No Effect of Chronotype on Hunger or Snack Consumption during a Night Shift with Acute Sleep Deprivation
Abstract
:1. Introduction
2. Materials and Methods
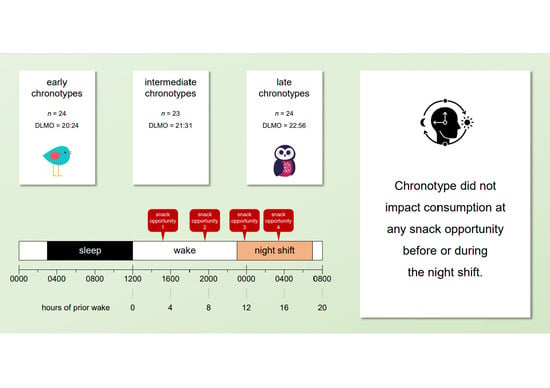
2.1. Participants
2.2. Design
2.3. Procedure
2.4. Measures
2.4.1. Circadian Phase
2.4.2. Hunger, Prospective Consumption, Desire to Eat Fruit, and Desire to Eat Fast Food
2.4.3. Snack Consumption
2.4.4. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alterman, T.; Luckhaupt, S.E.; Dahlhamer, J.M.; Ward, B.W.; Calvert, G.M. Prevalence rates of work organization characteristics among workers in the U.S.: Data from the 2010 National Health Interview Survey. Am. J. Ind. Med. 2013, 56, 647–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eurofound. Fifth European Working Conditions Survey; Publications Office of the European Union: Luxembourg, 2012. [Google Scholar]
- Åkerstedt, T. Is there an optimal sleep-wake pattern in shift work? Scand. J. Work. Environ. Health 1998, 24, 18–27. [Google Scholar] [PubMed]
- Wright, K.P., Jr.; Bogan, R.K.; Wyatt, J.K. Shift work and the assessment and management of shift work disorder (SWD). Sleep Med. Rev. 2013, 17, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Santhi, N.; Horowitz, T.S.; Duffy, J.F.; Czeisler, C.A. Acute sleep deprivation and circadian misalignment associated with transition onto the first night of work impairs visual selective attention. PLoS ONE 2007, 2, e1233. [Google Scholar] [CrossRef] [Green Version]
- Lamond, N.; Dorrian, J.; Burgess, H.; Holmes, A.; Roach, G.; McCulloch, K.; Fletcher, A.; Dawson, D. Adaptation of performance during a week of simulated night work. Ergonomics 2004, 47, 154–165. [Google Scholar] [CrossRef]
- Roach, G.D.; Sach, E.J.; Reiter, A.M.; Dawson, D.; Sargent, C. The likelihood of crashing during a simulated post-work commute decreases across a week of consecutive night shifts. Chronobiol. Int. 2020, 37, 1425–1429. [Google Scholar] [CrossRef]
- Purnell, M.T.; Feyer, A.M.; Herbison, G.P. The impact of a nap opportunity during the night shift on the performance and alertness of 12-h shift workers. J. Sleep Res. 2002, 11, 219–227. [Google Scholar] [CrossRef]
- Vidacek, S.; Kaliterna, L.; Radosevic-Vidacek, B.; Folkard, S. Productivity on a weekly rotating shift system: Circadian adjustment and sleep deprivation effects? Ergonomics 1986, 29, 1583–1590. [Google Scholar] [CrossRef]
- Folkard, S. Is there a ‘best compromise’shift system? Ergonomics 1992, 35, 1453–1463. [Google Scholar] [CrossRef]
- Cain, S.W.; Filtness, A.J.; Phillips, C.L.; Anderson, C. Enhanced preference for high-fat foods following a simulated night shift. Scand. J. Work. Environ. Health 2015, 41, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Heath, G.; Roach, G.D.; Dorrian, J.; Ferguson, S.A.; Darwent, D.; Sargent, C. The effect of sleep restriction on snacking behaviour during a week of simulated shiftwork. Accid. Anal. Prev. 2012, 45, 62–67. [Google Scholar] [CrossRef]
- Buxton, O.M.; Cain, S.W.; O’Connor, S.P.; Porter, J.H.; Duffy, J.F.; Wang, W.; Czeisler, C.A.; Shea, S.A. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 2012, 4, 129ra143. [Google Scholar] [CrossRef] [Green Version]
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Roberts, A.L.; Chen, J.; Kelleman, M.; O’Keeffe, M.; RoyChoudhury, A.; Jones, P.J. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am. J. Clin. Nutr. 2011, 94, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Morikawa, Y.; Nakagawa, H.; Miura, K.; Soyama, Y.; Ishizaki, M.; Kido, T.; Naruse, Y.; Suwazono, Y.; Nogawa, K. Effect of shift work on body mass index and metabolic parameters. Scand. J. Work. Environ. Health 2007, 33, 45–50. [Google Scholar] [CrossRef]
- Knutsson, A.; Kempe, A. Shift work and diabetes—A systematic review. Chronobiol. Int. 2014, 31, 1146–1151. [Google Scholar] [CrossRef]
- De Bacquer, D.; Van Risseghem, M.; Clays, E.; Kittel, F.; De Backer, G.; Braeckman, L. Rotating shift work and the metabolic syndrome: A prospective study. Int. J. Epidemiol. 2009, 38, 848–854. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, J.; Buckley, P.; Edwards, B.; Reilly, T. Measurement of, and some reasons for, differences in eating habits between night and day workers. Chronobiol. Int. 2003, 20, 1075–1092. [Google Scholar] [CrossRef]
- Nedeltcheva, A.V.; Kilkus, J.M.; Imperial, J.; Kasza, K.; Schoeller, D.A.; Penev, P.D. Sleep curtailment is accompanied by increased intake of calories from snacks. Am. J. Clin. Nutr. 2009, 89, 126–133. [Google Scholar] [CrossRef]
- Sargent, C.; Zhou, X.; Matthews, R.W.; Darwent, D.; Roach, G.D. Daily Rhythms of Hunger and Satiety in Healthy Men during One Week of Sleep Restriction and Circadian Misalignment. Int. J. Environ. Res. Public Health 2016, 13, 170. [Google Scholar] [CrossRef] [Green Version]
- Scheer, F.A.; Morris, C.J.; Shea, S.A. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity (Silver Spring) 2013, 21, 421–423. [Google Scholar] [CrossRef]
- Gamble, K.L.; Young, M.E. Circadian biology: The early bird catches the morning shift. Curr. Biol. 2015, 25, R269–R271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantermann, T.; Eastman, C.I. Circadian phase, circadian period and chronotype are reproducible over months. Chronobiol. Int. 2018, 35, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Juda, M.; Vetter, C.; Roenneberg, T. Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J. Biol. Rhythms 2013, 28, 141–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.; Tanigawa, T.; Takahashi, M.; Mutou, K.; Tachibana, N.; Kage, Y.; Iso, H. Shiftwork locus of control, situational and behavioural effects on sleepiness and fatigue in shiftworkers. Ind. Health 2005, 43, 151–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamble, K.L.; Motsinger-Reif, A.A.; Hida, A.; Borsetti, H.M.; Servick, S.V.; Ciarleglio, C.M.; Robbins, S.; Hicks, J.; Carver, K.; Hamilton, N.; et al. Shift work in nurses: Contribution of phenotypes and genotypes to adaptation. PLoS ONE 2011, 6, e18395. [Google Scholar] [CrossRef] [Green Version]
- Vetter, C.; Fischer, D.; Matera, J.L.; Roenneberg, T. Aligning work and circadian time in shift workers improves sleep and reduces circadian disruption. Curr. Biol. 2015, 25, 907–911. [Google Scholar] [CrossRef] [Green Version]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef]
- Lack, L.; Bailey, M.; Lovato, N.; Wright, H. Chronotype differences in circadian rhythms of temperature, melatonin, and sleepiness as measured in a modified constant routine protocol. Nat. Sci. Sleep 2009, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Patterson, F.; Malone, S.K.; Lozano, A.; Grandner, M.A.; Hanlon, A.L. Smoking, Screen-Based Sedentary Behavior, and Diet Associated with Habitual Sleep Duration and Chronotype: Data from the UK Biobank. Ann. Behav. Med. 2016, 50, 715–726. [Google Scholar] [CrossRef] [Green Version]
- Kanerva, N.; Kronholm, E.; Partonen, T.; Ovaskainen, M.L.; Kaartinen, N.E.; Konttinen, H.; Broms, U.; Mannisto, S. Tendency toward eveningness is associated with unhealthy dietary habits. Chronobiol. Int. 2012, 29, 920–927. [Google Scholar] [CrossRef]
- Mazri, F.H.; Manaf, Z.A.; Shahar, S.; Mat Ludin, A.F. The Association between Chronotype and Dietary Pattern among Adults: A Scoping Review. Int. J. Environ. Res. Public Health 2019, 17, 68. [Google Scholar] [CrossRef] [Green Version]
- Burgess, H.J.; Eastman, C.I. The dim light melatonin onset following fixed and free sleep schedules. J. Sleep Res. 2005, 14, 229–237. [Google Scholar] [CrossRef]
- Voultsios, A.; Kennaway, D.J.; Dawson, D. Salivary melatonin as a circadian phase marker: Validation and comparison to plasma melatonin. J. Biol. Rhythms 1997, 12, 457–466. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Stubbs, R.J.; Hughes, D.A.; Johnstone, A.M.; Rowley, E.; Reid, C.; Elia, M.; Stratton, R.; Delargy, H.; King, N.; Blundell, J.E. The use of visual analogue scales to assess motivation to eat in human subjects: A review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br. J. Nutr. 2000, 84, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Roenneberg, T.; Keller, L.K.; Fischer, D.; Matera, J.L.; Vetter, C.; Winnebeck, E.C. Human activity and rest in situ. Methods Enzymol. 2015, 552, 257–283. [Google Scholar]
- Dijk, D.J.; Duffy, J.F.; Czeisler, C.A. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J. Sleep Res. 1992, 1, 112–117. [Google Scholar] [CrossRef]
- Persson, M.; Mårtensson, J. Situations influencing habits in diet and exercise among nurses working night shift. J. Nurs. Manag. 2006, 14, 414–423. [Google Scholar] [CrossRef]
- Knauth, P.; Landau, K.; Droge, C.; Schwitteck, M.; Widynski, M.; Rutenfranz, J. Duration of sleep depending on the type of shift work. Int. Arch. Occup. Environ. Health 1980, 46, 167–177. [Google Scholar] [CrossRef] [PubMed]


| Chronotype Main Effect | Time of Day Main Effect | Chronotype × Time of Day Interaction Effect | |
|---|---|---|---|
| Hunger | F (2.67) = 0.23, p = 0.799 | F (1.8, 121.3) = 0.54, p = 0.564 | F (3.6, 121.3) = 0.43, p = 0.767 |
| Prospective Consumption | F (2.67) = 0.03, p = 0.975 | F (2.134) = 6.77, p = 0.002 | F (4.134) = 0.22, p = 0.927 |
| Desire to eat Fruit | F (2.67) = 1.81, p = 0.172 | F (2.134) = 26.05, p < 0.001 | F (4.134) = 0.50, p = 0.734 |
| Desire to eat Fast Food | F (2.67) = 0.74, p = 0.482 | F (2.134) = 35.23, p < 0.001 | F (4.134) = 1.17, p = 0.325 |
| Snack Consumption | F (2.68) = 0.88, p = 0.420 | F (2.72, 185.1)= 4.00, p = 0.011 | F (5.4, 185.1) = 0.87, p = 0.511 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiter, A.M.; Roach, G.D.; Sargent, C. No Effect of Chronotype on Hunger or Snack Consumption during a Night Shift with Acute Sleep Deprivation. Nutrients 2022, 14, 1324. https://doi.org/10.3390/nu14071324
Reiter AM, Roach GD, Sargent C. No Effect of Chronotype on Hunger or Snack Consumption during a Night Shift with Acute Sleep Deprivation. Nutrients. 2022; 14(7):1324. https://doi.org/10.3390/nu14071324
Chicago/Turabian StyleReiter, Andrew M., Gregory D. Roach, and Charli Sargent. 2022. "No Effect of Chronotype on Hunger or Snack Consumption during a Night Shift with Acute Sleep Deprivation" Nutrients 14, no. 7: 1324. https://doi.org/10.3390/nu14071324
APA StyleReiter, A. M., Roach, G. D., & Sargent, C. (2022). No Effect of Chronotype on Hunger or Snack Consumption during a Night Shift with Acute Sleep Deprivation. Nutrients, 14(7), 1324. https://doi.org/10.3390/nu14071324