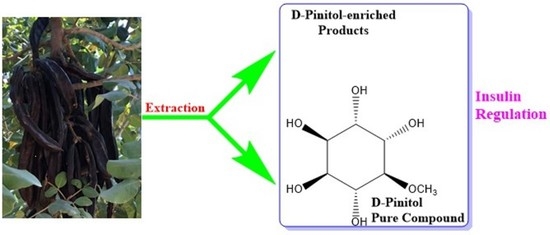
D-Pinitol—Active Natural Product from Carob with Notable Insulin Regulation
Abstract
:1. Introduction
1.1. Carob: The Faithful Companion of Humanity
1.2. Insulin Resistance in Type 2 Diabetes
1.3. Treatment of Insulin Resistance with Natural Products
2. Inositols—A Brief Presentation
3. D-Pinitol: Occurrence, Isolation, and Properties
4. D-Pinitol as Insulin Regulator
5. Discussion
- (A)
- D-Pinitol content of Carob (pods) is the highest of all plants [64].
- (B)
- D-Pinitol-containing products of Carob such as molasses, have important health benefits [157].
- (C)
- Compared with most other natural products that have insulin-regulation activity, such as polyphenols, D-Pinitol is more stable in biological gastric conditions [48]. This property increases its bioavailability in the human body.
- (D)
- (E)
- (F)
- D-Pinitol has wide range of medicinal activities (Table 5), so it is a multi-functional natural product. This property increases its potential as a drug.
6. Conclusions and Future Horizons
Funding
Conflicts of Interest
References
- Azab, A. Carob (Ceratonia siliqua): Super Food and Medicine. Literature Update. Eur. Chem. Bull. 2020, 9, 306–312. [Google Scholar] [CrossRef]
- Brassesco, M.E.; Brandao, T.; Silva, C.; Pintado, M. Carob bean (Ceratonia siliqua L.): A new perspective for functional food. Trends Food Sci. Technol. 2021, 114, 310–322. [Google Scholar] [CrossRef]
- Azab, A. Carob (Ceratonia siliqua): Health, medicine and chemistry. Eur. Chem. Bull. 2017, 6, 456–469. [Google Scholar] [CrossRef] [Green Version]
- Ben Ayache, S.; Behija Saafi, E.; Emhemmed, F.; Flamini, G.; Achour, L.; Muller, C.D. Biological activities of aqueous extracts from Carob plant (Ceratonia siliqua L.) by antioxidant, analgesic and proapoptotic properties evaluation. Molecules 2020, 25, 3120. [Google Scholar] [CrossRef]
- Khalifa, A.B. Herbs: Nature’s Pharmacy, 1st ed.; Arab Cultural Center: Casablanca, Morocco, 2004; pp. 286–288. [Google Scholar]
- Saad, B.; Said, O. Greco-Arab and Islamic Herbal Medicine; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; p. 308. [Google Scholar] [CrossRef]
- Nasar-Abbas, S.M.; E-Huma, Z.; Vu, T.H.; Khan, M.K.; Esbenshade, H.; Jayasena, V. Carob kibble: A bioactive-rich food ingredient. Compr. Rev. Food Sci. Food Saf. 2016, 15, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Lakkab, I.; El Hajaji, H.; Lachkar, N.; El Bali, B.; Lachkar, M.; Ciobica, A. Phytochemistry, bioactivity: Suggestion of Ceratonia siliqua L. as neurodegenerative disease therapy. J. Complement. Integr. Med. 2018, 15, 20180013. [Google Scholar] [CrossRef]
- Zhu, B.J.; Zayed, M.Z.; Zhu, H.-X.; Zhao, J.; Li, S.-P. Functional polysaccharides of carob fruit: A review. Chin. Med. 2019, 14, 40. [Google Scholar] [CrossRef] [Green Version]
- Rtibi, K.; Selmi, S.; Grami, D.; Amri, M.; Eto, B.; El-Benna, J.; Sebai, H.; Marzouki, L. Chemical constituents and pharmacological actions of carob pods and leaves (Ceratonia siliqua L.) on the gastrointestinal tract: A review. Biomed. Pharmacother. 2017, 93, 522–528. [Google Scholar] [CrossRef]
- Papagiannopoulos, M.; Wollseifen, H.R.; Mellenthin, A.; Haber, B.; Galensa, R. Identification and quantification of polyphenols in carob fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MS. J. Agric. Food Chem. 2004, 52, 3784–3791. [Google Scholar] [CrossRef]
- Gohar, A.; Gedara, S.R.; Baraka, H.N. New acylated flavonol glycoside from Ceratonia siliqua L. seeds. J. Med. Plants Res. 2009, 3, 424–428. [Google Scholar] [CrossRef]
- Cavdarova, M.; Makris, D.P. Extraction Kinetics of Phenolics from Carob (Ceratonia siliqua L.) Kibbles Using Environmentally Benign Solvents. Waste Biomass Valori. 2014, 5, 773–779. [Google Scholar] [CrossRef]
- Benković, M.; Bosiljkov, T.; Semić, A.; Ježek, D.; Srečec, S. Influence of Carob Flour and Carob Bean Gum on Rheological Properties of Cocoa and Carob Pastry Fillings. Foods 2019, 8, 66. [Google Scholar] [CrossRef] [Green Version]
- Santonocito, D.; Granata, G.; Geraci, C.; Panico, A.; Siciliano, E.A.; Raciti, G.; Puglia, C. Carob Seeds: Food Waste or Source of Bioactive Compounds? Pharmaceutics 2020, 12, 1090. [Google Scholar] [CrossRef]
- Reed, J.; Bain, S.; Kanamarlapudi, V. A Review of Current Trends with Type 2 Diabetes Epidemiology, Aetiology, Pathogenesis, Treatments and Future Perspectives. Diabetes Metab. Syndr. Obes. 2021, 14, 3567–3602. [Google Scholar] [CrossRef]
- Ganasegeran, K.; Hor, C.P.; Jamil, M.F.; Loh, H.C.; Noor, J.M.; Hamid, N.A.; Suppiah, P.D.; Abdul Manaf, M.R.; Ch’ng, A.S.; Looi, I. A Systematic Review of the Economic Burden of Type 2 Diabetes in Malaysia. Int. J. Environ. Res. Public Health 2020, 17, 5723. [Google Scholar] [CrossRef]
- Rodríguez, J.E.; Campbell, K.M. Racial and Ethnic Disparities in Prevalence and Care of Patients with Type 2 Diabetes. Clin. Diabetes 2017, 35, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Peleg, O. The Relationship between Type 2 Diabetes, Differentiation of Self, and Emotional Distress: Jews and Arabs in Israel. Nutrients 2022, 14, 39. [Google Scholar] [CrossRef]
- Kahn, C.R. The molecular mechanism of insulin action. Annu. Rev. Med. 1985, 36, 429–451. [Google Scholar] [CrossRef]
- Ingle, P.V.; Yin, S.B.; Ying, B.J.; Leong, B.K.; Xin, T.Z.; Hwa, L.T.; Mun, L.T. Current Trends in Pharmacological Treatment of Type II Diabetes Mellitus. Int. J. Pharm. Res. Rev. 2018, 7, 1–15. [Google Scholar]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [Green Version]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Ahmad, F.; Misra, L.; Gupta, V.K.; Darokar, M.P.; Prakash, O.; Khan, F.; Shukla, R. Synergistic effect of (+)-pinitol from Saraca asoca with β-lactam antibiotics and studies on the in silico possible mechanism. J. Kor. Diabetes Assoc. 2005, 29, 344–351, Reprinted in J. Assian Nat. Prod. Res. 2015, 18, 72–183. [Google Scholar] [CrossRef]
- James, D.E.; Stöckli, J.; Birnbaum, M.J. The aetiology and molecular landscape of insulin resistance. Nat. Rev. Mol. Cell Biol. 2021, 22, 751–771. [Google Scholar] [CrossRef]
- Banks, W.A.; Rhea, E.M. The Blood-Brain Barrier, Oxidative Stress, and Insulin Resistance. Antioxidants 2021, 10, 1695. [Google Scholar] [CrossRef]
- Reading, C.L.; Stickney, D.R.; Flores-Riveros, J.; Destiche, D.A.; Ahlem, C.N.; Cefalu, W.T.; Frincke, J.M. A synthetic anti-inflammatory sterol improves insulin sensitivity in insulin-resistant obese impaired glucose tolerance subjects. Obesity 2013, 21, E343–E349. [Google Scholar] [CrossRef]
- Ravindran, R.; Mitra, K.; Arumugam, S.K.; Doble, M. Preparation of Curdlan sulphate—Chitosan nanoparticles as a drug carrier to target Mycobacterium smegmatis infected macrophages. Carbohydr. Polym. 2021, 258, 117686. [Google Scholar] [CrossRef]
- Xue, S.; Yin, J.; Shao, J.; Yu, Y.; Yang, L.; Wang, Y.; Xie, M.; Fussenegger, M.; Ye, H. A Synthetic-Biology-Inspired Therapeutic Strategy for Targeting and Treating Hepatogenous Diabetes. Mol. Ther. 2017, 25, 443–455. [Google Scholar] [CrossRef] [Green Version]
- Vieira, R.; Souto, S.B.; Sánchez-López, E.; Machado, A.L.; Severino, P.; Jose, S.; Santini, A.; Fortuna, A.; García, M.L.; Silva, A.M.; et al. Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome-Review of Classical and New Compounds: Part-I. Pharmaceuticals 2019, 12, 152. [Google Scholar] [CrossRef] [Green Version]
- Saadeldeen, F.S.; Niu, Y.; Wang, H.; Zhou, L.; Meng, L.; Chen, S.; Sun-Waterhouse, D.; Waterhouse, G.I.; Liu, Z.; Kang, W. Natural Products: Regulating Glucose Metabolism and Improving Insulin Resistance. Food Sci. Hum. Wellness 2020, 9, 214–228. [Google Scholar] [CrossRef]
- Li, J.; Bai, L.; Wei, F.; Zhao, J.; Wang, D.; Xiao, Y.; Yan, W.; Wei, J. Therapeutic Mechanisms of Herbal Medicines against Insulin Resistance: A Review. Front. Pharmacol. 2019, 10, 661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alaaeldin, R.; Abdel-Rahman, I.A.; Hassan, H.A.; Youssef, N.; Allam, A.E.; Abdelwahab, S.F.; Zhao, Q.L.; Fathy, M. Carpachromene Ameliorates Insulin Resistance in HepG2 Cells via Modulating IR/IRS1/PI3k/Akt/GSK3/FoxO1 Pathway. Molecules 2021, 26, 7629. [Google Scholar] [CrossRef] [PubMed]
- Deenadayalan, A.; Subramanian, V.; Paramasivan, V.; Veeraraghavan, V.P.; Rengasamy, G.; Sadagopan, J.C.; Rajagopal, P.; Jayaraman, S. Stevioside Attenuates Insulin Resistance in Skeletal Muscle by Facilitating IR/IRS-1/Akt/GLUT 4 Signaling Pathways: An In Vivo and In Silico Approach. Molecules 2021, 26, 7689. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Lamora, H.; Marrero, P.F.; Haro, D.; Relat, J. A Mixture of Pure, Isolated Polyphenols Worsens the Insulin Resistance and Induces Kidney and Liver Fibrosis Markers in Diet-Induced Obese Mice. Antioxidants 2022, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Ostenhof, O.; Pittner, F. The biosynthesis of myo-inositol and its isomers. Can. J. Chem. 1982, 60, 1863–1871. [Google Scholar] [CrossRef]
- Watkins, O.C.; Yong, H.E.; Sharma, N.; Chan, S.-Y. A review of the role of inositols in conditions of insulin dysregulation and in uncomplicated and pathological pregnancy. Crit. Rev. Food Sci. Nutr. 2022, 62, 1626–1673. [Google Scholar] [CrossRef]
- Kennington, A.S.; Hill, C.R.; Craig, J.; Bogardus, C.; Raz, I.; Ortmeyer, H.K.; Hansen, B.C.; Romero, G.; Larner, J. Low urinary chiro-inositol excretion in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1990, 323, 373–378. [Google Scholar] [CrossRef]
- Hallman, M.; Bry, K.; Hoppu, K.; Lappi, M.; Pohjavuori, M. Inositol supplementation in premature infants with respiratory distress syndrome. N. Engl. J. Med. 1992, 326, 1233–1239. [Google Scholar] [CrossRef]
- Asplin, I.; Galasko, G.; Larner, J. Chiro-inositol deficiency and insulin resistance: A comparison of the chiro-inositol- and the myo-inositol-containing insulin mediators isolated from urine, hemodialysate, and muscle of control and type II diabetic subjects. Proc. Natl. Acad. Sci. USA 1993, 90, 5924–5928. [Google Scholar] [CrossRef] [Green Version]
- Levine, J. Controlled trials of inositol in psychiatry. Eur. Neuropsychopharmacol. 1997, 7, 147–155. [Google Scholar] [CrossRef]
- Nestler, J.E.; Jakubowicz, D.J.; Reamer, P.; Gunn, R.D.; Allan, G. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. N. Engl. J. Med. 1999, 340, 1314–1320. [Google Scholar] [CrossRef] [Green Version]
- McLaurin, J.; Golomb, R.; Jurewicz, A.; Antel, J.P.; Fraser, P.E. Inositol stereoisomers stabilize an oligomeric aggregate of Alzheimer amyloid beta peptide and inhibit Aβ-induced toxicity. J. Biol. Chem. 2000, 275, 18495–18502. [Google Scholar] [CrossRef] [Green Version]
- Jung, T.S.; Hahm, J.R.; Kim, J.J.; Jung, J.H.; Kang, M.Y.; Moon, S.W.; Lee, K.W.; Kim, H.C.; Lee, J.D.; Kim, J.H.; et al. Determination of urinary Myo-/chiro-inositol ratios from Korean diabetes patients. Yonsei Med. J. 2005, 46, 532–538. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, N.R.; Lessa, L.M.; Kerntopf, M.R.; Sousa, C.M.; Alves, R.S.; Queiroz, M.G.; Price, J.; Heimark, D.B.; Larner, J.; Du, X.; et al. Inositols prevent and reverse endothelial dysfunction in diabetic rat and rabbit vasculature metabolically and by scavenging superoxide. Proc. Natl. Acad. Sci. USA 2006, 103, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Michell, R.H. Evolution of the diverse biological roles of inositols. Biochem. Soc. Symp. 2007, 74, 223–246. [Google Scholar] [CrossRef] [Green Version]
- Michell, R.H. Inositol derivatives: Evolution and functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 151–161. [Google Scholar] [CrossRef]
- Bizzarri, M.; Carlomagno, G. Inositol: History of an effective therapy for Polycystic Ovary Syndrome. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1896–1903. [Google Scholar] [PubMed]
- Mancini, M.; Andreassi, A.; Salvioni, M.; Pelliccione, F.; Mantellassi, G.; Banderali, G. Myoinositol and D-Chiro Inositol in Improving Insulin Resistance in Obese Male Children: Preliminary Data. Int. J. Endocrinol. 2016, 8720342. [Google Scholar] [CrossRef] [Green Version]
- Kalra, B.; Kalra, S.; Sharma, J.B. The inositols and polycystic ovary syndrome. Indian J. Endocrinol. Metab. 2016, 20, 720–724. [Google Scholar] [CrossRef]
- Hanna, R.; Wehbe, T.; Abou Jaoude, E. Metabolic Effects of D-Chiro-Inositol and Myo-Inositol in Polycystic Ovary Syndrome. Int. J. Clin. Endocrinol. Metab. 2017, 3, 029–033. [Google Scholar] [CrossRef]
- Orrù, B.; Circo, R.; Logoteta, P.; Petousis, S.; Carlomagno, G. Finding the best therapeutic approach for PCOS: The importance of inositol(s) bioavailability. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 83–88. [Google Scholar] [PubMed]
- Showell, M.G.; Mackenzie-Proctor, R.; Jordan, V.; Hodgson, R.; Farquhar, C. Inositol for subfertile women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2018, 12, CD012378. [Google Scholar] [CrossRef]
- Miñambres, I.; Cuixart, G.; Gonçalves, A.; Corcoy, R. Effects of inositol on glucose homeostasis: Systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2019, 38, 1146–1152. [Google Scholar] [CrossRef]
- Chhetri, D.R. Myo-Inositol and Its Derivatives: Their Emerging Role in the Treatment of Human Diseases. Front Pharmacol. 2019, 10, 1172. [Google Scholar] [CrossRef] [Green Version]
- Roseff, S.; Montenegro, M. Inositol Treatment for PCOS Should Be Science-Based and Not Arbitrary. Int. J. Endocrinol. 2020, 2020, 6461254. [Google Scholar] [CrossRef] [Green Version]
- Merviel, P.; James, P.; Bouée, S.; Le Guillou, M.; Rince, C.; Nachtergaele, C.; Kerla, V. Impact of myo-inositol treatment in women with polycystic ovary syndrome in assisted reproductive technologies. Reprod. Health 2021, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Watkins, O.C.; Chu, A.H.; Cutfield, W.; Godfrey, K.M.; Yong, H.E.; Chan, S.Y. Myo-inositol: A potential prophylaxis against premature onset of labour and preterm birth. Nutr. Res. Rev. 2021, 34, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cantelmi, T.; Lambiase, E.; Unfer, V.R.; Gambioli, R.; Unfer, V. Inositol treatment for psychological symptoms in Polycystic Ovary Syndrome women. Eur. Rev. Med. Pharmacol Sci. 2021, 25, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional Components of Carob Fruit: Linking the Chemical and Biological Space. Int. J. Mol. Sci. 2016, 17, 1875. [Google Scholar] [CrossRef] [PubMed]
- Skøt, L.; Egsgaard, H. Identification of ononitol and O-methyl-scyllo-inositol in pea root nodules. Planta 1984, 161, 32–36. [Google Scholar] [CrossRef]
- Sheveleva, E.; Chmara, W.; Bohnert, H.J.; Jensen, R.G. Increased Salt and Drought Tolerance by D-Ononitol Production in Transgenic Nicotiana tabacum L. Plant Physiol. 1997, 115, 1211–1219. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.I.; Kim, J.C.; Joo, H.J.; Jung, S.H.; Kim, J.J. Determination of total chiro-inositol content in selected natural materials and evaluation of the antihyperglycemic effect of pinitol isolated from soybean and carob. Food Sci. Biotechnol. 2005, 14, 441–445. [Google Scholar]
- Negishi, O.; Mun’im, A.; Negishi, Y. Content of methylated inositols in familiar edible plants. J. Agric. Food Chem. 2015, 63, 2683–2688. [Google Scholar] [CrossRef]
- Qiu, J.; Yan, X.; Liao, Y.; Yu, D.; Wen, C.; Xiang, Z. An UPLC-MS/MS method for quantification of D-pinitol in rat plasma and its application to a pharmacokinetic and bioavailability study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1163, 122498. [Google Scholar] [CrossRef]
- Fenili, D.; Weng, Y.Q.; Aubert, I.; Nitz, M.; McLaurin, J. Sodium/myo-Inositol transporters: Substrate transport requirements and regional brain expression in the TgCRND8 mouse model of amyloid pathology. PLoS ONE 2011, 6, e24032. [Google Scholar] [CrossRef] [Green Version]
- Pitt, J.; Thorner, M.; Brautigan, D.; Larner, J.; Klein, W.L. Protection against the synaptic targeting and toxicity of Alzheimer’s-associated Aβ oligomers by insulin mimetic chiro-inositols. FASEB J. 2013, 27, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Olivares-Martín, M.; Bañuelos-Hortigüela, O.; Pallàs, M. Synergistic Neuroprotective Effects of a Natural Product Mixture against AD Hallmarks and Cognitive Decline in Caenorhabditis elegans and an SAMP8 Mice Model. Nutrients 2021, 13, 2411. [Google Scholar] [CrossRef]
- Hada, B.; Yoo, M.R.; Seong, K.M.; Jin, Y.W.; Myeong, H.K.; Min, K.J. D-chiro-inositol and pinitol extend the life span of Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, R.; Chakrapani, G.; Mitra, K.; Doble, M. Inhibitory activity of traditional plants against Mycobacterium smegmatis and their action on Filamenting temperature sensitive mutant Z (FtsZ)-A cell division protein. PLoS ONE 2020, 15, e0232482. [Google Scholar] [CrossRef]
- Sethi, G.; Ahn, K.S.; Sung, B.; Aggarwal, B.B. Pinitol targets nuclear factor-kappaB activation pathway leading to inhibition of gene products associated with proliferation, apoptosis, invasion, and angiogenesis. Mol. Cancer Ther. 2008, 7, 1604–1614. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.H.; Tan, T.W.; Tsai, T.H.; Chen, C.C.; Hsieh, T.F.; Lee, S.S.; Liu, H.H.; Chen, W.C.; Tang, C.H. D-pinitol inhibits prostate cancer metastasis through inhibition of αVβ3 integrin by modulating FAK, c-Src and NF-κB pathways. Int. J. Mol. Sci. 2013, 14, 9790–9802. [Google Scholar] [CrossRef] [PubMed]
- Rengarajan, T.; Nandakumar, N.; Rajendran, P.; Haribabu, L.; Nishigaki, I.; Balasubramanian, M.P. D-pinitol promotes apoptosis in MCF-7 cells via induction of p53 and Bax and inhibition of Bcl-2 and NF-κB. Asian Pac. J. Cancer Prev. 2014, 15, 1757–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayasooriya, R.G.; Kang, G.-H.; Park, S.R.; Choi, Y.-H.; Kim, G.-Y. Pinitol Suppresses Tumor Necrosis Factor-α-Induced Invasion of Prostate Cancer LNCaP Cells by Inhibiting Nuclear Factor-κB-Mediated Matrix Metalloproteinase-9 Expression. Trop. J. Pharm. Res. 2015, 14, 1357–1364. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.-C.; Bang, T.-H.; Kang, H.-M.; Park, B.-S.; Kim, I.-R. Anticancer effects of D-pinitol in human oral squamous carcinoma cells. Int. J. Oral Biol. 2020, 45, 152–161. [Google Scholar] [CrossRef]
- Yao, X.; Shi, K.; Yang, Y.; Gu, X.; Tan, W.; Wang, Q.; Gao, X.; Veeraraghavan, V.P.; Mohan, S.K.; Jin, S. D-Pinitol treatment induced the apoptosis in human leukemia MOLT-4 cells by improved apoptotic signaling pathway. Saudi J. Biol. Sci. 2020, 27, 2134–2138. [Google Scholar] [CrossRef]
- Rengarajan, T.; Jagadeesan, A.J.; Balamurugan, A.; Balasubramanian, M.P. Chemotherapeutic potential of D-Pinitol against 7, 12 dimethylbenz (a) (DMBA) induced mammary carcinoma in Sprague Dawley rats. Int. J. Pharm. BioSci. 2011, 2, 232–241. [Google Scholar]
- Rengarajan, T.; Nandakumar, N.; Balasubramanian, M.P. D-Pinitol a low-molecular cyclitol prevents 7,12-Dimethylbenz a anthracene induced experimental breast cancer through regulating anti-apoptotic protein Bcl-2, mitochondrial and carbohydrate key metabolizing enzymes. Biomed. Prevent. Nutr. 2012, 2, 25–30. [Google Scholar] [CrossRef]
- Rengarajan, T.; Nandakumar, N.; Balasubramanian, M.P. D-Pinitol attenuates 7, 12 dimethylbenz a anthracene induced hazards through modulating protein bound carbohydrates, adenosine triphosphatases and lysosomal enzymes during experimental mammary carcinogenesis. J. Exp. Ther. Oncol. 2012, 10, 39–49. [Google Scholar]
- Rengarajan, T.; Nandakumar, N.; Balasubramanian, M.P. D-Pinitol prevents rat breast carcinogenesis induced by 7, 12 -Dimethylbenz aanthracene through inhibition of Bcl-2 and induction of p53, caspase-3 proteins and modulation of hepatic biotransformation enzymes and antioxidants. Biomed. Prevent. Nutr. 2013, 3, 31–41. [Google Scholar] [CrossRef]
- Venkatachalam, S.; Boobathi, L.; Balasubramanian, B.M. D-Pinitol Prevents Rat Colon Carcinogenesis Induced by Azoxymethane through Free Radical Formation Induced Cell Damage and Affects Enzymes and Antioxidants. Res. J. Pharm. Technol. 2014, 7, 845–849. [Google Scholar]
- Rengarajan, T.; Nandakumar, N.; Rajendran, P.; Ganesh, M.K.; Balasubramanian, M.P.; Nishigaki, I. D-pinitol mitigates tumor growth by modulating interleukins and hormones and induces apoptosis in rat breast carcinogenesis through inhibition of NF-κB. J. Physiol. Biochem. 2015, 71, 191–204. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, Y.; Su, J.; Wang, M.; Wu, X.; Su, Z.; Yi, X.; Wei, L.; Jian Cai, J.; Sun, Z. Therapeutic role of D-pinitol on experimental colitis via activating Nrf2/ARE and PPAR-γ/NF-κB signaling pathways. Food Funct. 2021, 12, 2554–2568. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Alba-Betancourt, C.; Rocha-González, E.; Ruiz-Arredondo, A.; Zapata-Morales, J.R.; Gasca-Martínez, D.; Pérez-Gutiérrez, S. Neuropharmacological effects of d-pinitol and its possible mechanisms of action. J. Food Biochem. 2019, 43, e13070. [Google Scholar] [CrossRef]
- Narayanan, C.R.; Joshi, D.D.; Muhumdar, A.M.; Dhekne, V.V. Pinitol—A new anti-diabetic compound from the leaves of Bougainvillea spectabilis. Curr. Sci. 1987, 56, 139–141. [Google Scholar]
- Sivakumar, S.; Subramanian, S.P. D-pinitol attenuates the impaired activities of hepatic key enzymes in carbohydrate metabolism of streptozotocin-induced diabetic rats. Gen. Physiol. Biophys. 2009, 28, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, S.; Subramanian, S.P. Pancreatic tissue protective nature of D-Pinitol studied in streptozotocin-mediated oxidative stress in experimental diabetic rats. Eur. J. Pharmacol. 2009, 622, 65–70. [Google Scholar] [CrossRef]
- Dang, N.T.; Mukai, R.; Yoshida, K.; Ashida, H. D-pinitol and myo-inositol stimulate translocation of glucose transporter 4 in skeletal muscle of C57BL/6 mice. Biosci. Biotechnol. Biochem. 2010, 74, 1062–1067. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.H.; Lee, C.C.; Wu, S.C. Ice plant (Mesembryanthemum crystallinum) improves hyperglycaemia and memory impairments in a Wistar rat model of streptozotocin-induced diabetes. J. Sci Food Agric. 2014, 94, 2266–2273. [Google Scholar] [CrossRef]
- Huang, B.; Wang, Z.; Park, J.H.; Ryu, O.H.; Choi, M.K.; Lee, J.Y.; Kang, Y.H.; Lim, S.S. Anti-diabetic effect of purple corn extract on C57BL/KsJ db/db mice. Nutr. Res. Pract. 2015, 9, 22–29. [Google Scholar] [CrossRef]
- Srivastava, K.; Dubey, A.; Tiwari, M.; Dubey, A. To evaluate the synergistic effect of Pinitol with Glimepride in diabetic Wistar rats. J. Crit. Rev. 2020, 7, 2058–2062. [Google Scholar]
- Kim, J.I.; Kim, J.C.; Kang, M.J.; Lee, M.S.; Kim, J.J.; Cha, I.J. Effects of pinitol isolated from soybeans on glycaemic control and cardiovascular risk factors in Korean patients with type II diabetes mellitus: A randomized controlled study. Eur. J. Clin. Nutr. 2005, 59, 456–458. [Google Scholar] [CrossRef]
- Kang, M.J.; Kim, J.I.; Yoon, S.Y.; Kim, J.C.; Cha, I.J. Pinitol from soybeans reduces postprandial blood glucose in patients with type 2 diabetes mellitus. J. Med. Food 2006, 9, 182–186. [Google Scholar] [CrossRef]
- Kim, M.J.; Yoo, K.H.; Kim, J.H.; Seo, Y.T.; Ha, B.W.; Kho, J.H.; Shin, Y.G.; Chung, C.H. Effect of pinitol on glucose metabolism and adipocytokines in uncontrolled type 2 diabetes. Diabetes Res. Clin. Pract. 2007, 77, S247–S251. [Google Scholar] [CrossRef]
- Hernández-Mijares, A.; Bañuls, C.; Peris, J.E.; Monzó, N.; Jover, A.; Bellod, L.; Victor, V.M.; Rocha, M. A single acute dose of pinitol from a naturally-occurring food ingredient decreases hyperglycaemia and circulating insulin levels in healthy subjects. Food Chem. 2013, 141, 1267–1272. [Google Scholar] [CrossRef]
- Lambert, C.; Cubedo, J.; Padró, T.; Vilahur, G.; López-Bernal, S.; Rocha, M.; Hernández-Mijares, A.; Badimon, L. Effects of a Carob-Pod-Derived Sweetener on Glucose Metabolism. Nutrients 2018, 10, 271. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Sakuraba, K.; Wada, T.; Watabane, N.; Wada, S.; Kitabayashi, Y.; Sunohara, M. Single-Dose Pinitol Ingestion Suppresses Post-Prandial Glucose Levels: A Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. Nat. Prod. Commun. 2019, 14, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.K.; Tewari, S.P. Theoretical evaluation of the bioactivity of plant-derived natural molecule D-Pinitol and other derived structure. AIP Conf. Proc. 2019, 2142, 150019. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Zapata-Morales, J.R.; Arana-Argáez, V.; Torres-Romero, J.C.; Ramírez-Villanueva, E.; Pérez-Medina, S.E.; Ramírez-Morales, M.A.; Juárez-Méndez, M.A.; Infante-Barrios, Y.P.; Martínez-Gutiérrez, F.; et al. Pharmacological and toxicological study of a chemical-standardized ethanol extract of the branches and leaves from Eysenhardtia polystachya (Ortega) Sarg. (Fabaceae). J. Ethnopharmacol. 2018, 224, 314–322. [Google Scholar] [CrossRef]
- Koh, E.S.; Kim, S.; Kim, M.; Hong, Y.A.; Shin, S.J.; Park, C.W.; Chang, Y.S.; Chung, S.; Kim, H.S. D-Pinitol alleviates cyclosporine A-induced renal tubulointerstitial fibrosis via activating Sirt1 and Nrf2 antioxidant pathways. Int. J. Mol. Med. 2018, 41, 1826–1834. [Google Scholar] [CrossRef]
- Geethan, P.K.; Prince, P.S. Antihyperlipidemic effect of D-pinitol on streptozotocin-induced diabetic Wistar rats. J. Biochem. Mol. Toxicol. 2008, 22, 220–224. [Google Scholar] [CrossRef]
- Singh, R.K.; Pandey, B.L.; Tripathi, M.; Pandey, V.B. Anti-inflammatory effect of (+)-pinitol. Fitoterapia 2001, 72, 168–170. [Google Scholar] [CrossRef]
- Kim, J.C.; Shin, J.Y.; Shin, D.H.; Kim, S.H.; Park, S.H.; Park, R.D.; Park, S.C.; Kim, Y.B.; Shin, Y.C. Synergistic antiinflammatory effects of pinitol and glucosamine in rats. Phytother. Res. 2005, 19, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.; Palsamy, P.; Subramanian, S.P. Impact of D-pinitol on the attenuation of proinflammatory cytokines, hyperglycemia-mediated oxidative stress and protection of kidney tissue ultrastructure in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2010, 188, 237–245. [Google Scholar] [CrossRef]
- Zheng, K.; Zhao, Z.; Lin, N.; Wu, Y.; Xu, Y.; Zhang, W. Protective Effect of Pinitol Against Inflammatory Mediators of Rheumatoid Arthritis via Inhibition of Protein Tyrosine Phosphatase Non-Receptor Type 22 (PTPN22). Med. Sci. Monit. 2017, 23, 1923–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.S.; Lee, W.H.; Kwon, E.Y.; Kang, M.A.; Lee, M.K.; Park, Y.B.; Jeon, S.M. Effects of soy pinitol on the pro-inflammatory cytokines and scavenger receptors in oxidized low-density lipoprotein-treated THP-1 macrophages. J. Med. Food. 2007, 10, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Eser, F.; Altundag, E.M.; Gedik, G.; Demirtas, I.; Onal, A.; Selvi, B. Anti-inflammatory effect of D-pinitol isolated from the leaves of Colutea cilicica Boiss et Bal. on K562 cells. Turk. J. Biochem. 2017, 42, 445–450. [Google Scholar] [CrossRef]
- Kong, J.; Du, Z.; Dong, L. Pinitol Prevents Lipopolysaccharide (LPS)-Induced Inflammatory Responses in BV2 Microglia Mediated by TREM2. Neurotox. Res. 2020, 38, 96–104. [Google Scholar] [CrossRef]
- López-Domènech, S.; Bañuls, C.; de Marañón, A.M.; Abab-Jiménez, Z.; Morillas, C.; Gómez-Abril, S.Á.; Rovira-Llopis, S.; Víctor, V.M.; Hernández-Mijares, A.; Rocha, M. Pinitol alleviates systemic inflammatory cytokines in human obesity by a mechanism involving unfolded protein response and sirtuin 1. Clin. Nutr. 2018, 37, 2036–2044. [Google Scholar] [CrossRef]
- Navarro, J.A.; Decara, J.; Medina-Vera, D.; Tovar, R.; Suarez, J.; Pavón, J.; Serrano, A.; Vida, M.; Gutierrez-Adan, A.; Sanjuan, C.; et al. D-Pinitol from Ceratonia siliqua Is an Orally Active Natural Inositol That Reduces Pancreas Insulin Secretion and Increases Circulating Ghrelin Levels in Wistar Rats. Nutrients 2020, 12, 2030. [Google Scholar] [CrossRef]
- Yu, J.; Choi, S.; Park, E.S.; Shin, B.; Yu, J.; Lee, S.H.; Takami, M.; Kang, J.S.; Meong, H.; Rho, J. D-chiro-inositol negatively regulates the formation of multinucleated osteoclasts by down-regulating NFATc1. J. Clin. Immunol. 2012, 32, 1360–1371. [Google Scholar] [CrossRef]
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Balasubramanian, M.P.; Nishigaki, I. Free radical scavenging and antioxidant activity of D-pinitol against 7, 12 dimethylbenz(a) anthracene induced breast cancer in sprague dawley rats. Asian Pac. J. Trop. Dis. 2014, 4, 384–390. [Google Scholar] [CrossRef]
- Ma, J.; Feng, S.; Ai, D.; Liu, Y.; Yang, X. D-Pinitol Ameliorates Imiquimod-Induced PsoriasisLike Skin Inflammation in a Mouse Model via the NF-κB Pathway. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 285–295. [Google Scholar] [CrossRef]
- Suresh, K.G.; Manivannan, R.; Nivetha, B. In-silico docking analysis of phytochemicals from mimosa pudica l. Leaves as an antiviral agent against herpes simplex virus type I. Int. J. Biomed. NanoLet. 2021, 1, 1–9. [Google Scholar]
- Lee, J.S.; Lee, C.M.; Jeong, Y.I.; Jung, I.D.; Kim, B.H.; Seong, E.Y.; Kim, J.I.; Choi, I.W.; Chung, H.Y.; Park, Y.M. D-pinitol regulates Th1/Th2 balance via suppressing Th2 immune response in ovalbumin-induced asthma. FEBS Lett. 2007, 581, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.C.; Chuang, S.M.; Tang, C.H. D-pinitol inhibits RANKL-induced osteoclastogenesis. Int. Immunopharmacol. 2012, 12, 494–500. [Google Scholar] [CrossRef]
- Sudha, M.; Vetrichelvan, T. Protective effect of D-Pinitol isolated from aerial parts of soybean plants on haematological profile against Doxorubicin-induced cyto-toxicity in mice. Int. J. Pharm. Sci. Res. 2021, 12, 2926–2932. [Google Scholar] [CrossRef]
- Li, X.L.; Xu, M.; Yu, F.; Fu, C.L.; Yu, X.; Cheng, M.; Gao, H.Q. Effects of D-pinitol on myocardial apoptosis and fibrosis in streptozocin-induced aging-accelerated mice. J. Food Biochem. 2021, 45, e13669. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, Y.; LV, X.; Feng, Z. Elucidation of the mechanism of action of pinitol against pressure overload-induced cardiac hypertrophy and fibrosis in an animal model of aortic stenosis. Biosci. Biotechnol. Biochem. 2021, 85, 643–655. [Google Scholar] [CrossRef]
- Cordero, C.P.; Pinzon, R.; Aristizabal, F.A. Cytotoxicity of bixin, rutin, pinitol B and ent-16-kauren-19-oic acid isolated from Colombian plants. Rev. Col. Cienc. Quím. Farm. 2003, 32, 137–140. [Google Scholar]
- Alonso-Castro, A.J.; Alba-Betancourt, C.; Yáñez-Barrientos, E.; Luna-Rocha, C.; Páramo-Castillo, A.S.; Aragón-Martínez, O.H.; Zapata-Morales, J.R.; Cruz-Jiménez, G.; Gasca-Martínez, D.; González-Ibarra, A.A.; et al. Diuretic activity and neuropharmacological effects of an ethanol extract from Senna septemtrionalis (Viv.) H.S. Irwin & Barneby (Fabaceae). J. Ethnopharmacol. 2019, 239, 111923. [Google Scholar] [CrossRef]
- Sudha, M.; Vetrichelvan, T. Genoprotective effect of D-Pinitol isolated from aerial parts of Soybean plants against Doxorubicin-induced genotoxicity evaluated by in vitro comet assay in Vero cell lines. Int. J. Res. Pharm. Sci. 2021, 12, 1379–1384. [Google Scholar] [CrossRef]
- Lee, E.; Lim, Y.; Kwon, S.W.; Kwon, O. Pinitol consumption improves liver health status by reducing oxidative stress and fatty acid accumulation in subjects with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. J. Nutr. Biochem. 2019, 68, 33–41. [Google Scholar] [CrossRef]
- Ostlund, R.E.; Seemayer, R.; Gupta, S.; Kimmel, R.; Ostlund, E.L.; Sherman, W.R. A stereospecific myo-inositol/D-chiro-inositol transporter in HepG2 liver cells. Identification with D-chiro-3-3Hinositol. J. Biol. Chem. 1996, 271, 10073–10078. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Park, C.M.; Cho, C.W.; Song, Y.S. Protective effect of pinitol against D-galactosamine-induced hepatotoxicity in rats fed on a high-fat diet. Biosci. Biotechnol. Biochem. 2008, 72, 1657–1666. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, S.; Palsamy, P.; Subramanian, S.P. Attenuation of oxidative stress and alteration of hepatic tissue ultrastructure by D-pinitol in streptozotocin-induced diabetic rats. Free Radic. Res. 2010, 44, 668–678. [Google Scholar] [CrossRef]
- Magielse, J.; Arcoraci, T.; Breynaert, A.; van Dooren, I.; Kanyanga, C.; Fransen, E.; Van Hoof, V.; Vlietinck, A.; Apers, S.; Pieters, L.; et al. Antihepatotoxic activity of a quantified Desmodium adscendens decoction and D-pinitol against chemically-induced liver damage in rats. J. Ethnopharmacol. 2013, 146, 250–256. [Google Scholar] [CrossRef]
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Lokeshkumar, B.; Balasubramanian, M.P. D-Pinitol Protects Against Carbon Tetrachloride-Induced Hepatotoxicity in Rats. J. Environ. Pathol. Toxicol. Oncol. 2015, 34, 287–298. [Google Scholar] [CrossRef]
- Yan, L.; Luo, H.; Li, X.; Li, Y. d-Pinitol protects against endoplasmic reticulum stress and apoptosis in hepatic ischemia-reperfusion injury via modulation of AFT4-CHOP/GRP78 and caspase-3 signaling pathways. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211032098. [Google Scholar] [CrossRef]
- Da Silva, J.A.; Da Silva, A.C.; Figueiredo, L.S.; Araujo, T.R.; Freitas, I.N.; Carneiro, E.M.; Ribeiro, E.S.; Ribeiro, R.A. D-Pinitol Increases Insulin Secretion and Regulates Hepatic Lipid Metabolism in Msg-Obese Mice. An. Acad. Bras. Cienc. 2020, 92, e20201382. [Google Scholar] [CrossRef]
- Muñoz, C.X.; Johnson, E.C.; Kunces, L.J.; McKenzie, A.L.; Wininger, M.; Butts, C.L.; Caldwell, A.; Seal, A.; McDermott, B.P.; Vingren, J.; et al. Impact of Nutrient Intake on Hydration Biomarkers Following Exercise and Rehydration Using a Clustering-Based Approach. Nutrients 2020, 12, 1276. [Google Scholar] [CrossRef]
- Adams, W.M.; Wininger, M.; Zaplatosch, M.E.; Hevel, D.J.; Maher, J.P.; McGuirt, J.T. Influence of Nutrient Intake on 24 Hour Urinary Hydration Biomarkers Using a Clustering-Based Approach. Nutrients 2020, 12, 2933. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.N.; Silva, J.F.; Silva, G.C.; Lemos, V.S.; Cortes, S.F. Activation of eNOS by D-pinitol Induces an Endothelium-Dependent Vasodilatation in Mouse Mesenteric Artery. Front. Pharmacol. 2018, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Jung, I.D.; Jeong, Y.I.; Lee, C.M.; Shin, Y.K.; Lee, S.Y.; Suh, D.S.; Yoon, M.S.; Lee, K.S.; Choi, Y.H.; et al. D-pinitol inhibits Th1 polarization via the suppression of dendritic cells. Int. Immunopharmacol. 2007, 7, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.J.; Lee, J.W.; Shim, S.B.; Jee, S.W.; Lee, S.H.; Woo, J.M.; Lee, C.K.; Hwang, D.Y. GATA binding protein 3 overexpression and suppression significantly contribute to the regulation of allergic skin inflammation. Int. J. Mol. Med. 2011, 28, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Gupta, K.K.; Bani, S. The immunosuppressive effects of Agyrolobium roseum and pinitol in experimental animals. Int. Immunopharmacol. 2011, 11, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Brautigan, D.L.; Brown, M.; Grindrod, S.; Chinigo, G.; Kruszewski, A.; Lukasik, S.M.; Bushweller, J.H.; Horal, M.; Keller, S.; Tamura, S.; et al. Allosteric activation of protein phosphatase 2C by D-chiro-inositol-galactosamine, a putative mediator mimetic of insulin action. Biochemistry 2005, 44, 11067–11173. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.H.; Yoon, J.H.; Li, H.; Kang, J.H.; Ji, H.S.; Park, K.H.; Shin, D.H.; Park, H.Y.; Jeong, T.S. Pterocarpan-enriched soy leaf extract ameliorates insulin sensitivity and pancreatic β-cell proliferation in type 2 diabetic mice. Molecules 2014, 19, 18493–18510. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, M.; Wu, T.; Xu, M.; Cai, H.; Zhang, Z. Effects of D-Pinitol on Insulin Resistance through the PI3K/Akt Signaling Pathway in Type 2 Diabetes Mellitus Rats. J. Agric. Food Chem. 2015, 63, 6019–6026. [Google Scholar] [CrossRef]
- Medina-Vera, D.; Navarro, J.A.; Tovar, R.; Rosell-Valle, C.; Gutiérrez-Adan, A.; Ledesma, J.C.; Sanjuan, C.; Pavón, F.J.; Baixeras, E.; Rodríguez de Fonseca, F.; et al. Activation of PI3K/Akt Signaling Pathway in Rat Hypothalamus Induced by an Acute Oral Administration of D-Pinitol. Nutrients 2021, 13, 2268. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, K.S.; Lee, S.K.; Min, K.W.; Han, K.A.; Kim, Y.K.; Ku, B.J. Effects of pinitol on glycemic control, insulin resistance and adipocytokine levels in patients with type 2 diabetes mellitus. Ann. Nutr. Metab. 2012, 60, 1–5. [Google Scholar] [CrossRef]
- Do, G.M.; Choi, M.S.; Kim, H.J.; Woo, M.N.; Lee, M.K.; Jeon, S.M. Soy pinitol acts partly as an insulin sensitizer or insulin mediator in 3T3-L1 preadipocytes. Genes Nutr. 2008, 2, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Siracusa, L.; Occhiuto, C.; Molonia, M.S.; Cimino, F.; Palumbo, M.; Saija, A.; Speciale, A.; Rocco, C.; Ruberto, G.; Cristani, M. A pinitol-rich Glycyrrhiza glabra L. leaf extract as functional supplement with potential in the prevention of endothelial dysfunction through improving insulin signalling. Arch. Physiol. Biochem. 2020, 1–10. [Google Scholar] [CrossRef]
- Vasaikar, N.; Mahajan, U.; Patil, K.R.; Suchal, K.; Patil, C.R.; Ojha, S.; Goyal, S.N. D-pinitol attenuates cisplatin-induced nephrotoxicity in rats: Impact on pro-inflammatory cytokines. Chem. Biol. Interact. 2018, 290, 6–11. [Google Scholar] [CrossRef]
- Farias, V.X.; Macêdo, F.H.; Oquendo, M.B.; Tomé, A.R.; Báo, S.N.; Cintra, D.O.; Santos, C.F.; Albuquerque, A.A.; Heimark, D.B.; Larner, J.; et al. Chronic treatment with D-chiro-inositol prevents autonomic and somatic neuropathy in STZ-induced diabetic mice. Diabetes Obes. Metab. 2011, 13, 243–250. [Google Scholar] [CrossRef]
- Dong, W.; Zhao, S.; Wen, S.; Dong, C.; Chen, Q.; Gong, T.; Chen, W.; Liu, W.; Mu, L.; Shan, H.; et al. A preclinical randomized controlled study of ischemia treated with Ginkgo biloba extracts: Are complex components beneficial for treating acute stroke? Curr. Res. Transl. Med. 2020, 68, 197–203. [Google Scholar] [CrossRef]
- An, Y.; Li, J.; Liu, Y.; Fan, M.; Tian, W. Protective effect of D-pinitol on the experimental spinal cord injury in rats. Metab. Brain Dis. 2020, 35, 473–482. [Google Scholar] [CrossRef]
- Sakata, K.; Kawasaki, H.; Suzuki, T.; Ito, K.; Negishi, O.; Tsuno, T.; Tsuno, H.; Yamazaki, Y.; Ishida, N. Inositols affect the mating circadian rhythm of Drosophila melanogaster. Front. Pharmacol. 2005, 6, 111. [Google Scholar] [CrossRef] [Green Version]
- Rahaman, M.S.; Yamasaki, S.; Binte Hossain, K.F.; Hosokawa, T.; Saito, T.; Kurasaki, M. Effects of curcumin, D-pinitol alone or in combination in cytotoxicity induced by arsenic in PC12 Cells. Food Chem. Toxicol. 2020, 144, 111577. [Google Scholar] [CrossRef]
- Juneja, K.; Mishra, R.; Chauhan, S.; Gupta, S.; Roy, P.; Sircar, D. Metabolite profiling and wound-healing activity of Boerhavia diffusa leaf extracts using in vitro and in vivo models. J. Trad. Complement. Med. 2020, 10, 52–59. [Google Scholar] [CrossRef]
- López-Gambero, A.J.; Sanjuan, C.; Serrano-Castro, P.J.; Suárez, J.; Rodríguez de Fonseca, F. The Biomedical Uses of Inositols: A Nutraceutical Approach to Metabolic Dysfunction in Aging and Neurodegenerative Diseases. Biomedicines 2020, 8, 295. [Google Scholar] [CrossRef]
- Srivastava, K.; Tiwari, M.; Dubey, A.; Dubey, A. D-Pinitol—A Natural Phytomolecule and its Pharmacological effect. Int. J. Pharm. Life Sci. 2020, 11, 6609–6623. [Google Scholar]
- Antonowski, T.; Osowski, A.; Lahuta, L.; Górecki, R.; Rynkiewicz, A.; Wojtkiewicz, J. Health-Promoting Properties of Selected Cyclitols for Metabolic Syndrome and Diabetes. Nutrients 2019, 11, 2314. [Google Scholar] [CrossRef] [Green Version]
- Kalekar, S.A.; Munshi, R.P.; Bhalerao, S.S.; Thatte, U.M. Insulin sensitizing effect of 3 Indian medicinal plants: An in vitro study. Indian J. Pharmacol. 2013, 45, 30–33. [Google Scholar] [CrossRef]
- Stadlbauer, V.; Neuhauser, C.; Aumiller, T.; Stallinger, A.; Iken, M.; Weghuber, J. Identification of Insulin-Mimetic Plant Extracts: From an In Vitro High-Content Screen to Blood Glucose Reduction in Live Animals. Molecules 2021, 26, 4346. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Agapiou, A.; Giannopoulos, S.; Kokkinofta, R. Nutritional characterization of carobs and traditional carob products. Food Sci. Nutr. 2018, 6, 2151–2161. [Google Scholar] [CrossRef]
- Kim, I.-S.; Kim, C.-H.; Yang, W.-S. Physiologically Active Molecules and Functional Properties of Soybeans in Human Health—A Current Perspective. Int. J. Mol. Sci. 2021, 22, 4054. [Google Scholar] [CrossRef]
- Tewari, R.; Gupta, M.; Ahmad, F.; Rout, P.K.; Misra, L.; Patwardhan, A.; Vasudeva, R. Extraction, quantification and antioxidant activities of flavonoids, polyphenols and pinitol from wild and cultivated Saraca asoca bark using RP-HPLC-PDA-RI method. Ind. Crops Prod. 2017, 103, 73–80. [Google Scholar] [CrossRef]








| Component | Proportion (%) |
|---|---|
| Moisture | 6.3–7.6 |
| Protein | 1.7–5.9 |
| Ash | 2.3–3.2 |
| Fat | 0.2–4.4 |
| Total dietary fiber | 11.7–47 |
| Starch | 0.1 |
| Total carbohydrates | 42–86 |
| Fructose | 2–7.4 |
| Glucose | 3–7.3 |
| Sucrose | 15–34 |
| D-Pinitol | 5.5 |
| Effect Type | Role of Insulin |
|---|---|
| Metabolic | Stimulation of glucose transport and metabolism |
| Stimulation of glycogen synthesis | |
| Stimulation of lipogenesis | |
| Inhibition of lipolysis | |
| Stimulation of ion flux | |
| Growth-promoting | Stimulation of DNA synthesis |
| Stimulation of cell growth and differentiation | |
| Metabolic & Growth-promoting | Stimulation of amino acid influx |
| Stimulation of protein synthesis | |
| Inhibition of protein degradation | |
| Stimulation of RNA synthesis |
| Molecular Mechanism | Roles in Insulin Resistance |
|---|---|
| Upregulation of PTP1B [25] | Reverses insulin-induced phosphorylation in tyrosine residues of IRS-1 and so impairs insulin signal transduction |
| Inflammatory mediators and adipokines | Activation of IKKβ/NF-κB and JNK pathways, serine phosphorylation of IRS-1 in the site of 307, declines GLUT-4 expression, reduces IRS-1 expression via ERK1/2, induce IRS degradation through SOCS1- and SOCS3-dependent mechanisms |
| Free radical overload | Activates several serine–threonine kinase pathways, i.e., IKKβ/NF-κB and JNK, IRS degradation, suppresses GLUT-4 expression and localization in cell membrane, decreases insulin-induced IRS-1 and PIP-kinase relocation between cytoplasm and microsomes, decreases PKB phosphorylation, serine phosphorylation at site of serine 307 of IRS-1, activates inflammatory responses |
| Defects in serine phosphorylation of IRS-1 | Decrease in insulin receptor phosphorylation, phosphorylation in serine 307 which blocks signaling |
| Obesity and adipocytes importance | Decrease in insulin receptor phosphorylation, phosphorylation in serine 307 which blocks signaling |
| Accelerated insulin degradation | Autoimmune antibodies against insulin or abnormal insulin structure due to mutation |
| Mitochondrial dysfunction | Induces oxidative stress, impairs insulin signaling |
| Reduced the capacity of receptors to binding to insulin | Decrease in number of insulin receptors, reduction in functional receptors due to mutation, autoimmune antibodies against insulin receptors |
| Mutations of GLUT-4 | Point mutation changes normal modification of GLUT-4, inhibits glucose entering into dependent cells and impairs subsequent signaling pathways |
| ER stress | Disrupts proper protein folding leading to accumulation of misfolded proteins |
| Property Short Description | Type of Publication | Ref., Year |
|---|---|---|
| Insulin regulation in human diabetics | research | [39], 1990 |
| Treatment respiratory disorders in infants | research | [40], 1992 |
| Insulin regulation in human diabetics | research | [41], 1993 |
| Treatments of psychiatric disorders | review | [42], 1997 |
| Treatment of polycystic ovary syndrome (PCOS) | research | [43], 1999 |
| Treatment of Alzheimer disease, in vitro | research | [44], 2000 |
| Insulin regulation in human diabetics | research | [45], 2005 |
| Treatment of endothelial dysfunction, antioxidant, animal model | research | [46], 2006 |
| Biological roles | review | [47], 2007 |
| Derivatives and their functions | review | [48], 2008 |
| Treatment of PCOS | review | [49], 2014 |
| Insulin regulation in obese male children | research | [50], 2016 |
| Treatment of PCOS | review | [51], 2016 |
| Treatment of PCOS | research | [52], 2017 |
| Bioavailability for treatment of PCOS | review | [53], 2017 |
| Treatment of PCOS in subfertile women | review | [54], 2018 |
| Effects on glucose homeostasis | review | [55], 2019 |
| General presentation of medicinal activities | review | [56], 2019 |
| Treatment of PCOS | review | [57], 2020 |
| Treatment of PCOS, with other technologies | review | [58], 2021 |
| Treatment of preterm birth | review | [59], 2021 |
| Treatment of psychological symptoms in PCOS | review | [60], 2021 |
| Insulin regulation in pregnancy | review | [38], 2022 |
| Activity/Property | Testing Method | Ref. |
|---|---|---|
| Anti-Alzheimer | In vivo, mice | [67] |
| Anti-Alzheimer | In vitro, hippocampal cultures | [68] |
| Anti-Alzheimer | In vivo, C. elegans, mice | [69] |
| Antiaging | In vivo, D. Melanogaster | [70] |
| Antibacterial | M. smegmatis | [71] |
| Anticancer | In vitro, human cancer cells | [72,73,74,75,76,77] |
| Anticancer | In vivo, rats | [78,79,80,81,82,83] |
| Anti-colitis | In vivo, rats | [84] |
| Antidepressant | In vivo, mice | [85] |
| Antidiabetic | In vivo, mice/rats | [86,87,88,89,90,91,92] |
| Antidiabetic | In vivo, humans | [93,94,95,96,97,98] |
| Antidiabetic | Theoretical evaluation | [99] |
| Antidiarrheal | In vivo, mice | [100] |
| Antifibrotic | In vivo, mice | [101] |
| Antihyperlipidemic | In vivo, rats | [64,102] |
| Anti-inflammatory | In vivo, mice/rats | [103,104,105,106] |
| Anti-inflammatory | In vitro, Human cells | [72,107,108] |
| Anti-inflammatory | In vitro, BV2 microglial cells | [109] |
| Antinociceptive | In vivo, mice | [100] |
| Anti-obesity | In vivo, humans | [110] |
| Anti-obesity | In vivo, rats | [111] |
| Anti-osteoclastic | In vitro, UAMS32 cells | [112] |
| Antioxidant | In vivo, rats | [78,81,82,88,113] |
| Anti-psoriatic | In vivo, mice | [114] |
| Antiviral | Theoretical evaluation | [115] |
| Asthma treatment | In vivo, mice | [116] |
| Bone protection | In vitro, Bone marrow cell lines, rats | [117] |
| Bone protection | In vivo, rats | [118] |
| Cardioprotective | In vivo, humans | [93] |
| Cardioprotective | In vivo, mice/rats | [119,120] |
| Cytotoxic | In vitro, human cancer cell lines | [121] |
| Diuretic | In vivo, mice | [122] |
| Geno-protective | In vitro, monkey liver cell lines | [123] |
| Hepatoprotective | In vivo, humans | [124] |
| Hepatoprotective | In vivo, mice/rats | [125,126,127,128,129,130,131] |
| Hydration biomarker | In vivo, humans | [132,133] |
| Hypotensive | In vivo, mice | [134] |
| Immuno-protective | Theoretical evaluation | [99] |
| Immuno-protective | In vivo, mice | [116,135,136] |
| Immunosuppressive | In vivo, mice | [137] |
| Insulin regulation | In vivo, mice/rats | [111,131,138,139,140,141] |
| Insulin regulation | In vivo, humans | [96,142] |
| Insulin regulation | In vitro, 3T3-L1, HUVEC cells | [143,144] |
| Memory enhancement | In vivo, rats | [90] |
| Nanoparticles loaded | In vitro, against M. smegmatis | [29] |
| Nephroprotective | In vivo, mice/rats | [105,145] |
| Neuroprotective | In vivo, mice/rats | [85,122,146,147,148] |
| Sleep enhancer | In vivo, D. melanogaster, in vitro PC12 cells | [149] |
| Synergism w/ curcumin | In vitro, PC12 cells, against As+3 toxicity | [150] |
| Wound healing | In vivo, rats, in vitro, HaCaT cells | [151] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azab, A. D-Pinitol—Active Natural Product from Carob with Notable Insulin Regulation. Nutrients 2022, 14, 1453. https://doi.org/10.3390/nu14071453
Azab A. D-Pinitol—Active Natural Product from Carob with Notable Insulin Regulation. Nutrients. 2022; 14(7):1453. https://doi.org/10.3390/nu14071453
Chicago/Turabian StyleAzab, Abdullatif. 2022. "D-Pinitol—Active Natural Product from Carob with Notable Insulin Regulation" Nutrients 14, no. 7: 1453. https://doi.org/10.3390/nu14071453
APA StyleAzab, A. (2022). D-Pinitol—Active Natural Product from Carob with Notable Insulin Regulation. Nutrients, 14(7), 1453. https://doi.org/10.3390/nu14071453