Sweetness of Chilean Infants’ Diets: Methodology and Description
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of Original and Current Study
2.2. Sample
2.3. Anthropometry
2.4. Dietary Assessment
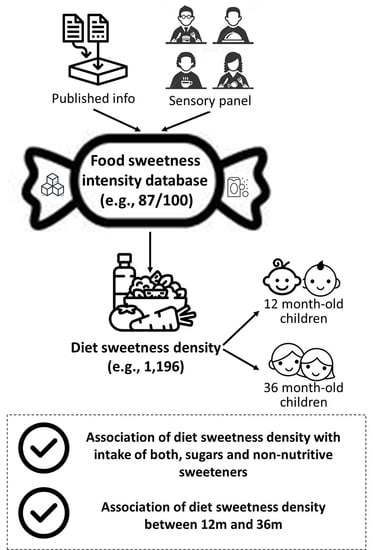
2.5. Sweetness Intensity Value in Food and Beverages
2.5.1. Food Sweetness Intensity Databases
2.5.2. Sensory Evaluation of Sweetness
2.6. Estimation of Sweetness Density of Diet
2.7. Statistical Analysis
3. Results
3.1. Construction of the Food Sweetness Intensity Database
3.2. Sweetness Density Estimation of Child Diet at 12 and 36 Months of Age
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Selection Tests and Training for Panel
Appendix B. Sweetness Density Using Grams as Denominator
[All beverage densities are assumed to be 1 g/mL]
| 12-Month-Old | 36-Month-Old | |
|---|---|---|
| Sweetness density using grams, median (p25th–p75th) | ||
| Overall # | 815 (600–1.164) | 1.362 (1.001–1.847) |
| Males # | 851 (626–1.179) | 1.392 (1.056–1.903) |
| Females # | 756 (574–1.124) | 1.295 (929–1.794) |
| Normal weight # | 795 (615–1.164) | 1.322 (976–1.885) |
| Overweight # | 852 (597–1.124) | 1.420 (1.011–1.817) |
| Obese # | 625 (513–1.268) | 1.343 (1.056–1.794) |
| Association between both sweetness density indicators, beta coefficient (95% CI) * | 1.35 (1.28; 1.41) | 1.15 (1.05; 1.25) |
| Association sweetness density using grams with sugars intake, beta coefficient (95% CI) | 11.6 (6.8; 16.5) | 22.9 (15.4; 30.4) |
| Association sweetness density using grams with NNS intake, beta coefficient (95% CI) | 191.8 (103.4; 280.2) | 553.3 (414.9; 691.7) |
References
- WHO. Guideline: Sugars Intake for Adults and Children; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- WHO. Diet, Nutrition and the Prevention of Chronic Diseases. World Health Organization Technical Report Series. 2003; Volume 916. Available online: http://apps.who.int/iris/bitstream/handle/10665/42665/WHO_TRS_916.pdf;jsessionid=0E3A57A192BC6DBF23472200BCF4BE93?sequence=1 (accessed on 28 January 2022).
- Popkin, B.; Hawkes, C. The sweetening of the global diet, particularly beverages: Patterns, trends and policy responses for diabetes prevention. Lancet Diabetes Endocrinol. 2015, 4, 174–186. [Google Scholar] [CrossRef] [Green Version]
- Azaïs-Braesco, V.; Sluik, D.; Maillot, M.; Kok, F.; Moreno, L.A. A review of total & added sugar intakes and dietary sources in Europe. Nutr. J. 2017, 16, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisberg, M.; Kovalskys, I.; Gómez, G.; Rigotti, A.; Sanabria, L.Y.C.; García, M.C.Y.; Torres, R.G.P.; Herrera-Cuenca, M.; Zimberg, I.Z.; Koletzko, B.; et al. Total and Added Sugar Intake: Assessment in Eight Latin American Countries. Nutrients 2018, 10, 389. [Google Scholar] [CrossRef] [Green Version]
- Drewnowski, A.; Rehm, C.D. Consumption of added sugars among us children and adults by food purchase location and food source. Am. J. Clin. Nutr. 2014, 100, 901–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fidler Mis, N.; Braegger, C.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Hulst, J.; Indrio, F.; Lapillonne, A.; et al. ESPGHAN Committee on Nutrition: Sugar in Infants, Children and Adolescents: A Position Paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 681–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Campos, M.; González, M.S.J.; Martín, J.D.; Comité de Nutrición de la Asociación Española de Pediatría. Uso de azúcares y edulcorantes en la alimentación del niño. Recomendaciones del Comité de Nutrición de la Asociación Española de Pediatría. An. Pediatría 2015, 83, 353.e1–353.e7. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; De Banate, M.A.; Rother, K.I. Artificial Sweeteners: A systematic review of metabolic effects in youth. Int. J. Pediatr. Obes. 2010, 5, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Ventura, A.K.; Mennella, J.A. Innate and learned preferences for sweet taste during childhood. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, R.; Fonseca, E.; Simon, S.A. The neuroscience of sugars in taste, gut-reward, feeding circuits, and obesity. Cell. Mol. Life Sci. 2020, 77, 3469–3502. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K. Why do we like sweet taste: A bitter tale? Physiol. Behav. 2016, 164, 432–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Regional Office for Europe. Policy Statement and Recommended Actions for Lowering Sugar Intake and Reducing Prevalence of Type 2 Diabetes and Obesity in the Eastern Mediterranean Region; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- PAHO; WHO. Nutrient Profile Model; WHO: Washington, DC, USA, 2016; Available online: http://iris.paho.org/xmlui/handle/123456789/18622 (accessed on 28 January 2022).
- Appleton, K.; Tuorila, H.; Bertenshaw, E.; De Graaf, C.; Mela, D. Sweet taste exposure and the subsequent acceptance and preference for sweet taste in the diet: Systematic review of the published literature. Am. J. Clin. Nutr. 2018, 107, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Danyliw, A.D.; Vatanparast, H.; Nikpartow, N.; Whiting, S.J. Beverage intake patterns of Canadian children and adolescents. Public Health Nutr. 2011, 14, 1961–1969. [Google Scholar] [CrossRef] [Green Version]
- Divert, C.; Chabanet, C.; Schoumacker, R.; Martin, C.; Lange, C.; Issanchou, S.; Nicklaus, S. Relation between sweet food consumption and liking for sweet taste in French children. Food Qual. Prefer. 2017, 56, 18–27. [Google Scholar] [CrossRef]
- Yuan, W.L.; Lange, C.; Schwartz, C.; Martin, C.; Chabanet, C.; de Lauzon-Guillain, B.; Nicklaus, S. Infant dietary exposures to sweetness and fattiness increase during the first year of life and are associated with feeding practices. J. Nutr. 2016, 146, 2334–2342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zancheta Ricardo, C.; Corvalán, C.; Smith Taillie, L.; Quitral, V.; Reyes, M. Changes in the Use of Non-nutritive Sweeteners in the Chilean Food and Beverage Supply After the Implementation of the Food Labeling and Advertising Law. Front. Nutr. 2021, 8, 773450. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.M.; Delahunty, C.M.; Baxter, I.A. Descriptive sensory analysis: Past, present and future. Food Res. Int. 2001, 34, 461–471. [Google Scholar] [CrossRef]
- Lease, H.; Hendrie, G.A.; Poelman, A.A.M.; Delahunty, C.; Cox, D.N. A Sensory-Diet database: A tool to characterize the sensory qualities of diets. Food Qual. Prefer. 2016, 49, 20–32. [Google Scholar] [CrossRef]
- Martin, C.; Visalli, M.; Lange, C.; Schlich, P.; Issanchou, S. Creation of a food taste database using an in-home “taste” profile method. Food Qual. Prefer. 2014, 36, 70–80. [Google Scholar] [CrossRef]
- van Langeveld, A.W.; Teo, P.S.; de Vries, J.H.; Feskens, E.J.; de Graaf, C.; Mars, M. Dietary taste patterns by sex and weight status in the Netherlands. Br. J. Nutr. 2018, 119, 1195–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gluckman, P.D.; Buklijas, T.; Hanson, M.A. The Developmental Origins of Health and Disease (DOHaD) Concept: Past, Present, and Future. In The Epigenome and Developmental Origins of Health and Disease; Academic Press: Cambridge, MA, USA, 2015; pp. 1–15. [Google Scholar]
- Toro-Campos, R.; Algarín, C.; Peirano, P.; Peña, M.; Murguia-Peniche, T.; Wu, S.S.; Uauy, R. Effect of feeding mode on infant growth and cognitive function: Study protocol of the Chilean infant Nutrition randomized controlled Trial (ChiNuT). BMC Pediatrics 2020, 20, 225. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; WHO Child Growth Standards: Geneva, Switzerland, 2006. [Google Scholar]
- World Health Organization. Obesity and Overweight. 2021. Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 28 January 2022).
- FAO. Dietary Assessment. A Resource Guide to Method Selection and Application in Low Resource Settings. 2018. Available online: http://www.fao.org/3/I9940EN/i9940en.pdf (accessed on 28 January 2022).
- Jensen, M.L.; Corvalán, C.; Reyes, M.; Popkin, B.M.; Taillie, L.S. Snacking patterns among Chilean children and adolescents: Is there potential for improvement? Public Health Nutr. 2019, 22, 2803–2812. [Google Scholar] [CrossRef] [PubMed]
- USDA. Agriculture Research Service USDA. National Nutrient Database for Standard Reference Release 28. Available online: https://data.nal.usda.gov/dataset/composition-foods-raw-processed-prepared-usda-national-nutrient-database-standard-reference-release-28-0 (accessed on 28 January 2022).
- Faculty of Chemical and Pharmaceutical Sciences, Department of Food Sciences and Chemical Technology, University of Chile. Table of Chemical Composition of Chilean Foods. Eighth Edition 1990. Available online: https://libros.uchile.cl/files/presses/1/monographs/426/submission/proof/files/assets/common/downloads/publication.pdf (accessed on 28 January 2022).
- Kanter, R.; Reyes, M.; Corvalán, C. Photographic methods for measuring packaged food and beverage products in supermarkets. Curr. Dev. Nutr. 2017, 1, e001016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanter, R.; Reyes, M.; Swinburn, B.; Vandevijvere, S.; Corvalán, C. The food supply prior to the implementation of the chilean law of food labeling and advertising. Nutrients 2019, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Health of Chile, University of Chile. National Survey of Food Consumption; University of Chile: Santiago, Chile, 2014; Available online: http://web.minsal.cl/sites/default/files/ENCA-INFORME_FINAL.pdf (accessed on 28 January 2022).
- Venegas Hargous, C.; Reyes, M.; Smith Taillie, L.; González, C.G.; Corvalán, C. Consumption of non-nutritive sweeteners by pre-schoolers of the food and environment Chilean cohort (FECHIC) before the implementation of the Chilean food labelling and advertising law. Nutr. J. 2020, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Chilean Ministry of Health. Food Sanitary Regulation; Decree 977; Chilean Ministry of Health: Santiago, Chile, 1996. [Google Scholar]
- McDaniel, M.R.; Barker, E.; Lederer, C.L. Sensory characterization of human milk. J. Dairy Sci. 1989, 72, 1149–1158. [Google Scholar] [CrossRef]
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 2007.
- ISO 8586:2012; Sensory Analysis—General Guidance for Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2012.
- Cox, D.N.; Hendrie, G.A.; Lease, H.J.; Rebuli, M.A.; Barnes, M. How does fatty mouthfeel, saltiness or sweetness of diets contribute to dietary energy intake? Appetite 2018, 131, 36–43. [Google Scholar] [CrossRef]
- Ministry of Health of Chile. Feeding Guide for Children under 2 Years of Age. Feeding Guide to Adolescence, 4th ed.; Ministry of Health of Chile: Santiago, Chile, 2015. [Google Scholar]
- Reyes, M.; Smith Taillie, L.; Popkin, B.; Kanter, R.; Vandevijvere, S.; Corvalan, C. Changes in the amount of nutrient of packaged foods and beverages after the initial implementation of the Chilean Law of Food Labelling and Advertising: A nonexperimental prospective study. PLoS Med. 2020, 17, e1003220. [Google Scholar] [CrossRef]
- Araya, C.; Corvalán, C.; Cediel, G.; Taillie, L.S.; Reyes, M. Ultra-Processed Food Consumption Among Chilean Preschoolers Is Associated with Diets Promoting Non-communicable Diseases. Front. Nutr. 2021, 8, 601526. [Google Scholar] [CrossRef]
- Nguyen, A.N.; van Langeveld, A.W.; de Vries, J.H.; Ikram, M.A.; de Graaf, C.; Mars, M.; Voortman, T. Dietary taste patterns in early childhood: The Generation R Study. Am. J. Clin. Nutr. 2021, 113, 63–69. [Google Scholar] [CrossRef]
- Deglaire, A.; Méjean, C.; Castetbon, K.; Kesse-Guyot, E.; Hercberg, S.; Schlich, P. Associations between weight status and liking scores for sweet, salt and fat according to the gender in adults (The Nutrinet-Santé study). Eur. J. Clin. Nutr. 2015, 69, 40–46. [Google Scholar] [CrossRef]
- Guinard, J.-X. Sensory and consumer testing with children. Trends Food Sci. Technol. 2001, 11, 273–283. [Google Scholar] [CrossRef]
- De Graaf, C.; Zandstra, E.H. Sweetness intensity and pleasantness in children, adolescents, and adults. Physiol. Behav. 1999, 67, 513–520. [Google Scholar] [CrossRef]
- Naska, A.; Lagiou, A.; Lagiou, P. Dietary assessment methods in epidemiological research: Current state of the art and future prospects. F1000Research 2017, 6, 926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djekic, I.; Lorenzo, J.; Munekata, P.; Gagaoua, M.; Tomasevic, I. Review on characteristics of trained sensory panels in food science. J. Texture Stud. 2021, 52, 501–509. [Google Scholar] [CrossRef] [PubMed]
| Product | Sweetness Intensity Value Reported in Primary Database | Sweetness Intensity Value Measured in Current Study |
|---|---|---|
| Strawberry jam | 74 | 71 |
| Orange juice | 31 | 32 |
| Whole milk | 12 | 12 |
| Food or Liquid | Sweetness Intensity Value |
|---|---|
| Condensed milk † | 88 |
| Jam † | 74 |
| Flavorings (e.g., chocolate powder) ¥ | 65 |
| Carmel “Manjar” * | 64 |
| Soda (regular and diet) ¥ | 51 |
| Ice cream ¥ | 46 |
| Cola drinks ¥ | 44 |
| Breakfast cereal ¥ | 41 |
| Juice (regular and diet) ¥ | 40 |
| Sweet purees * | 38 |
| Cookies ¥ | 35 |
| Fruits ¥ | 30 |
| Banana ¥ | 30 |
| Yogurt ¥ | 30 |
| Apple ¥ | 20 |
| Milk ¥ | 12 |
| Peanuts † | 8 |
| Beef † | 4 |
| Bread † | 4 |
| Natural pasta † | 3 |
| Cheese † | 2 |
| Oil † | 1 |
| 12 Months | 36 Months | p-Value ** | |
|---|---|---|---|
| Female, % | 49.3 | 49.3 | - |
| Male, % | 50.7 | 50.7 | - |
| BMI z-score, mean ± SE | 0.74 ± 0.89 | 0.82 ± 1.09 | <0.01 |
| Normal weight, n (%) | 273 (62.6) | 262 (60.1) | <0.01 |
| Overweight, n (%) | 137 (31.4) | 129 (29.6) | |
| Obesity, n (%) | 26 (6.0) | 45 (10.3) | |
| Maternal education ≤ 12 years, n (%) | 191 (43.8) | 172 (39.4) | <0.01 |
| Maternal education > 12 years, n (%) | 245 (56.2) | 264 (60.6) | |
| Maternal weight status: Normal weight, n (%) | 112 (28.1) | 73 (19.9) | <0.01 |
| Maternal weight status: Overweight, n (%) | 122 (30.7) | 125 (34.1) | |
| Maternal weight status: Obese, n (%) | 164 (41.2) | 169 (46.0) |
| Predictor Variable | Sweetness Density | |
|---|---|---|
| 12 Months | 36 Months | |
| Total sugars, % of calories | 24.6 [17.3–31.9] * | 44.7 [33.2–56.1] * |
| Consumed non-nutritive sweetener † | 243.8 [107.4–380.2] * | 715.5 [494.8–936.2] * |
| Food Groups | 12 Months | 36 Months |
|---|---|---|
| Fruits | 27.3 | 13.7 |
| Beverages | 19.3 | 32.2 |
| Vegetables/algae and mushrooms | 17.7 | 5.7 |
| Baby foods | 13.3 | 2.7 |
| Dairy and substitutes (e.g., soy/almond drink) | 10.8 | 28.6 |
| Sugars and candy | 3.6 | 6.2 |
| Grains and bread | 3.2 | 6.0 |
| Meat and substitutes (e.g., soy) | 2.1 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, C.G.; Corvalán, C.; Reyes, M. Sweetness of Chilean Infants’ Diets: Methodology and Description. Nutrients 2022, 14, 1447. https://doi.org/10.3390/nu14071447
González CG, Corvalán C, Reyes M. Sweetness of Chilean Infants’ Diets: Methodology and Description. Nutrients. 2022; 14(7):1447. https://doi.org/10.3390/nu14071447
Chicago/Turabian StyleGonzález, Carmen Gloria, Camila Corvalán, and Marcela Reyes. 2022. "Sweetness of Chilean Infants’ Diets: Methodology and Description" Nutrients 14, no. 7: 1447. https://doi.org/10.3390/nu14071447
APA StyleGonzález, C. G., Corvalán, C., & Reyes, M. (2022). Sweetness of Chilean Infants’ Diets: Methodology and Description. Nutrients, 14(7), 1447. https://doi.org/10.3390/nu14071447