: Are we mistaking the scaffolding for the building? Neuropharmacology 2012, 62, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, B.L.; van Praag, H.; Gage, F.H. Adult brain neurogenesis and psychiatry: A novel theory of depression. Mol. Psychiatry 2000, 5, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Mira, R.G.; Tapia-Rojas, C.; Pérez, M.J.; Jara, C.; Vergara, E.H.; Quintanilla, R.A.; Cerpa, W. Alcohol impairs hippocampal function: From NMDA receptor synaptic transmission to mitochondrial function. Drug Alcohol Depend. 2019, 205, 107628. [Google Scholar] [CrossRef] [PubMed]
- Malberg, J.; Schechter, L. Increasing hippocampal neurogenesis: A novel mechanism for antidepressant drugs. CPD 2005, 11, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C. Neurogenesis and antidepressant action. Cell Tissue Res. 2019, 377, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and Synaptic Plasticity, Cognitive Function, and Dysfunction. In Neurotrophic Factors; Lewin, G.R., Carter, B.D., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 220, pp. 223–250. [Google Scholar] [CrossRef]
- Yang, W.; Liu, M.; Zhang, Q.; Zhang, J.; Chen, J.; Chen, Q.; Suo, L. Knockdown of MiR-124 reduces depression-like behavior by targeting CREB1 and BDNF. CNR 2020, 17, 196–203. [Google Scholar] [CrossRef]
- Luo, L.; Li, C.; Du, X.; Shi, Q.; Huang, Q.; Xu, X.; Wang, Q. Effect of aerobic exercise on BDNF/ProBDNF expression in the ischemic hippocampus and depression recovery of rats after stroke. Behav. Brain Res. 2019, 362, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.W.; Chaudhury, D.; Han, M.-H.; Nestler, E.J. Role of mesolimbic brain-derived neurotrophic factor in depression. Biol. Psychiatry 2019, 86, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Seroogy, K.B.; Lundgren, K.H.; Tran, T.M.D.; Guthrie, K.M.; Isackson, P.J.; Gall, C.M. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 MRNAs. J. Comp. Neurol. 1994, 342, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wu, X.; Hu, X.; Wang, T.; Jin, F. Recognizing depression from the microbiota–gut–brain axis. Int. J. Mol. Sci. 2018, 19, 1592. [Google Scholar] [CrossRef] [Green Version]
- Forsythe, P.; Sudo, N.; Dinan, T.; Taylor, V.H.; Bienenstock, J. Mood and gut feelings. Brain Behav. Immun. 2010, 24, 9–16. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Brain-gut-microbiota axis and mental health. Psychosom. Med. 2017, 79, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.R.; Goel, R.; Seungbum, K.; Richards, E.M.; Holbert, R.C.; Pepine, C.J.; Raizada, M.K. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma lps and altered gut microbiome in anxiety or depression. Gut 2018, 67, 1555–1557. [Google Scholar] [CrossRef]
- Harhaj, N.S.; Antonetti, D.A. Regulation of tight junctions and loss of barrier function in pathophysiology. Int. J. Biochem. Cell Biol. 2004, 36, 1206–1237. [Google Scholar] [CrossRef] [PubMed]
- Vetrano, S.; Rescigno, M.; Rosaria Cera, M.; Correale, C.; Rumio, C.; Doni, A.; Fantini, M.; Sturm, A.; Borroni, E.; Repici, A.; et al. Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology 2008, 135, 173–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Cao, F.; Liu, Q.; Li, X.; Xu, G.; Liu, G.; Zhang, Y.; Yang, X.; Yi, S.; Xu, F.; et al. Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behav. Brain Res. 2019, 364, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, L.; Chang, L.; Pu, Y.; Qu, Y.; Hashimoto, K. A Key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl. Psychiatry 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Luo, C.; Zhang, W.; Chen, Y.; He, J.; Zhao, Q.; Zuo, R.; Wu, Y. Pro- and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: Involvement in depression. Behav. Brain Res. 2011, 225, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, B.; Chen, X.; Zhang, J.; Yuan, S. A key role of gut microbiota-vagus nerve/spleen axis in sleep deprivation-mediated aggravation of systemic inflammation after LPS administration. Life Sci. 2021, 265, 118736. [Google Scholar] [CrossRef] [PubMed]
- Saand, A.R.; Yu, F.; Chen, J.; Chou, S.H.-Y. Systemic inflammation in hemorrhagic strokes—A novel neurological sign and therapeutic target? J. Cereb. Blood Flow Metab. 2019, 39, 959–988. [Google Scholar] [CrossRef] [PubMed]
- Kevil, C.G.; Oshima, T.; Alexander, B.; Coe, L.L.; Alexander, J.S. H2O2—Mediated permeability: Role of MAPK and occludin. Am. J. Physiol. Cell Physiol. 2000, 279, C21–C30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nusrat, A.; Giry, M.; Turner, J.R.; Colgan, S.P.; Parkos, C.A.; Carnes, D.; Lemichez, E.; Boquet, P.; Madara, J.L. Rho Protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc. Natl. Acad. Sci. USA 1995, 92, 10629–10633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elamin, E.; Masclee, A.; Dekker, J.; Jonkers, D. Ethanol disrupts intestinal epithelial tight junction integrity through intracellular calcium-mediated Rho/ROCK activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G677–G685. [Google Scholar] [CrossRef] [Green Version]
- Rowart, P.; Wu, J.; Caplan, M.; Jouret, F. Implications of AMPK in the formation of epithelial tight junctions. Int. J. Mol. Sci. 2018, 19, 2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Z.; Huang, L.; Yin, P.; Liu, F.; Liu, Y.; Zhang, Z.; Lin, J.; Zou, W.; Li, C. L-Arginine alleviates heat stress-induced intestinal epithelial barrier damage by promoting expression of tight junction proteins via the AMPK pathway. Mol. Biol. Rep. 2019, 46, 6435–6451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.-J.; Sun, X.; Du, M. AMPK in regulation of apical junctions and barrier function of intestinal epithelium. Tissue Barriers 2018, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, J.; You, Q.; He, S.; Meng, Q.; Gao, J.; Wu, X.; Shen, Y.; Sun, Y.; Wu, X.; et al. Activating AMPK to restore tight junction assembly in intestinal epithelium and to attenuate experimental colitis by metformin. Front. Pharmacol. 2018, 9, 761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-J.; Ahmad, F.; Philp, A.; Baar, K.; Williams, T.; Luo, H.; Ke, H.; Rehmann, H.; Taussig, R.; Brown, A.L.; et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting CAMP phosphodiesterases. Cell 2012, 148, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Liu, W.; Fan, Y.-Z.; He, D.-L.; Li, L. PrLZ increases prostate cancer docetaxel resistance by inhibiting LKB1/AMPK-mediated autophagy. Theranostics 2018, 8, 109–123. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Dong, F.; Liu, X.; Xu, J.; Wu, X.; Liu, W.; Zheng, Y. Crosstalk of oxidative damage, apoptosis, and autophagy under endoplasmic reticulum (ER) stress involved in thifluzamide-induced liver damage in zebrafish (Danio rerio). Environ. Pollut. 2018, 243, 1904–1911. [Google Scholar] [CrossRef]
- Chen, Z.; Nie, S.-D.; Qu, M.-L.; Zhou, D.; Wu, L.-Y.; Shi, X.-J.; Ma, L.-R.; Li, X.; Zhou, S.-L.; Wang, S.; et al. The autophagic degradation of cav-1 contributes to pa-induced apoptosis and inflammation of astrocytes. Cell Death Dis. 2018, 9, 771. [Google Scholar] [CrossRef] [Green Version]







| Group | n | DCI |
|---|---|---|
| Control | 6 | 0.03 ± 0.05 |
| Model | 6 | 0.11 ± 0.07 * |
| Propolis | 6 | 0.04 ± 0.05 △ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Guo, P.; Wang, Y.; Teng, X.; Zhang, H.; Sun, L.; Xue, M.; Liang, H. Propolis Ameliorates Alcohol-Induced Depressive Symptoms in C57BL/6J Mice by Regulating Intestinal Mucosal Barrier Function and Inflammatory Reaction. Nutrients 2022, 14, 1213. https://doi.org/10.3390/nu14061213
Wang P, Guo P, Wang Y, Teng X, Zhang H, Sun L, Xue M, Liang H. Propolis Ameliorates Alcohol-Induced Depressive Symptoms in C57BL/6J Mice by Regulating Intestinal Mucosal Barrier Function and Inflammatory Reaction. Nutrients. 2022; 14(6):1213. https://doi.org/10.3390/nu14061213
Chicago/Turabian StyleWang, Peng, Peiyu Guo, Yanhui Wang, Xiangyun Teng, Huaqi Zhang, Lirui Sun, Meilan Xue, and Hui Liang. 2022. "Propolis Ameliorates Alcohol-Induced Depressive Symptoms in C57BL/6J Mice by Regulating Intestinal Mucosal Barrier Function and Inflammatory Reaction" Nutrients 14, no. 6: 1213. https://doi.org/10.3390/nu14061213
APA StyleWang, P., Guo, P., Wang, Y., Teng, X., Zhang, H., Sun, L., Xue, M., & Liang, H. (2022). Propolis Ameliorates Alcohol-Induced Depressive Symptoms in C57BL/6J Mice by Regulating Intestinal Mucosal Barrier Function and Inflammatory Reaction. Nutrients, 14(6), 1213. https://doi.org/10.3390/nu14061213