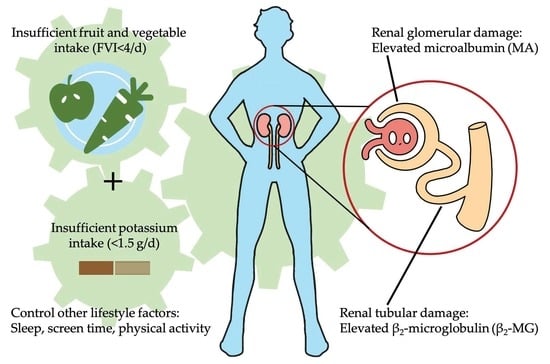
Insufficient Fruit and Vegetable Intake and Low Potassium Intake Aggravate Early Renal Damage in Children: A Longitudinal Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Anthropometric Measurements
2.3. Blood Pressure Measurements
2.4. Urine Collection and Measurements
2.5. Lifestyle Covariates and Definition
2.6. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics
3.2. Binary Regression of Renal Damage Indicators, FVI, and Potassium Indicators
3.3. Multivariable Regression of Renal Damage Indicators with Insufficient FVI and Insufficient Potassium Intake
3.4. Visualization of Early Renal Damage with Insufficient FVI and Quantiles of Potassium Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Msambichaka, B.; Eze, I.C.; Abdul, R.; Abdulla, S.; Klatser, P.; Tanner, M.; Kaushik, R.; Geubbels, E.; Probst-Hensch, N. Insufficient Fruit and Vegetable Intake in a Low- and Middle-Income Setting: A Population-Based Survey in Semi-Urban Tanzania. Nutrients 2018, 10, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, T.; Carrero, J.J.; McCulloch, C.; Burrows, N.R.; Siegel, K.R.; Morgenstern, H.; Saran, R.; Powe, N.R. Dietary Factors and Prevention: Risk of End-Stage Kidney Disease by Fruit and Vegetable Consumption. Am. J. Nephrol. 2021, 52, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Hodder, R.K.; O’Brien, K.M.; Tzelepis, F.; Wyse, R.J.; Wolfenden, L. Interventions for increasing fruit and vegetable consumption in children aged five years and under. Cochrane Database Syst. Rev. 2020, 5, Cd008552. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Fly, A.D. USDA Fresh Fruit and Vegetable Program Is More Effective in Town and Rural Schools Than Those in More Populated Communities. J. Sch. Health 2016, 86, 769–777. [Google Scholar] [CrossRef]
- Xu, P.P.; Hu, X.Q.; Pan, H.; Yang, T.T.; Li, L.; Cao, W.; Gan, Q.; Xu, J.; Zhang, Q. The status of vegetables and fruits consumption of children aged 6 to 17-year-old from 2010 to 2012, China. Chin. J. Prev. Med. 2018, 52, 552–555. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.-L.; Yang, T.-T.; Xu, P.-P.; Li, L.; Cao, W.; Gan, Q.; Pan, H.; Wang, H.-L.; Hu, X.-Q.; et al. Changes of the dietary intake of vegetable and fruit among Chinese children aged 6–17 in 1982 and 2012. Chin. J. Dis. Control Prev. 2021, 25, 509–514. [Google Scholar] [CrossRef]
- Woodruff, R.C.; Zhao, L.; Ahuja, J.K.C.; Gillespie, C.; Goldman, J.; Harris, D.M.; Jackson, S.L.; Moshfegh, A.; Rhodes, D.; Sebastian, R.S.; et al. Top Food Category Contributors to Sodium and Potassium Intake—United States, 2015–2016. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Toba, K.; Hosojima, M.; Kabasawa, H.; Kuwahara, S.; Murayama, T.; Yamamoto-Kabasawa, K.; Kaseda, R.; Wada, E.; Watanabe, R.; Tanabe, N.; et al. Higher estimated net endogenous acid production with lower intake of fruits and vegetables based on a dietary survey is associated with the progression of chronic kidney disease. BMC Nephrol. 2019, 20, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrero, J.J.; González-Ortiz, A.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P.; et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef]
- Gunn, C.A.; Weber, J.L.; Coad, J.; Kruger, M.C. Increasing fruits and vegetables in midlife women: A feasibility study. Nutr. Res. 2013, 33, 543–551. [Google Scholar] [CrossRef]
- Ko, B.-J.; Chang, Y.; Ryu, S.; Kim, E.M.; Lee, M.Y.; Hyun, Y.Y.; Lee, K.-B. Dietary acid load and chronic kidney disease in elderly adults: Protein and potassium intake. PLoS ONE 2017, 12, e0185069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malnic, G.; Giebisch, G.; Muto, S.; Wang, W.; Bailey, M.A.; Satlin, L.M. Chapter 49—Regulation of K+ Excretion. In Seldin and Giebisch’s the Kidney, 5th ed.; Alpern, R.J., Moe, O.W., Caplan, M., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 1659–1715. [Google Scholar] [CrossRef]
- Wu, H.Y.; Huang, J.W.; Peng, Y.S.; Hung, K.Y.; Wu, K.D.; Lai, M.S.; Chien, K.L. Microalbuminuria screening for detecting chronic kidney disease in the general population: A systematic review. Ren. Fail. 2013, 35, 607–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadam, P.; Yachha, M.; Srivastava, G.; Pillai, A.; Pandita, A. Urinary beta-2 microglobulin as an early predictive biomarker of acute kidney injury in neonates with perinatal asphyxia. Eur. J. Pediatr. 2022, 181, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.T.; Tan, N.; Lu, H.Y.; Xu, X.Y.; Yu, Y.Y. Early renal injury indicators can help evaluate renal injury in patients with chronic hepatitis B with long-term nucleos(t)ide therapy. World J. Clin. Cases 2020, 8, 6306–6314. [Google Scholar] [CrossRef] [PubMed]
- Kieneker, L.M.; Bakker, S.J.; de Boer, R.A.; Navis, G.J.; Gansevoort, R.T.; Joosten, M.M. Low potassium excretion but not high sodium excretion is associated with increased risk of developing chronic kidney disease. Kidney Int. 2016, 90, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Elfassy, T.; Zhang, L.; Raij, L.; Bibbins-Domingo, K.; Lewis, C.E.; Allen, N.B.; Liu, K.J.; Peralta, C.A.; Odden, M.C.; Zeki Al Hazzouri, A. Results of the CARDIA study suggest that higher dietary potassium may be kidney protective. Kidney Int. 2020, 98, 187–194. [Google Scholar] [CrossRef]
- Li, M.; Shu, W.; Zunong, J.; Amaerjiang, N.; Xiao, H.; Li, D.; Vermund, S.H.; Hu, Y. Predictors of non-alcoholic fatty liver disease in children. Pediatr. Res. 2021. [Google Scholar] [CrossRef]
- Meng, L.; Ma, L.; Sun, C. The Estimating Modole Building of 24-h Urine Excretion from the Casual Urinary Electrolytic Concentrations Based on Clinical Big Data. Eur. J. Pediatr. 2016, 175, 1866–1867. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Guideline: Potassium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Chinese Society of Nutrition. Dietary Guidelines for Chinese Residents, 1st ed.; People’s Medical Publishing House: Beijing, China, 2016; p. 336. [Google Scholar]
- Barton, K.T.; Kakajiwala, A.; Dietzen, D.J.; Goss, C.W.; Gu, H.; Dharnidharka, V.R. Using the newer Kidney Disease: Improving Global Outcomes criteria, beta-2-microglobulin levels associate with severity of acute kidney injury. Clin. Kidney J. 2018, 11, 797–802. [Google Scholar] [CrossRef] [Green Version]
- Shatat, I.F.; Qanungo, S.; Hudson, S.; Laken, M.A.; Hailpern, S.M. Changes in Urine Microalbumin-to-Creatinine Ratio in Children with Sickle Cell Disease over Time. Front. Pediatr. 2016, 4, 106. [Google Scholar] [CrossRef] [Green Version]
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; García, A.; Pérez-Rodrigo, C.; Aranceta, J. Food, youth and the Mediterranean diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in children and adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Angeles-Agdeppa, I.; Lenighan, Y.M.; Jacquier, E.F.; Toledo, M.B.; Capanzana, M.V. The Impact of Wealth Status on Food Intake Patterns in Filipino School-Aged Children and Adolescents. Nutrients 2019, 11, 2910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pengpid, S.; Peltzer, K. Fruit and Vegetable Consumption is Protective from Short Sleep and Poor Sleep Quality Among University Students from 28 Countries. Nat. Sci. Sleep 2020, 12, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Elsenburg, L.K.; Corpeleijn, E.; van Sluijs, E.M.; Atkin, A.J. Clustering and correlates of multiple health behaviours in 9–10 year old children. PLoS ONE 2014, 9, e99498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, B.; Zou, Z.; Song, Y.; Hu, P.; Luo, D.; Wen, B.; Gao, D.; Wang, X.; Yang, Z.; Ma, Y.; et al. Adolescent Health and Healthy China 2030: A Review. J. Adolesc. Health Off. Publ. Soc. Adolesc. Med. 2020, 67, S24–S31. [Google Scholar] [CrossRef] [PubMed]
- Shook, R.P.; Halpin, K.; Carlson, J.A.; Davis, A.; Dean, K.; Papa, A.; Sherman, A.K.; Noel-MacDonnell, J.R.; Summar, S.; Krueger, G.; et al. Adherence With Multiple National Healthy Lifestyle Recommendations in a Large Pediatric Center Electronic Health Record and Reduced Risk of Obesity. Mayo Clin. Proc. 2018, 93, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, X.; Wang, W.; Wang, Y.; Qu, H.; Zhao, Y.; Lin, D. Clustering of multiple lifestyle behaviors among migrant, left-behind and local adolescents in China: A cross-sectional study. BMC Public Health 2021, 21, 542. [Google Scholar] [CrossRef]
- Amaerjiang, N.; Li, M.; Xiao, H.; Zunong, J.; Li, Z.; Huang, D.; Vermund, S.H.; Pérez-Escamilla, R.; Jiang, X.; Hu, Y. Dehydration Status Aggravates Early Renal Impairment in Children: A Longitudinal Study. Nutrients 2022, 14, 335. [Google Scholar] [CrossRef]
- Goraya, N.; Wesson, D.E. Management of the Metabolic Acidosis of Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2017, 24, 298–304. [Google Scholar] [CrossRef]


| Factors | Total | Sufficient FVI (≥4/d) | Insufficient FVI (<4/d) | p |
|---|---|---|---|---|
| Sex 1 | 0.018 | |||
| Boy [n (%)] | 956 (50.0) | 465 (47.3) | 491 (52.7) | |
| Girl [n (%)] | 958 (50.0) | 518 (52.7) | 440 (47.3) | |
| Age (year) 2 | 6.6 ± 0.3 | 6.6 ± 0.3 | 6.6 ± 0.3 | 0.09 |
| Height (cm) 2 | 122.5 ± 5.3 | 122.3 ± 5.4 | 122.6 ± 5.3 | 0.25 |
| Weight (kg) 2 | 24.8 ± 5.9 | 24.7 ± 5.9 | 24.8 ± 5.9 | 0.72 |
| BMI (kg/m2) 2 | 16.4 ± 2.9 | 16.4 ± 2.9 | 16.4 ± 2.9 | 0.94 |
| SBP (mmHg) 2 | 101 ± 8 | 101 ± 8 | 101 ± 9 | 0.27 |
| DBP (mmHg) 2 | 56 ± 6 | 56 ± 6 | 56 ± 6 | 0.93 |
| Short sleep (<10 h/d) 1 | 1441 (75.3) | 706 (71.8) | 735 (78.9) | <0.001 |
| Long screen time (≥2 h/d) 1 | 95 (5.0) | 37 (3.8) | 58 (6.2) | 0.013 |
| Insufficient physical activity (<1 h/d) 1 | 1451 (75.8) | 739 (75.2) | 712 (76.5) | 0.51 |
| Spot urinary potassium excretion (mmol/L) 2 | ||||
| Wave 1 | 27.36 ± 12.53 | 27.41 ± 12.54 | 27.30 ± 12.53 | 0.85 |
| Wave 2 | 30.42 ± 12.30 | 30.34 ± 12.47 | 30.50 ± 12.12 | 0.77 |
| Wave 3 | 26.59 ± 12.86 | 26.82 ± 13.00 | 26.36 ± 12.74 | 0.45 |
| Wave 4 | 28.00 ± 13.56 | 28.26 ± 13.45 | 27.73 ± 13.68 | 0.46 |
| Estimated 24 h urinary potassium excretion (mg/d) 2 | ||||
| Wave 1 | 1253.5 ± 370.3 | 1272.4 ± 371.1 | 1233.6 ± 368.7 | 0.022 |
| Wave 2 | 1363.1 ± 260.2 | 1383.4 ± 267.0 | 1342.4 ± 251.5 | <0.001 |
| Wave 3 | 1116.7 ± 276.5 | 1134.8 ± 270.8 | 1097.9 ± 281.3 | 0.005 |
| Wave 4 | 1114.4 ± 266.6 | 1131.0 ± 263.3 | 1097.3 ± 269.1 | 0.017 |
| Estimated 24 h potassium intake (g/d) 2 | ||||
| Wave 1 | 1.63 ± 0.48 | 1.65 ± 0.48 | 1.60 ± 0.48 | 0.022 |
| Wave 2 | 1.77 ± 0.34 | 1.80 ± 0.35 | 1.75 ± 0.33 | <0.001 |
| Wave 3 | 1.45 ± 0.36 | 1.48 ± 0.35 | 1.43 ± 0.37 | 0.005 |
| Wave 4 | 1.45 ± 0.35 | 1.47 ± 0.34 | 1.43 ± 0.35 | 0.016 |
| Spot urinary β2-MG excretion (mg/L) 3 | ||||
| Wave 1 | 0.08 (0.04–0.13) | 0.07 (0.04–0.12) | 0.08 (0.05–0.13) | 0.011 |
| Wave 2 | 0.15 (0.12–0.18) | 0.14 (0.12–0.18) | 0.15 (0.12–0.19) | 0.066 |
| Wave 3 | 0.16 (0.13–0.21) | 0.16 (0.13–0.20) | 0.16 (0.13–0.21) | 0.19 |
| Wave 4 | 0.16 (0.13–0.21) | 0.16 (0.13–0.21) | 0.16 (0.13–0.21) | 0.52 |
| Spot urinary MA excretion (mg/L) 3 | ||||
| Wave 1 | 9.13 (6.45–12.56) | 8.85 (6.36–12.18) | 9.28 (6.53–12.96) | 0.019 |
| Wave 2 | 6.70 (6.10–8.50) | 6.60 (6.10–8.30) | 6.70 (6.10–8.90) | 0.027 |
| Wave 3 | 7.00 (6.20–9.10) | 6.90 (6.20–9.00) | 7.00 (6.20–9.40) | 0.35 |
| Wave 4 | 6.80 (6.00–8.90) | 6.70 (6.00–8.80) | 6.85 (6.00–9.10) | 0.16 |
| Dependent Variable | Independent Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| Estimate (95%CI) | p | Estimate (95%CI) | p | Estimate (95%CI) | p | ||
| Estimated pota-ssium intake | Insufficient FVI | −0.049 (−0.071, −0.027) | <0.001 | −0.049 (−0.071, −0.027) | <0.001 | −0.050 (−0.072, −0.027) | <0.001 |
| Spot urinary potassium | 0.013 (0.012, 0.014) | <0.001 | 0.013 (0.012, 0.014) | <0.001 | 0.013 (0.012, 0.014) | <0.001 | |
| β2-MG | Insufficient FVI | 0.011 (0.004, 0.018) | 0.003 | 0.011 (0.004, 0.018) | 0.004 | 0.012 (0.005, 0.020) | <0.001 |
| Estimated potassium intake | −0.043 (−0.052, −0.034) | <0.001 | −0.042 (−0.052, −0.033) | <0.001 | −0.042 (−0.052, −0.033) | <0.001 | |
| MA | Insufficient FVI | 0.616 (−0.020, 1.253) | 0.058 | 0.669 (0.034, 1.304) | 0.039 | 0.717 (0.075, 1.359) | 0.029 |
| Estimated potassium intake | −1.837 (−2.656, −1.019) | <0.001 | −1.753 (−2.574, −0.933) | <0.001 | −1.778 (−2.600, −0.956) | <0.001 | |
| Dependent Variable | Independent Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| Estimate (95%CI) | p | Estimate (95%CI) | p | Estimate (95%CI) | p | ||
| β2-MG | Intercept | 0.142 (0.104, 0.180) | <0.001 | 0.090 (−0.004, 0.184) | 0.061 | 0.043 (−0.058, 0.145) | 0.40 |
| Insufficient FVI | 0.009 (0.002, 0.017) | 0.010 | 0.009 (0.002, 0.017) | 0.011 | 0.011 (0.004, 0.018) | 0.003 | |
| Insufficient potassium intake | 0.031 (0.023, 0.039) | <0.001 | 0.031 (0.023, 0.038) | <0.001 | 0.031 (0.023, 0.038) | <0.001 | |
| MA | Intercept | 8.940 (7.911, 9.969) | <0.001 | 6.614 (−0.662, 13.890) | 0.075 | 2.228 (−5.736, 10.193) | 0.58 |
| Insufficient FVI | 0.558 (−0.077, 1.193) | 0.085 | 0.612 (−0.022, 1.246) | 0.059 | 0.658 (0.017, 1.299) | 0.044 | |
| Insufficient potassium intake | 1.255 (0.563, 1.947) | <0.001 | 1.171 (0.479, 1.863) | <0.001 | 1.185 (0.492, 1.878) | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Amaerjiang, N.; Li, Z.; Xiao, H.; Zunong, J.; Gao, L.; Vermund, S.H.; Hu, Y. Insufficient Fruit and Vegetable Intake and Low Potassium Intake Aggravate Early Renal Damage in Children: A Longitudinal Study. Nutrients 2022, 14, 1228. https://doi.org/10.3390/nu14061228
Li M, Amaerjiang N, Li Z, Xiao H, Zunong J, Gao L, Vermund SH, Hu Y. Insufficient Fruit and Vegetable Intake and Low Potassium Intake Aggravate Early Renal Damage in Children: A Longitudinal Study. Nutrients. 2022; 14(6):1228. https://doi.org/10.3390/nu14061228
Chicago/Turabian StyleLi, Menglong, Nubiya Amaerjiang, Ziang Li, Huidi Xiao, Jiawulan Zunong, Lifang Gao, Sten H. Vermund, and Yifei Hu. 2022. "Insufficient Fruit and Vegetable Intake and Low Potassium Intake Aggravate Early Renal Damage in Children: A Longitudinal Study" Nutrients 14, no. 6: 1228. https://doi.org/10.3390/nu14061228
APA StyleLi, M., Amaerjiang, N., Li, Z., Xiao, H., Zunong, J., Gao, L., Vermund, S. H., & Hu, Y. (2022). Insufficient Fruit and Vegetable Intake and Low Potassium Intake Aggravate Early Renal Damage in Children: A Longitudinal Study. Nutrients, 14(6), 1228. https://doi.org/10.3390/nu14061228