Use of the β-Glucan-Producing Lactic Acid Bacteria Strains Levilactobacillus brevis and Pediococcus claussenii for Sourdough Fermentation—Chemical Characterization and Chemopreventive Potential of In Situ-Enriched Wheat and Rye Sourdoughs and Breads
Abstract
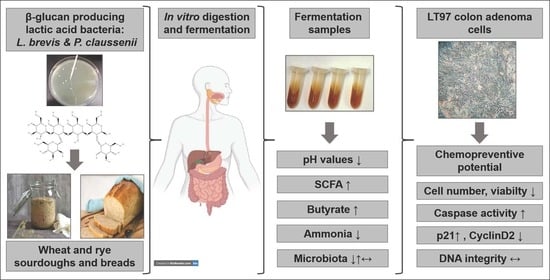
:1. Introduction
2. Materials and Methods
2.1. Cultivation of L. brevis and P. claussenii Strains and Preparation of Sourdoughs and Breads
2.2. Quantification of Main Nutrients in Sourdoughs and Breads
2.2.1. Dietary Fiber
2.2.2. Crude Protein
2.2.3. Crude Fat
2.3. Quantification of Bacterial β-Glucan in Sourdoughs and Breads
2.4. In Vitro Digestion and Fermentation
2.5. Determination of Short-Chain Fatty Acid Concentrations
2.6. Determination of Ammonia Concentrations
2.7. Quantification of Bacteria Species via 16SrDNA Amplicon Sequencing of Fermentation Pellets
2.8. Cell Culture
2.9. DAPI Assay
2.10. MTT Assay
2.11. Comet Assay
2.12. Caspase Assay
2.13. Analyses of mRNA Expression by qPCR
2.13.1. Isolation of Total RNA
2.13.2. Synthesis of cDNA
2.13.3. qPCR Experiments
2.14. Analyses of Protein Expression by Western Blotting
2.15. Statistical Analyses
3. Results
3.1. Quantification of Main Nutrients in Sourdoughs and Breads
3.2. Quantification of β-Glucan in Sourdoughs and Breads
3.3. Characterization of Fermentation Samples Obtained from Sourdoughs and Breads
3.3.1. pH Values
3.3.2. Concentrations of SCFA
3.3.3. Concentrations of Ammonia
3.4. Modulation of Bacteria Species in FP
3.5. Cytotoxic Effects of FS
3.6. Genotoxic and Antigenotoxic Effects of FS
3.7. Induction of Caspase Activity by FS
3.8. Effects of FS on the mRNA Expression of Cell Cycle- and Detoxification-Relevant Target Genes
3.9. Effects of FS on the Expression of Cell Cycle- and Detoxification-Relevant Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arendt, E.K.; Ryan, L.A.; Dal Bello, F. Impact of sourdough on the texture of bread. Food Microbiol. 2007, 24, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Chavan, R.S.; Chavan, S.R. Sourdough Technology-A Traditional Way for Wholesome Foods: A Review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 170–183. [Google Scholar] [CrossRef]
- De Vuyst, L.; Comasio, A.; Kerrebroeck, S.V. Sourdough production: Fermentation strategies, microbial ecology, and use of non-flour ingredients. Crit. Rev. Food Sci. Nutr. 2021, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Contributions of exopolysaccharides from lactic acid bacteria as biotechnological tools in food, pharmaceutical, and medical applications. Int. J. Biol. Macromol. 2021, 173, 79–89. [Google Scholar] [CrossRef]
- Galle, S.; Schwab, C.; Arendt, E.K.; Ganzle, M.G. Structural and rheological characterisation of heteropolysaccharides produced by lactic acid bacteria in wheat and sorghum sourdough. Food Microbiol. 2011, 28, 547–553. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef]
- Oleksy, M.; Klewicka, E. Exopolysaccharides produced by Lactobacillus sp.: Biosynthesis and applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 450–462. [Google Scholar] [CrossRef]
- Prete, R.; Alam, M.K.; Perpetuini, G.; Perla, C.; Pittia, P.; Corsetti, A. Lactic Acid Bacteria Exopolysaccharides Producers: A Sustainable Tool for Functional Foods. Foods 2021, 10, 1653. [Google Scholar] [CrossRef]
- Galle, S.; Arendt, E.K. Exopolysaccharides from sourdough lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2014, 54, 891–901. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Fraunhofer, M.E.; Geissler, A.J.; Wefers, D.; Bunzel, M.; Jakob, F.; Vogel, R.F. Characterization of beta-glucan formation by Lactobacillus brevis TMW 1.2112 isolated from slimy spoiled beer. Int. J. Biol. Macromol. 2018, 107, 874–881. [Google Scholar] [CrossRef]
- Bockwoldt, J.A.; Fellermeier, J.; Steffens, E.; Vogel, R.F.; Ehrmann, M.A. beta-Glucan Production by Levilactobacillus brevis and Pediococcus claussenii for In Situ Enriched Rye and Wheat Sourdough Breads. Foods 2021, 10, 547. [Google Scholar] [CrossRef]
- Bockwoldt, J.A.; Stahl, L.; Ehrmann, M.A.; Vogel, R.F.; Jakob, F. Persistence and beta-glucan formation of beer-spoiling lactic acid bacteria in wheat and rye sourdoughs. Food Microbiol. 2020, 91, 103539. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Choi, J.S. Clinical and Physiological Perspectives of beta-Glucans: The Past, Present, and Future. Int. J. Mol. Sci. 2017, 18, 1906. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood LDL-cholesterol concentrations (ID 1236, 1299), increase in satiety leading to a reduction in energy intake (ID 851, 852. EFSA J. 2011, 9, 2207–2221. [Google Scholar] [CrossRef] [Green Version]
- Schlörmann, W.; Glei, M. Potential health benefits of beta-glucan from barley und oat. Ernahr. Umsch. 2017, 64, M555–M559. [Google Scholar] [CrossRef]
- Tosh, S.M. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur. J. Clin. Nutr. 2013, 67, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, A.; Beck, E.J.; Tosh, S.; Wolever, T.M. Cholesterol-lowering effects of oat beta-glucan: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Schlörmann, W.; Keller, F.; Zetzmann, S.; Lorkowski, S.; Dawczynski, C.; Glei, M. Impact of processing degree on fermentation profile and chemopreventive effects of oat and waxy barley in LT97 colon adenoma cells. Eur. Food Res. Technol. 2021, 247, 569–578. [Google Scholar] [CrossRef]
- Schlörmann, W.; Atanasov, J.; Lorkowski, S.; Dawczynski, C.; Glei, M. Study on chemopreventive effects of raw and roasted beta-glucan-rich waxy winter barley using an in vitro human colon digestion model. Food Funct. 2020, 11, 2626–2638. [Google Scholar] [CrossRef]
- Hussain, P.R.; Rather, S.A.; Suradkar, P.P. Structural characterization and evaluation of antioxidant, anticancer and hypoglycemic activity of radiation degraded oat (Avena sativa) beta- glucan. Radiat. Phys. Chem. 2018, 144, 218–230. [Google Scholar] [CrossRef]
- Shah, A.; Ahmad, M.; Ashwar, B.A.; Gani, A.; Masoodi, F.A.; Wani, I.A.; Wani, S.M.; Gani, A. Effect of gamma-irradiation on structure and nutraceutical potential of beta-D-glucan from barley (Hordeum vulgare). Int. J. Biol. Macromol. 2015, 72, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Stack, H.M.; Kearney, N.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Association of beta-glucan endogenous production with increased stress tolerance of intestinal lactobacilli. Appl. Environ. Microbiol. 2010, 76, 500–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, P.; Lopez, P.; Capozzi, V.; de Palencia, P.F.; Duenas, M.T.; Spano, G.; Fiocco, D. Beta-glucans improve growth, viability and colonization of probiotic microorganisms. Int. J. Mol. Sci. 2012, 13, 6026–6039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Notararigo, S.; de Las Casas-Engel, M.; de Palencia, P.F.; Corbi, A.L.; Lopez, P. Immunomodulation of human macrophages and myeloid cells by 2-substituted (1-3)-beta-D-glucan from P. parvulus 2.6. Carbohydr. Polym. 2014, 112, 109–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlörmann, W.; Bockwoldt, J.A.; Mayr, M.F.; Lorkowski, S.; Dawczynski, C.; Rohn, S.; Ehrmann, M.A.; Glei, M. Fermentation profile, cholesterol-reducing properties and chemopreventive potential of beta-glucans from Levilactobacillus brevis and Pediococcus claussenii—A comparative study with beta-glucans from different sources. Food Funct. 2021, 12, 10615–10631. [Google Scholar] [CrossRef] [PubMed]
- Latimer, G.W. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Lee, S.C.; Prosky, L.; De Vries, J.W. Determination of Total, Soluble, and Insoluble Dietary Fiber in Foods—Enzymatic-Gravimetric Method, MES-TRIS Buffer: Collaborative Study. J. AOAC Int. 1992, 75, 395–416. [Google Scholar] [CrossRef]
- Kjeldahl, J. A New Method for the Determination of Nitrogen in Organic Matter. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef] [Green Version]
- Stoldt, W. Deutsche Lebensmittel-Rundschau: Zeitschrift für Lebensmittelkunde und Lebensmittelrecht. Dtsch. Lebensm. Rundsch. 1949, 45, 41–46. [Google Scholar]
- Werning, M.L.; Perez-Ramos, A.; Fernandez de Palencia, P.; Mohedano, M.L.; Duenas, M.T.; Prieto, A.; Lopez, P. A specific immunological method to detect and quantify bacterial 2-substituted (1,3)-beta-D-glucan. Carbohydr. Polym. 2014, 113, 39–45. [Google Scholar] [CrossRef]
- Duenas-Chasco, M.T.; Rodriguez-Carvajal, M.A.; Tejero Mateo, P.; Franco-Rodriguez, G.; Espartero, J.L.; Irastorza-Iribas, A.; Gil-Serrano, A.M. Structural analysis of the exopolysaccharide produced by Pediococcus damnosus 2.6. Carbohydr. Res. 1997, 303, 453–458. [Google Scholar] [CrossRef]
- Notararigo, S.; Nacher-Vazquez, M.; Ibarburu, I.; Werning, M.L.; de Palencia, P.F.; Duenas, M.T.; Aznar, R.; Lopez, P.; Prieto, A. Comparative analysis of production and purification of homo- and hetero-polysaccharides produced by lactic acid bacteria. Carbohydr. Polym. 2013, 93, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Barry, J.L.; Hoebler, C.; Macfarlane, G.T.; Macfarlane, S.; Mathers, J.C.; Reed, K.A.; Mortensen, P.B.; Nordgaard, I.; Rowland, I.R.; Rumney, C.J. Estimation of the fermentability of dietary fibre in vitro: A European interlaboratory study. Br. J. Nutr. 1995, 74, 303–322. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.; Friedrich, U.; Vogelsang, H.; Jahreis, G. Lactobacillus acidophilus 74-2 and Bifidobacterium animalis subsp lactis DGCC 420 modulate unspecific cellular immune response in healthy adults. Eur. J. Clin. Nutr 2008, 62, 584–593. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Joseph, D.; Kapfhammer, M.; Giritli, S.; Horn, M.; Haller, D.; Clavel, T. IMNGS: A comprehensive open resource of processed 16S rRNA microbial profiles for ecology and diversity studies. Sci. Rep. 2016, 6, 33721. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Lagkouvardos, I.; Fischer, S.; Kumar, N.; Clavel, T. Rhea: A transparent and modular R pipeline for microbial profiling based on 16S rRNA gene amplicons. PeerJ 2017, 5, e2836. [Google Scholar] [CrossRef] [Green Version]
- Richter, M.; Jurek, D.; Wrba, F.; Kaserer, K.; Wurzer, G.; Karner-Hanusch, J.; Marian, B. Cells obtained from colorectal microadenomas mirror early premalignant growth patterns in vitro. Eur. J. Cancer 2002, 38, 1937–1945. [Google Scholar] [CrossRef]
- Schlörmann, W.; Lamberty, J.; Ludwig, D.; Lorkowski, S.; Glei, M. In vitro-fermented raw and roasted walnuts induce expression of CAT and GSTT2 genes, growth inhibition, and apoptosis in LT97 colon adenoma cells. Nutr. Res. 2017, 47, 72–80. [Google Scholar] [CrossRef]
- Schlörmann, W.; Hiller, B.; Jahns, F.; Zöger, R.; Hennemeier, I.; Wilhelm, A.; Lindhauer, M.G.; Glei, M. Chemopreventive effects of in vitro digested and fermented bread in human colon cells. Eur. J. Nutr. 2012, 51, 827–839. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Wang, H.; Wang, F.; Tao, X.; Cheng, H. Ammonia-containing dimethyl sulfoxide: An improved solvent for the dissolution of formazan crystals in the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay. Anal. Biochem. 2012, 421, 324–326. [Google Scholar] [CrossRef]
- Glei, M.; Fischer, S.; Lamberty, J.; Ludwig, D.; Lorkowski, S.; Schlormann, W. Chemopreventive Potential of In Vitro Fermented Raw and Roasted Hazelnuts in LT97 Colon Adenoma Cells. Anticancer Res. 2018, 38, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Rieu, I.; Powers, S.J. Real-time quantitative RT-PCR: Design, calculations, and statistics. Plant Cell 2009, 21, 1031–1033. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lau, S.W.; Chong, A.Q.; Chin, N.L.; Talib, R.A.; Basha, R.K. Sourdough Microbiome Comparison and Benefits. Microorganisms 2021, 9, 1355. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Pelaez, J.; Paesani, C.; Gomez, M. Sourdough Technology as a Tool for the Development of Healthier Grain-Based Products: An Update. Agronomy 2020, 10, 1962. [Google Scholar] [CrossRef]
- Oerlemans, M.M.P.; Akkerman, R.; Ferrari, M.; Walvoort, M.T.C.; de Vos, P. Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health. J. Funct. Foods 2021, 76, 104289. [Google Scholar] [CrossRef]
- Atanasov, J.; Schlormann, W.; Trautvetter, U.; Glei, M. The Effects of beta-Glucans on Intestinal Health. Ernahr. Umsch. 2020, 67, M140–M147. [Google Scholar]
- Schlörmann, W.; Lamberty, J.; Lorkowski, S.; Ludwig, D.; Mothes, H.; Saupe, C.; Glei, M. Chemopreventive potential of in vitro fermented nuts in LT97 colon adenoma and primary epithelial colon cells. Mol. Carcinog. 2017, 56, 1461–1471. [Google Scholar] [CrossRef]
- De Vuyst, L.; Degeest, B. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol. Rev. 1999, 23, 153–177. [Google Scholar] [CrossRef]
- Perez-Ramos, A.; Mohedano, M.L.; Lopez, P.; Spano, G.; Fiocco, D.; Russo, P.; Capozzi, V. In Situ beta-Glucan Fortification of Cereal-Based Matrices by Pediococcus parvulus 2.6: Technological Aspects and Prebiotic Potential. Int. J. Mol. Sci. 2017, 18, 1588. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, M.; Rizzello, C.G.; Alfonsi, G.; Arnault, P.; Cappelle, S.; Di Cagno, R.; Gobbetti, M. Use of sourdough lactobacilli and oat fibre to decrease the glycaemic index of white wheat bread. Br. J. Nutr. 2007, 98, 1196–1205. [Google Scholar] [CrossRef] [Green Version]
- Hansen, H.B.; Andreasen, M.F.; Nielsen, M.M.; Larsen, L.M.; Knudsen, K.E.B.; Meyer, A.S.; Christensen, L.P.; Hansen, A. Changes in dietary fibre, phenolic acids and activity of endogenous enzymes during rye bread-making. Eur. Food Res. Technol. 2002, 214, 33–42. [Google Scholar] [CrossRef]
- Johansson, C.G.; Siljestrom, M.; Asp, N.G. Dietary fibre in bread and corresponding flours--formation of resistant starch during baking. Z. Lebensm. Unters. Forsch. 1984, 179, 24–28. [Google Scholar] [CrossRef]
- Mihhalevski, A.; Nisamedtinov, I.; Halvin, K.; Oseka, A.; Paalme, T. Stability of B-complex vitamins and dietary fiber during rye sourdough bread production. J. Cereal Sci. 2013, 57, 30–38. [Google Scholar] [CrossRef]
- Saa, D.T.; Di Silvestro, R.; Dinelli, G.; Gianotti, A. Effect of sourdough fermentation and baking process severity on dietary fibre and phenolic compounds of immature wheat flour bread. Lwt-Food Sci. Technol. 2017, 83, 26–32. [Google Scholar] [CrossRef]
- Demirkesen-Bicak, H.; Arici, M.; Yaman, M.; Karasu, S.; Sagdic, O. Effect of Different Fermentation Condition on Estimated Glycemic Index, In Vitro Starch Digestibility, and Textural and Sensory Properties of Sourdough Bread. Foods 2021, 10, 514. [Google Scholar] [CrossRef]
- Johansson, D.P.; Gutierrez, J.L.V.; Landberg, R.; Alminger, M.; Langton, M. Impact of food processing on rye product properties and their in vitro digestion. Eur. J. Nutr. 2018, 57, 1651–1666. [Google Scholar] [CrossRef]
- Nordlund, E.; Katina, K.; Mykkanen, H.; Poutanen, K. Distinct Characteristics of Rye and Wheat Breads Impact on Their in Vitro Gastric Disintegration and in Vivo Glucose and Insulin Responses. Foods 2016, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Glei, M.; Zetzmann, S.; Lorkowski, S.; Dawczynski, C.; Schlormann, W. Chemopreventive effects of raw and roasted oat flakes after in vitro fermentation with human faecal microbiota. Int. J. Food Sci. Nutr. 2021, 72, 57–69. [Google Scholar] [CrossRef]
- Schlörmann, W.; Birringer, M.; Lochner, A.; Lorkowski, S.; Richter, I.; Rohrer, C.; Glei, M. In vitro fermentation of nuts results in the formation of butyrate and c9, t11 conjugated linoleic acid as chemopreventive metabolites. Eur. J. Nutr. 2016, 55, 2063–2073. [Google Scholar] [CrossRef]
- Borowicki, A.; Stein, K.; Scharlau, D.; Scheu, K.; Brenner-Weiss, G.; Obst, U.; Hollmann, J.; Lindhauer, M.; Wachter, N.; Glei, M. Fermented wheat aleurone inhibits growth and induces apoptosis in human HT29 colon adenocarcinoma cells. Br. J. Nutr. 2010, 103, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Hiller, B.; Schlormann, W.; Glei, M.; Lindhauer, M.G. Comparative study of colorectal health related compounds in different types of bread Analysis of bread samples pre and post digestion in a batch fermentation model of the human intestine. Food Chem. 2011, 125, 1202–1212. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Scharlau, D.; Borowicki, A.; Habermann, N.; Hofmann, T.; Klenow, S.; Miene, C.; Munjal, U.; Stein, K.; Glei, M. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat. Res. 2009, 682, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Beyer-Sehlmeyer, G.; Glei, M.; Hartmann, E.; Hughes, R.; Persin, C.; Bohm, V.; Rowland, I.; Schubert, R.; Jahreis, G.; Pool-Zobel, B.L. Butyrate is only one of several growth inhibitors produced during gut flora-mediated fermentation of dietary fibre sources. Br. J. Nutr. 2003, 90, 1057–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kautenburger, T.; Beyer-Sehlmeyer, G.; Festag, G.; Haag, N.; Kuhler, S.; Kuchler, A.; Weise, A.; Marian, B.; Peters, W.H.; Liehr, T.; et al. The gut fermentation product butyrate, a chemopreventive agent, suppresses glutathione S-transferase theta (hGSTT1) and cell growth more in human colon adenoma (LT97) than tumor (HT29) cells. J. Cancer Res. Clin. Oncol. 2005, 131, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.; Magee, E.A.; Bingham, S. Protein degradation in the large intestine: Relevance to colorectal cancer. Curr. Issues Intest. Microbiol. 2000, 1, 51–58. [Google Scholar]
- Windey, K.; De Preter, V.; Verbeke, K. Relevance of protein fermentation to gut health. Mol. Nutr. Food Res. 2012, 56, 184–196. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.M.; Wichienchot, S.; He, X.W.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of beta-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Myhrstad, M.C.W.; Tunsjo, H.; Charnock, C.; Telle-Hansen, V.H. Dietary Fiber, Gut Microbiota, and Metabolic Regulation-Current Status in Human Randomized Trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef] [Green Version]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Costabile, A.; Santarelli, S.; Claus, S.P.; Sanderson, J.; Hudspith, B.N.; Brostoff, J.; Ward, J.L.; Lovegrove, A.; Shewry, P.R.; Jones, H.E.; et al. Effect of breadmaking process on in vitro gut microbiota parameters in irritable bowel syndrome. PLoS ONE 2014, 9, e111225. [Google Scholar] [CrossRef]
- Korem, T.; Zeevi, D.; Zmora, N.; Weissbrod, O.; Bar, N.; Lotan-Pompan, M.; Avnit-Sagi, T.; Kosower, N.; Malka, G.; Rein, M.; et al. Bread Affects Clinical Parameters and Induces Gut Microbiome-Associated Personal Glycemic Responses. Cell Metab. 2017, 25, 1243–1253. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Hou, H.; Chen, D.; Zhang, K.; Zhang, W.; Liu, T.; Wang, S.; Dai, X.; Wang, B.; Zhong, W.; Cao, H. Gut microbiota-derived short-chain fatty acids and colorectal cancer: Ready for clinical translation? Cancer Lett. 2022, 526, 225–235. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Dubin, K.; Littmann, E.R.; Moody, T.U.; Fontana, E.; Seok, R.; Leiner, I.M.; Taur, Y.; Peled, J.U.; van den Brink, M.R.M.; et al. Inhibiting antibiotic-resistant Enterobacteriaceae by microbiota-mediated intracellular acidification. J. Exp. Med. 2019, 216, 84–98. [Google Scholar] [CrossRef]
- Hinnebusch, B.F.; Meng, S.; Wu, J.T.; Archer, S.Y.; Hodin, R.A. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J. Nutr. 2002, 132, 1012–1017. [Google Scholar] [CrossRef] [Green Version]
- Barnard, J.A.; Warwick, G. Butyrate rapidly induces growth inhibition and differentiation in HT-29 cells. Cell Growth Differ. 1993, 4, 495–501. [Google Scholar]
- Schlörmann, W.; Naumann, S.; Renner, C.; Glei, M. Influence of miRNA-106b and miRNA-135a on butyrate-regulated expression of p21 and Cyclin D2 in human colon adenoma cells. Genes Nutr. 2015, 10, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, H.; Taussig, D.P.; Cheng, W.H.; Johnson, L.K.; Hakkak, R. Butyrate Inhibits Cancerous HCT116 Colon Cell Proliferation but to a Lesser Extent in Noncancerous NCM460 Colon Cells. Nutrients 2017, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borowicki, A.; Stein, K.; Scharlau, D.; Glei, M. Fermentation supernatants of wheat (Triticum aestivum L.) aleurone beneficially modulate cancer progression in human colon cells. J. Agric. Food Chem. 2010, 58, 2001–2007. [Google Scholar] [CrossRef]
- Kopeina, G.S.; Zhivotovsky, B. Caspase-2 as a master regulator of genomic stability. Trends Cell Biol. 2021, 31, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Dorstyn, L.; Kumar, S. The p53-caspase-2 axis in the cell cycle and DNA damage response. Exp. Mol. Med. 2021, 53, 517–527. [Google Scholar] [CrossRef]
- O’Byrne, K.J.; Richard, D.J. Nucleolar caspase-2: Protecting us from DNA damage. J. Cell Biol. 2017, 216, 1521–1523. [Google Scholar] [CrossRef] [PubMed]
- Putaala, H.; Makivuokko, H.; Tiihonen, K.; Rautonen, N. Simulated colon fiber metabolome regulates genes involved in cell cycle, apoptosis, and energy metabolism in human colon cancer cells. Mol. Cell. Biochem. 2011, 357, 235–245. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, K.N.; Vitetta, L. Effects of Intestinal Microbial-Elaborated Butyrate on Oncogenic Signaling Pathways. Nutrients 2019, 11, 1026. [Google Scholar] [CrossRef] [Green Version]
- Siavoshian, S.; Segain, J.P.; Kornprobst, M.; Bonnet, C.; Cherbut, C.; Galmiche, J.P.; Blottiere, H.M. Butyrate and trichostatin A effects on the proliferation/differentiation of human intestinal epithelial cells: Induction of cyclin D3 and p21 expression. Gut 2000, 46, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Chai, F.; Evdokiou, A.; Young, G.P.; Zalewski, P.D. Involvement of p21(Waf1/Cip1) and its cleavage by DEVD-caspase during apoptosis of colorectal cancer cells induced by butyrate. Carcinogenesis 2000, 21, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Medina, V.; Edmonds, B.; Young, G.P.; James, R.; Appleton, S.; Zalewski, P.D. Induction of caspase-3 protease activity and apoptosis by butyrate and trichostatin A (inhibitors of histone deacetylase): Dependence on protein synthesis and synergy with a mitochiondrial cytochrome c-dependent pathway. Cancer Res. 1997, 57, 3697–3707. [Google Scholar]
- Glei, M.; Ludwig, D.; Lamberty, J.; Fischer, S.; Lorkowski, S.; Schlormann, W. Chemopreventive Potential of Raw and Roasted Pistachios Regarding Colon Carcinogenesis. Nutrients 2017, 9, 1368. [Google Scholar] [CrossRef] [Green Version]










| Genes | Sequence |
|---|---|
| Catalase | Forward 5′-TGG ACA AGT ACA ATG CTG AG-3′ |
| Reverse 5′-TTA CAC GGA TGA ACG CTA AG-3′ | |
| SOD2 | Forward 5′-GCC CTG GAA CCT CAC ATC AAC-3′ |
| Reverse 5′-CAA CGC CTC CTG GTA CTT CTC-3′ | |
| p21 | Forward 5′-CAC TGT CTT GTA CCC TTG TG-3′ |
| Reverse 5′-CTT CCT CTT GGA GAA GAT CAG-3′ | |
| Cyclin D2 | Forward 5′-CCA CCG ACT TTA AGT TTG CC-3′ |
| Reverse 5′-CTT TGA GAC AAT CCA CGT CTG-3′ | |
| β-Actin | Forward 5′-AGA GCC TCG CCT TTG CCG AT-3′ |
| Reverse 5′-CCC ACG ATG GAG GGG AAG AC-3′ | |
| GAPDH | Forward 5′-CAA CAG CGA CAC CCA CTC CT-3′ |
| Reverse 5′-CAC CCT GTT GCT GTA GCC AAA-3′ |
| Wheat | Rye | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Dietary Fiber [%] | Protein [%] | Fat [%] | Ash [%] | β-Glucan [µg/g] | Total Dietary Fiber [%] | Protein [%] | Fat [%] | Ash [%] | β-Glucan [µg/g] | |
| Sourdough | ||||||||||
| P. claussenii WT | 17.2 | 11.3 | 0.8 | 0.7 | 119.9 | 22.8 | 9.2 | 0.9 | 1.4 | 169.4 |
| P. claussenii M | 13.0 | 11.2 | 0.8 | 0.7 | 35.7 | 19.1 | 8.7 | 1.2 | 1.3 | 40.3 |
| L. brevis WT | 15.9 | 11.1 | 0.9 | 0.7 | 248.6 | 20.3 | 9.8 | 1.3 | 1.4 | 519.4 |
| L. brevis M | 15.5 | 10.9 | 0.8 | 0.7 | 25.0 | 20.0 | 9.6 | 1.4 | 1.4 | 27.6 |
| Bread | ||||||||||
| P. claussenii WT | 20.5 | 11.8 | 1.5 | 2.9 | 188.3 | 26.5 | 9.6 | 1.5 | 3.3 | 674.2 |
| P. claussenii M | 13.0 | 11.9 | 1.5 | 2.8 | 0.0 | 23.2 | 9.4 | 1.9 | 3.4 | 0.0 |
| L. brevis WT | 14.3 | 11.1 | 1.5 | 2.9 | 100.2 | 19.8 | 9.6 | 1.3 | 3.4 | 312.2 |
| L. brevis M | 16.5 | 11.5 | 1.4 | 2.8 | 30.1 | 19.3 | 10.1 | 1.7 | 3.4 | 0.0 |
| FS | pH (a) | SCFA [mmol/L] (a) | ∑ SCFAs [mmol/L] | Molar Ratio SCFAs [%] | Ammonia [mmol/L] (b) | ||
|---|---|---|---|---|---|---|---|
| Acetate | Propionate | Butyrate | Acetate:Propionate:Butyrate | ||||
| Controls | |||||||
| Blank | 6.7 | 23.1 | 8.6 | 6.4 | 38.0 | 60.6:22.5:16.9 | 19.7 ± 1.0 a |
| Synergy1® | 5.2 | 64.2 | 12.1 | 25.8 | 102.1 | 62.9:11.8:25.3 | 5.4 ± 0.3 b |
| Wheat sourdoughs | |||||||
| P. claussenii WT | 5.4 | 61.1 | 13.8 | 22.4 | 97.3 | 62.8:14.2:23.0 | 14.8 ± 1.1 c |
| P. claussenii M | 5.3 | 62.3 | 14.5 | 24.2 | 100.9 | 61.7:14.3:24.0 | 16.9 ± 0.8 c,d |
| L. brevis WT | 5.3 | 65.5 | 14.8 | 24.7 | 104.9 | 62.4:14.1:23.5 | 17.4 ± 0.4 a,d |
| L. brevis M | 5.4 | 61.0 | 13.9 | 22.2 | 97.1 | 62.9:14.3:22.8 | 19.0 ± 0.2 a,d |
| Rye sourdoughs | |||||||
| P. claussenii WT | 5.5 | 58.3 | 13.6 | 21.5 | 93.4 | 62.4:14.6:23.0 | 17.2 ± 0.5 c,d |
| P. claussenii M | 5.5 | 58.2 | 13.9 | 22.1 | 94.2 | 61.7:14.8:23.5 | 17.3 ± 1.3 c,d |
| L. brevis WT | 5.5 | 58.2 | 13.6 | 21.8 | 93.6 | 62.1:14.6:23.3 | 16.7 ± 0.4 c,d |
| L. brevis M | 5.5 | 54.5 | 13.5 | 20.9 | 88.9 | 61.3:15.2:23.5 | 16.2 ± 1.8 c,d |
| FS | pH (a) | SCFA [mmol/L] (a) | ∑ SCFAs [mmol/L] | Molar Ratio SCFAs [%] | Ammonia [mmol/L] (b) | ||
|---|---|---|---|---|---|---|---|
| Acetate | Propionate | Butyrate | Acetate:propionate:butyrate | ||||
| Controls | |||||||
| Blank | 6.5 | 23.9 | 8.7 | 5.5 | 38.1 | 62.7:22.8:14.5 | 13.6 ± 0.9 a |
| Synergy1® | 5.0 | 57.8 | 15.6 | 22.2 | 95.6 | 60.5:16.3:23.2 | 2.2 ± 0.3 b |
| Wheat breads | |||||||
| P. claussenii WT | 5.2 | 58.3 | 17.4 | 17.5 | 93.1 | 62.6:18.6:18.8 | 15.7 ± 1.4 a |
| P. claussenii M | 5.2 | 59.2 | 17.4 | 18.0 | 94.6 | 62.6:18.4:19.0 | 16.4 ± 0.6 a |
| L. brevis WT | 5.2 | 59.5 | 17.5 | 17.8 | 94.8 | 62.8:18.5:18.7 | 16.1 ± 0.8 a |
| L. brevis M | 5.2 | 58.3 | 17.3 | 17.4 | 93.1 | 62.7:18.6:18.7 | 17.0 ± 1.0 a |
| Rye breads | |||||||
| P. claussenii WT | 5.2 | 56.1 | 17.0 | 16.5 | 89.6 | 62.6:19.0:18.4 | 13.9 ± 2.1 a |
| P. claussenii M | 5.2 | 56.6 | 17.3 | 16.9 | 90.8 | 62.4:19.0:18.6 | 14.3 ± 1.6 a |
| L. brevis WT | 5.2 | 58.0 | 17.5 | 17.1 | 92.6 | 62.6:18.9:18.5 | 13.2 ± 3.6 a |
| L. brevis M | 5.2 | 55.9 | 17.3 | 16.7 | 89.9 | 62.2:19.2:18.6 | 13.8 ± 0.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlörmann, W.; Bockwoldt, J.A.; Hübner, S.M.; Wittwer, E.; Reiners, S.; Lorkowski, S.; Dawczynski, C.; Ehrmann, M.A.; Glei, M. Use of the β-Glucan-Producing Lactic Acid Bacteria Strains Levilactobacillus brevis and Pediococcus claussenii for Sourdough Fermentation—Chemical Characterization and Chemopreventive Potential of In Situ-Enriched Wheat and Rye Sourdoughs and Breads. Nutrients 2022, 14, 1510. https://doi.org/10.3390/nu14071510
Schlörmann W, Bockwoldt JA, Hübner SM, Wittwer E, Reiners S, Lorkowski S, Dawczynski C, Ehrmann MA, Glei M. Use of the β-Glucan-Producing Lactic Acid Bacteria Strains Levilactobacillus brevis and Pediococcus claussenii for Sourdough Fermentation—Chemical Characterization and Chemopreventive Potential of In Situ-Enriched Wheat and Rye Sourdoughs and Breads. Nutrients. 2022; 14(7):1510. https://doi.org/10.3390/nu14071510
Chicago/Turabian StyleSchlörmann, Wiebke, Julia A. Bockwoldt, Sabine M. Hübner, Elisa Wittwer, Sarah Reiners, Stefan Lorkowski, Christine Dawczynski, Matthias A. Ehrmann, and Michael Glei. 2022. "Use of the β-Glucan-Producing Lactic Acid Bacteria Strains Levilactobacillus brevis and Pediococcus claussenii for Sourdough Fermentation—Chemical Characterization and Chemopreventive Potential of In Situ-Enriched Wheat and Rye Sourdoughs and Breads" Nutrients 14, no. 7: 1510. https://doi.org/10.3390/nu14071510
APA StyleSchlörmann, W., Bockwoldt, J. A., Hübner, S. M., Wittwer, E., Reiners, S., Lorkowski, S., Dawczynski, C., Ehrmann, M. A., & Glei, M. (2022). Use of the β-Glucan-Producing Lactic Acid Bacteria Strains Levilactobacillus brevis and Pediococcus claussenii for Sourdough Fermentation—Chemical Characterization and Chemopreventive Potential of In Situ-Enriched Wheat and Rye Sourdoughs and Breads. Nutrients, 14(7), 1510. https://doi.org/10.3390/nu14071510