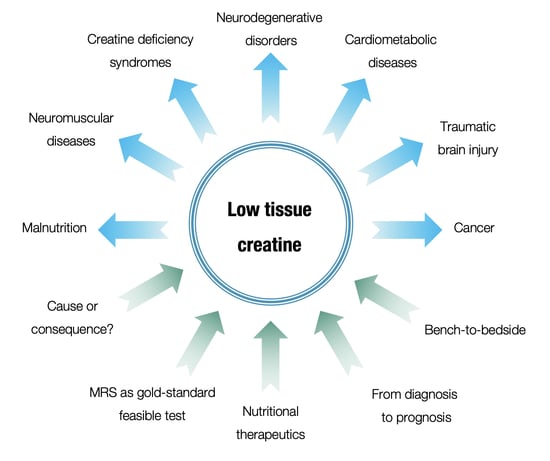
Low Tissue Creatine: A Therapeutic Target in Clinical Nutrition
Abstract
:1. Introduction
2. Creatine Shortfall in Clinical Medicine
| Pathology | Refs. |
|---|---|
| Autism spectrum disorder | [40,41,42,43,44,45] |
| Concussion and mild traumatic brain injury | [13,46] |
| Multiple sclerosis | [47,48,49,50,51] |
| Gyrate atrophy of the choroid and retina | [15,52] |
| Post-viral fatigue syndrome | [53,54] |
| Primary and secondary brain tumors | [17,18] |
| Neuromuscular disease | [19] |
| Facioscapulohumeral muscular dystrophy | [20] |
| Dilated cardiomyopathy | [21,24,25] |
| Aortic valve disease | [22] |
| Heart transplantation | [23] |
| Coronary disease | [26] |
| Chronic obstructive pulmonary disease | [27,28] |
| Lung cancer | [29] |
| Pancreatic cancer | [30] |
| Hepatitis C | [31] |
| Chronic HIV infection | [32] |
| Infant malnutrition | [33] |
3. Methods for Tissue Creatine Evaluation
4. Transition of Tissue Creatine Evaluation from Research to Routine Practice
5. Creatine in Clinical Nutrition
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallimann, T.; Dolder, M.; Schlattner, U.; Eder, M.; Hornemann, T.; Kraft, T.; Stolz, M. Creatine kinase: An enzyme with a central role in cellular energy metabolism. Magn. Reson. Mater. Phys. Biol. Med. 1998, 6, 116–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosnan, J.T.; Brosnan, M.E. Creatine: Endogenous Metabolite, Dietary, and Therapeutic Supplement. Annu. Rev. Nutr. 2007, 27, 241–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosnan, M.E.; Brosnan, J.T. The role of dietary creatine. Amino Acids 2016, 48, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Forbes, S.C. Perspective: Creatine, a Conditionally Essential Nutrient: Building the Case. Adv. Nutr. Int. Rev. J. 2021, 13, 34–37. [Google Scholar] [CrossRef] [PubMed]
- De Graf, R.A. In Vivo NMR Spectroscopy: Priniciples and Techniques, 3rd ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Wiedermann, D.; Schneider, J.; Fromme, A.; Thorwesten, L.; Möller, H.E. Creatine loading and resting skeletal muscle phosphocreatine flux: A saturation-transfer NMR study. Magn. Reson. Mater. Phys. Biol. Med. 2001, 13, 118–126. [Google Scholar] [CrossRef]
- Nakae, I.; Mitsunami, K.; Matsuo, S.; Matsumoto, T.; Morikawa, S.; Inubushi, T.; Koh, T.; Horie, M. Assessment of Myocardial Creatine Concentration in Dysfunctional Human Heart by Proton Magnetic Resonance Spectroscopy. Magn. Reson. Med. Sci. 2004, 3, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Mercimek-Andrews, S.; Salomons, G.S. Creatine Deficiency Syndromes. In GeneReviews [Internet]; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mirzaa, G., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2009. [Google Scholar]
- Sharer, J.D.; Bodamer, O.; Longo, N.; Tortorelli, S.; Wamelink, M.M.; Young, S. Laboratory diagnosis of creatine deficiency syndromes: A technical standard and guideline of the American College of Medical Genetics and Genomics. Genet. Med. 2017, 19, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Schulze, A. Creatine deficiency syndromes. Handb. Clin. Neurol. 2013, 113, 1837–1843. [Google Scholar] [CrossRef]
- Ford, T.C.; Crewther, D.P. A Comprehensive Review of the 1H-MRS Metabolite Spectrum in Autism Spectrum Disorder. Front. Mol. Neurosci. 2016, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Vagnozzi, R.; Signoretti, S.; Floris, R.; Marziali, S.; Manara, M.; Amorini, A.M.; Belli, A.; Di Pietro, V.; D’Urso, S.; Pastore, F.S.; et al. Decrease in N-Acetylaspartate Following Concussion May Be Coupled to Decrease in Creatine. J. Head Trauma Rehabil. 2013, 28, 284–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostojic, S.M. Creatine and multiple sclerosis. Nutr. Neurosci. 2020. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Nanto-Salonen, K.; Komu, M.; Lundbom, N.; Heinanen, K.; Alanen, A.; Sipila, I.; Simell, O. Reduced brain creatine in gyrate atrophy of the choroid and retina with hyperornithinemia. Neurology 1999, 53, 303. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S. Diagnostic and Pharmacological Potency of Creatine in Post-Viral Fatigue Syndrome. Nutrients 2021, 13, 503. [Google Scholar] [CrossRef] [PubMed]
- Chernov, M.F.; Hayashi, M.; Izawa, M.; Ono, Y.; Hori, T. Proton magnetic resonance spectroscopy (MRS) of metastatic brain tumors: Variations of metabolic profile. Int. J. Clin. Oncol. 2006, 11, 375–384. [Google Scholar] [CrossRef]
- Jia, C.; Li, Z.; Guo, D.; Zhang, Z.; Yu, J.; Jiang, G.; Xing, X.; Ji, S.; Jin, F. Brain Metastases of Non-Small Cell Lung Cancer: Magnetic Resonance Spectroscopy for Clinical Outcome Assessment in Patients with Stereotactic Radiotherapy. OncoTargets Ther. 2020, 13, 13087–13096. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Parise, G. Direct measurement of high-energy phosphate compounds in patients with neuromuscular disease. Muscle Nerve 1999, 22, 1228–1233. [Google Scholar] [CrossRef]
- Leung, D.G.; Wang, X.; Barker, P.B.; Carrino, J.A.; Wagner, K.R. Multivoxel proton magnetic resonance spectroscopy in facioscapulohumeral muscular dystrophy. Muscle Nerve 2018, 57, 958–963. [Google Scholar] [CrossRef]
- Hardy, C.J.; Weiss, R.G.; Bottomley, P.A.; Gerstenblith, G. Altered myocardial high-energy phosphate metabolites in patients with dilated cardiomyopathy. Am. Heart J. 1991, 122 Pt 1, 795–801. [Google Scholar] [CrossRef]
- Conway, M.; Allis, J.; Ouwerkerk, R.; Niioka, T.; Rajagopalan, B.; Radda, G. Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet 1991, 338, 973–976. [Google Scholar] [CrossRef]
- Evanochko, W.; Buchthal, S.D.; den Hollander, J.A.; Katholi, C.R.; Bourge, R.C.; Benza, R.L.; Kirklin, J.K.; Pohost, G.M. Cardiac transplant patients response to the 31P MRS stress test. J. Heart Lung Transplant. 2002, 21, 522–529. [Google Scholar] [CrossRef]
- Neubauer, S.; Krahe, T.; Schindler, R.; Horn, M.; Hillenbrand, H.; Entzeroth, C.; Mader, H.; Kromer, E.P.; Riegger, G.A.; Lackner, K. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation 1992, 86, 1810–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neubauer, S.; Horn, M.; Cramer, M.; Harre, K.; Newell, J.B.; Peters, W.; Pabst, T.; Ertl, G.; Hahn, D.; Ingwall, J.S.; et al. Myocardial Phosphocreatine-to-ATP Ratio Is a Predictor of Mortality in Patients with Dilated Cardiomyopathy. Circulation 1997, 96, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.D.; Shaw, L.J.; Buchthal, S.D.; Merz, C.N.B.; Kim, H.-W.; Scott, K.N.; Doyle, M.; Olson, M.B.; Pepine, C.J.; Hollander, J.D.; et al. Prognosis in Women With Myocardial Ischemia in the Absence of Obstructive Coronary Disease. Circulation 2004, 109, 2993–2999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payen, J.-F.; Wuyam, B.; Reutenauer, H.; Laurent, D.; Levy, P.; Le Bas, J.-F.; Benabid, A.L. Impairment of muscular metabolism in chronic respiratory failure. A human31P MRS study. NMR Biomed. 1991, 4, 41–45. [Google Scholar] [CrossRef]
- Mathur, R.; Cox, I.J.; Oatridge, A.; Shephard, D.T.; Shaw, R.J.; Taylor-Robinson, S.D. Cerebral Bioenergetics in Stable Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1999, 160, 1994–1999. [Google Scholar] [CrossRef] [Green Version]
- Hanaoka, H.; Yoshioka, Y.; Ito, I.; Niitu, K.; Yasuda, N. In vitro characterization of lung cancers by the use of1H nuclear magnetic resonance spectroscopy of tissue extracts and discriminant factor analysis. Magn. Reson. Med. 1993, 29, 436–440. [Google Scholar] [CrossRef]
- Chang, C.-K.; Shih, T.T.-F.; Tien, Y.-W.; Chang, M.-C.; Chang, Y.-T.; Yang, S.-H.; Cheng, M.-F.; Chen, B.-B. Metabolic Alterations in Pancreatic Cancer Detected by In Vivo 1H-MR Spectroscopy: Correlation with Normal Pancreas, PET Metabolic Activity, Clinical Stages, and Survival Outcome. Diagnostics 2021, 11, 1541. [Google Scholar] [CrossRef]
- Bladowska, J.; Zimny, A.; Knysz, B.; Małyszczak, K.; Kołtowska, A.; Szewczyk, P.; Gąsiorowski, J.; Furdal, M.; Sąsiadek, M.J. Evaluation of early cerebral metabolic, perfusion and microstructural changes in HCV-positive patients: A pilot study. J. Hepatol. 2013, 59, 651–657. [Google Scholar] [CrossRef]
- Boban, J.; Thurnher, M.M.; Brkic, S.; Lendak, D.; Ignjatovic, V.B.; Todorovic, A.; Kozic, D. Neurometabolic Remodeling in Chronic Hiv Infection: A Five-Year Follow-up Multi-Voxel Mrs Study. Sci. Rep. 2019, 9, 19799. [Google Scholar] [CrossRef]
- Cakir, M.; Senyuva, S.; Kul, S.; Sag, E.; Cansu, A.; Yucesan, F.B.; Yaman, S.O.; Orem, A. Neurocognitive Functions in Infants with Malnutrition; Relation with Long-chain Polyunsaturated Fatty Acids, Micronutrients Levels and Magnetic Resonance Spectroscopy. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Tsikas, D.; Bakker, S.J. Creatine is a Conditionally Essential Nutrient in Chronic Kidney Disease: A Hypothesis and Narrative Literature Review. Nutrients 2019, 11, 1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muccini, A.M.; Tran, N.T.; de Guingand, D.L.; Philip, M.; Della Gatta, P.A.; Galinsky, R.; Sherman, L.S.; Kelleher, M.A.; Palmer, K.R.; Berry, M.J.; et al. Creatine Metabolism in Female Reproduction, Pregnancy and Newborn Health. Nutrients 2021, 13, 490. [Google Scholar] [CrossRef] [PubMed]
- Mercimek-Mahmutoglu, S.; Stoeckler-Ipsiroglu, S.; Adami, A.; Appleton, R.; Araújo, H.C.; Duran, M.; Ensenauer, R.; Fernandez-Alvarez, E.; Garcia, P.; Grolik, C.; et al. GAMT deficiency: Features, treatment, and outcome in an inborn error of creatine synthesis. Neurology 2006, 67, 480–484. [Google Scholar] [CrossRef]
- Van De Kamp, J.; Mancini, G.; Pouwels, P.; Betsalel, O.; Van Dooren, S.; De Koning, I.; Steenweg, M.; Jakobs, C.; Van Der Knaap, M.; Salomons, G. Clinical features and X-inactivation in females heterozygous for creatine transporter defect. Clin. Genet. 2011, 79, 264–272. [Google Scholar] [CrossRef]
- Balestrino, M.; Adriano, E. Beyond sports: Efficacy and safety of creatine supplementation in pathological or paraphysiological conditions of brain and muscle. Med. Res. Rev. 2019, 39, 2427–2459. [Google Scholar] [CrossRef]
- Ostojic, S.M. Creatine as a food supplement for the general population. J. Funct. Foods 2021, 83, 104568. [Google Scholar] [CrossRef]
- Friedman, S.; Shaw, D.; Artru, A.; Richards, T.; Gardner, J.; Dawson, G.; Posse, S.; Dager, S. Regional brain chemical alterations in young children with autism spectrum disorder. Neurology 2003, 60, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Levitt, J.G.; O’Neill, J.; Blanton, R.E.; Smalley, S.; Fadale, D.; McCracken, J.T.; Guthrie, D.; Toga, A.W.; Alger, J.R. Proton magnetic resonance spectroscopic imaging of the brain in childhood autism. Biol. Psychiatry 2003, 54, 1355–1366. [Google Scholar] [CrossRef]
- Friedman, S.D.; Shaw, D.W.W.; Artru, A.A.; Dawson, G.; Petropoulos, H.; Dager, S.R. Gray and White Matter Brain Chemistry in Young Children With Autism. Arch. Gen. Psychiatry 2006, 63, 786–794. [Google Scholar] [CrossRef]
- Hardan, A.Y.; Minshew, N.J.; Melhem, N.M.; Srihari, S.; Jo, B.; Bansal, R.; Keshavan, M.S.; Stanley, J.A. An MRI and proton spectroscopy study of the thalamus in children with autism. Psychiatry Res. Neuroimaging 2008, 163, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinhans, N.M.; Richards, T.; Weaver, K.E.; Liang, O.; Dawson, G.; Aylward, E. Brief report: Biochemical correlates of clinical impairment in high functioning autism and Asperger’s disorder. J. Autism. Dev. Disord. 2009, 39, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A.; Erickson, L.C.; Zeeland, A.A.S.-V.; Koperski, S.; Haas, R.H.; Wallace, D.C.; Naviaux, R.K.; Lincoln, A.J.; Reiner, G.E.; Hamilton, G. Assessing bioenergetic compromise in autism spectrum disorder with 31P magnetic resonance spectroscopy: Preliminary report. J. Child Neurol. 2014, 29, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirov, I.; Fleysher, L.; Babb, J.; Silver, J.M.; Grossman, R.I.; Gonen, O. Characterizing ‘mild’ in traumatic brain injury with proton MR spectroscopy in the thalamus: Initial findings. Brain Inj. 2007, 21, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Cadoux-Hudson, T.A.; Kermode, A.; Rajagopalan, B.; Taylor, D.; Thompson, A.; Ormerod, I.E.; McDonald, W.I.; Radda, G.K. Biochemical changes within a multiple sclerosis plaque in vivo. J. Neurol. Neurosurg. Psychiatry 1991, 54, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, F.; Bauer, H.J.; Christen, H.-J.; Kruse, B.; Bruhn, H.; Frahm, J. Multiple sclerosis in childhood: Report of 15 cases. Brain Dev. 1991, 13, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Bruhn, H.; Frahm, J.; Merboldt, K.D.; Hanefeld, F.; Christen, H.J.; Kruse, B.; Bauer, H.J. Multiple sclerosis in children: Cerebral metabolic alterations monitored by localized proton magnetic resonance spectroscopy in vivo. Ann. Neurol. 1992, 32, 140–150. [Google Scholar] [CrossRef]
- Koschorek, F.; O’ermann, W.; Stelten, J.; Braunsdorf, W.E.; Steller, U.; Gremmel, H.; Leibfritz, D. High-resolution 1H NMR spectroscopy of cerebrospinal fluid in spinal diseases. Neurosurg. Rev. 1993, 16, 307–315. [Google Scholar] [CrossRef]
- Joanna, L.; James, P.; Anthony, A.; Garnette, R.S. Nuclear mag- netic resonance study of cerebrospinal fluid from patients with multiple sclerosis. Can. J. Neurol. Sci. 1993, 20, 194–198. [Google Scholar] [CrossRef] [Green Version]
- Valayannopoulos, V.; Boddaert, N.; Mention, K.; Touati, G.; Barbier, V.; Chabli, A.; Sedel, F.; Kaplan, J.; Dufier, J.; Seidenwurm, D.; et al. Secondary creatine deficiency in ornithine delta-aminotransferase deficiency. Mol. Genet. Metab. 2009, 97, 109–113. [Google Scholar] [CrossRef]
- Mueller, C.; Lin, J.; Sheriff, S.; Maudsley, A.A.; Younger, J.W. Evidence of widespread metabolite abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome: Assessment with whole-brain magnetic resonance spectroscopy. Brain Imaging Behav. 2020, 14, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Van Der Schaaf, M.E.; de Lange, F.; Schmits, I.C.; Geurts, D.E.; Roelofs, K.; Van Der Meer, J.W.; Toni, I.; Knoop, H. Prefrontal structure varies as a function of pain symptoms in chronic fatigue syndrome. Biol. Psychiatry 2017, 81, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Holloway, C.; Clarke, K. Is MR Spectroscopy of the Heart Ready for Humans? Heart Lung Circ. 2010, 19, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E.; Gant, N. The biochemistry of creatine. In Magnetic Resonance Spectroscopy; Stagg, C.J., Rothman, D.L., Eds.; Academic Press: London, UK, 2014; pp. 91–110. [Google Scholar]
- Lin, A.; Andronesi, O.; Bogner, W.; Choi, I.; Coello, E.; Cudalbu, C.; Juchem, C.; Kemp, G.J.; Kreis, R.; Krššák, M.; et al. Minimum reporting standards for in vivo magnetic resonance spectroscopy (MRSinMRS): Experts’ consensus recommendations. NMR Biomed. 2021, 34, e4484. [Google Scholar] [CrossRef]
- Inoue, T.; Kozawa, E.; Ishikawa, M.; Okada, H. Application of Magnetic Resonance Imaging in the Evaluation of Nutritional Status: A Literature Review with Focus on Dialysis Patients. Nutrients 2021, 13, 2037. [Google Scholar] [CrossRef]
- Selleck, M.J.; Senthil, M.; Wall, N.R. Making Meaningful Clinical Use of Biomarkers. Biomark. Insights 2017, 12, 1177271917715236. [Google Scholar] [CrossRef] [Green Version]
- Bakermans, A.J.; Boekholdt, S.M.; de Vries, D.K.; Reckman, Y.J.; Farag, E.S.; de Heer, P.; Uthman, L.; Denis, S.W.; Zuurbier, C.J.; Houtkooper, R.H.; et al. Quantification of Myocardial Creatine and Triglyceride Content in the Human Heart: Precision and Accuracy of in vivo Proton Magnetic Resonance Spectroscopy. J. Magn. Reson. Imaging 2021, 54, 411–420. [Google Scholar] [CrossRef]
- Gupta, A.; Houston, B. Cardiac 1H MR spectroscopy: Development of the past five decades and future perspectives. Heart Fail. Rev. 2021, 26, 839–859. [Google Scholar] [CrossRef]
- Alshammari, Q.T.; Salih, M.; Gameraddin, M.; Yousef, M.; Abdelmalik, B.; Loaz, O. Accuracy of Magnetic Resonance Spectroscopy in Discrimination of Neoplastic and Non-Neoplastic Brain Lesions. Curr. Med. Imaging 2021, 17, 904–910. [Google Scholar] [CrossRef]
- Server, A.; Josefsen, R.; Kulle, B.; Maehlen, J.; Schellhorn, T.; Gadmar, Ø.; Kumar, T.; Haakonsen, M.; Langberg, C.W.; Nakstad, P.H. Proton magnetic resonance spectroscopy in the distinction of high-grade cerebral gliomas from single metastatic brain tumors. Acta Radiol. 2010, 51, 316–325. [Google Scholar] [CrossRef]
- Barbagallo, G.; Arabia, G.; Morelli, M.; Nisticò, R.; Novellino, F.; Salsone, M.; Rocca, F.; Quattrone, A.; Caracciolo, M.; Sabatini, U.; et al. Thalamic neurometabolic alterations in tremulous Parkinson’s disease: A preliminary proton MR spectroscopy study. Park. Relat. Disord. 2017, 43, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Zanigni, S.; Testa, C.; Calandra-Buonaura, G.; Sambati, L.; Guarino, M.; Gabellini, A.; Evangelisti, S.; Cortelli, P.; Lodi, R.; Tonon, C. The contribution of cerebellar proton magnetic resonance spectroscopy in the differential diagnosis among parkinsonian syndromes. Park. Relat. Disord. 2015, 21, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Libero, L.E.; DeRamus, T.; Lahti, A.C.; Deshpande, G.; Kana, R.K. Multimodal neuroimaging based classification of autism spectrum disorder using anatomical, neurochemical, and white matter correlates. Cortex 2015, 66, 46–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Z.-H.; Li, C.-F.; Li, Z.-F.; Zhang, K.; Wang, Q.; Yu, D.-X. Preliminary study of 3T 1H MR spectroscopy in bone and soft tissue tumors. Chin. Med. J. 2009, 122, 39–43. [Google Scholar] [PubMed]
- Mowatt, G.; Scotland, G.; Boachie, C.; Cruickshank, M.; Ford, J.A.; Fraser, C.; Kurban, L.; Lam, T.B.; Padhani, A.; Royle, J.; et al. The diagnostic accuracy and cost-effectiveness of magnetic resonance spectroscopy and enhanced magnetic resonance imaging techniques in aiding the localisation of prostate abnormalities for biopsy: A systematic review and economic evaluation. Health Technol. Assess. 2013, 17, vii–xix, 1–281. [Google Scholar] [CrossRef] [Green Version]
- Izuhara, M.; Miura, S.; Otsuki, K.; Nagahama, M.; Hayashida, M.; Hashioka, S.; Asou, H.; Kitagaki, H.; Inagaki, M. Magnetic Resonance Spectroscopy in the Ventral Tegmental Area Distinguishes Responders to Suvorexant Prior to Treatment: A 4-Week Prospective Cohort Study. Front. Psychiatry 2021, 12, 714376. [Google Scholar] [CrossRef]
- Johnson, T.A.; Jinnah, H.A.; Kamatani, N. Shortage of cellular ATP as a cause of diseases and strategies to enhance ATP. Front. Pharmacol. 2019, 10, 98. [Google Scholar] [CrossRef]
- Chu, X.-Y.; Wang, G.; Zhang, H.-Y. ATP as an anti-aging agent: Beyond the energy reservoir. Drug Discov. Today 2021, 26, 2783–2785. [Google Scholar] [CrossRef]
- Leuzzi, V.; Bianchi, M.C.; Tosetti, M.; Carducci, C.; Cerquiglini, C.A.; Cioni, G.; Antonozzi, I. Brain creatine depletion: Guanidinoacetate methyltransferase deficiency (improving with creatine supplementation). Neurology 2000, 55, 1407–1410. [Google Scholar] [CrossRef]
- Bianchi, M.; Tosetti, M.; Battini, R.; Leuzzi, V.; Alessandri’, M.; Carducci, C.; Antonozzi, I.; Cioni, G. Treatment Monitoring of Brain Creatine Deficiency Syndromes: A 1H- and 31P-MR Spectroscopy Study. Am. J. Neuroradiol. 2007, 28, 548–554. [Google Scholar]
- Valayannopoulos, V.; Boddaert, N.; Chabli, A.; Barbier, V.; Desguerre, I.; Philippe, A.; Afenjar, A.; Mazzuca, M.; Cheillan, D.; Munnich, A.; et al. Treatment by oral creatine, L-arginine and L-glycine in six severely affected patients with creatine transporter defect. J. Inherit. Metab. Dis. 2012, 35, 151–157. [Google Scholar] [CrossRef]
- Barisic, N.; Bernert, G.; Ipsiroglu, O.; Stromberger, C.; Müller, T.; Gruber, S.; Prayer, D.; Moser, E.; Bittner, R.E.; Stöckler-Ipsiroglu, S. Effects of Oral Creatine Supplementation in a Patient with MELAS Phenotype and Associated Nephropathy. Neuropediatrics 2002, 33, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Byblow, W.; Gant, N. Creatine Supplementation Enhances Corticomotor Excitability and Cognitive Performance during Oxygen Deprivation. J. Neurosci. 2015, 35, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Stojanovic, M.; Drid, P.; Hoffman, J.R.; Sekulic, D.; Zenic, N. Supplementation with Guanidinoacetic Acid in Women with Chronic Fatigue Syndrome. Nutrients 2016, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Hersch, S.M.; Gevorkian, S.; Marder, K.; Moskowitz, C.; Feigin, A.; Cox, M.; Como, P.; Zimmerman, C.; Lin, M.; Zhang, L.; et al. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2’dG. Neurology 2006, 66, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Kondo, D.G.; Sung, Y.-H.; Hellem, T.L.; Fiedler, K.K.; Shi, X.; Jeong, E.-K.; Renshaw, P.F. Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: A 31-phosphorus magnetic resonance spectroscopy study. J. Affect. Disord. 2011, 135, 354–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, D.G.; Forrest, L.N.; Shi, X.; Sung, Y.-H.; Hellem, T.L.; Huber, R.S.; Renshaw, P.F. Creatine target engagement with brain bioenergetics: A dose-ranging phosphorus-31 magnetic resonance spectroscopy study of adolescent females with SSRI-resistant depression. Amino Acids 2016, 48, 1941–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellem, T.L.; Sung, Y.-H.; Shi, X.-F.; Pett, M.A.; Latendresse, G.; Morgan, J.; Huber, R.S.; Kuykendall, D.; Lundberg, K.J.; Renshaw, P.F. Creatine as a Novel Treatment for Depression in Females Using Methamphetamine: A Pilot Study. J. Dual Diagn. 2015, 11, 189–202. [Google Scholar] [CrossRef] [Green Version]
- Gordon, A.; Hultman, E.; Kaijser, L.; Kristjansson, S.; Rolf, C.J.; Nyquist, O.; Sylvén, C. Creatine supplementation in chronic heart failure increases skeletal muscle creatine phosphate and muscle performance. Cardiovasc. Res. 1995, 30, 413–418. [Google Scholar] [CrossRef]
- Willer, B.; Stucki, G.; Hoppeler, H.; Brühlmann, P.; Krähenbühl, S. Effects of creatine supplementation on muscle weakness in patients with rheumatoid arthritis. Rheumatology 2000, 39, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Kreider, R.B.; Stout, J.R. Creatine in Health and Disease. Nutrients 2021, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. GRAS Notice for Creatine Monohydrate. 2020. Available online: https://www.fda.gov/media/143525/download (accessed on 17 November 2021).
- Ostojic, S.M. Overcoming restraints of dietary creatine. Trends Food Sci. Technol. 2020, 100, 246–247. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostojic, S.M. Low Tissue Creatine: A Therapeutic Target in Clinical Nutrition. Nutrients 2022, 14, 1230. https://doi.org/10.3390/nu14061230
Ostojic SM. Low Tissue Creatine: A Therapeutic Target in Clinical Nutrition. Nutrients. 2022; 14(6):1230. https://doi.org/10.3390/nu14061230
Chicago/Turabian StyleOstojic, Sergej M. 2022. "Low Tissue Creatine: A Therapeutic Target in Clinical Nutrition" Nutrients 14, no. 6: 1230. https://doi.org/10.3390/nu14061230
APA StyleOstojic, S. M. (2022). Low Tissue Creatine: A Therapeutic Target in Clinical Nutrition. Nutrients, 14(6), 1230. https://doi.org/10.3390/nu14061230