Dietary Protein Patterns during Pregnancy Are Associated with Risk of Gestational Diabetes Mellitus in Chinese Pregnant Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Dietary Assessment
2.3. Assessment of GDM
2.4. Assessment of Covariates
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Dietary Intake Characteristics
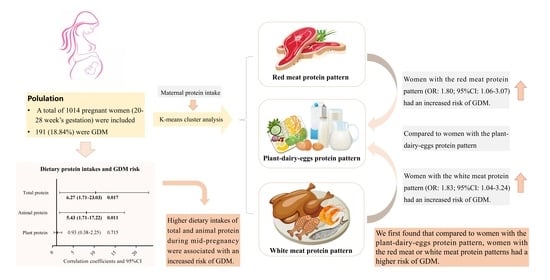
3.3. Association between Dietary Protein Intake and Risk of GDM
3.4. Association between Dietary Protein Patterns and Risk of GDM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2020, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association; Du, H.Y. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 37, S81–S90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Hehua, Z.; Yang, X.; Qing, C.; Shanyan, G.; Yuhong, Z. Dietary patterns and associations between air pollution and gestational diabetes mellitus. Environ. Int. 2021, 147, 106347. [Google Scholar] [CrossRef]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Sun, X.; Lu, L.; Liu, F.; Yuan, J. Prevalence of gestational diabetes mellitus in mainland China: A systematic review and meta-analysis. J. Diabetes Investig. 2019, 10, 154–162. [Google Scholar] [CrossRef]
- Juan, J.; Yang, H. Prevalence, Prevention, and Lifestyle Intervention of Gestational Diabetes Mellitus in China. Int. J. Environ. Res. Public Health 2020, 17, 9517. [Google Scholar] [CrossRef]
- Silva-Zolezzi, I.; Samuel, T.M.; Spieldenner, J. Maternal nutrition: Opportunities in the prevention of gestational diabetes. Nutr. Rev. 2017, 75, 32–50. [Google Scholar] [CrossRef]
- Schoenaker, D.A.; Mishra, G.D.; Callaway, L.K.; Soedamah-Muthu, S.S. The Role of Energy, Nutrients, Foods, and Dietary Patterns in the Development of Gestational Diabetes Mellitus: A Systematic Review of Observational Studies. Diabetes Care 2016, 39, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, F.; Lavigne, C.; Jacques, H.; Marette, A. Role of Dietary Proteins and Amino Acids in the Pathogenesis of Insulin Resistance. Annu. Rev. Nutr. 2007, 27, 293–310. [Google Scholar] [CrossRef]
- Mijatovic-Vukas, J.; Capling, L.; Cheng, S.; Stamatakis, E.; Louie, J.; Cheung, N.W.; Markovic, T.; Ross, G.; Senior, A.; Brand-Miller, J.C.; et al. Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, W.; Bowers, K.; Tobias, D.K.; Hu, F.B.; Zhang, C. Prepregnancy Dietary Protein Intake, Major Dietary Protein Sources, and the Risk of Gestational Diabetes Mellitus: A prospective cohort study. Diabetes Care 2013, 36, 2001–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, W.; Bowers, K.; Tobias, D.K.; Olsen, S.F.; Chavarro, J.; Vaag, A.; Kiely, M.; Zhang, C. Prepregnancy low-carbohydrate dietary pattern and risk of gestational diabetes mellitus: A prospective cohort study. Am. J. Clin. Nutr. 2014, 99, 1378–1384. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Gong, Y.; Zhang, X.; Yang, D.; Zhao, D.; Quan, L.; Zhou, R.; Bao, W.; Cheng, G. Dietary Protein Intake, Meat Consumption, and Dairy Consumption in the Year Preceding Pregnancy and During Pregnancy and Their Associations with the Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study in Southwest China. Front. Endocrinol. 2018, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.W.; Colega, M.; Cai, S.; Chan, Y.H.; Padmapriya, N.; Chen, L.-W.; Soh, S.-E.; Han, W.M.; Tan, K.H.; Lee, Y.S.; et al. Higher Maternal Dietary Protein Intake Is Associated with a Higher Risk of Gestational Diabetes Mellitus in a Multiethnic Asian Cohort. J. Nutr. 2017, 147, 653–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Chen, R.; Zhong, C.; Wu, J.; Li, X.; Li, Q.; Cui, W.; Yi, N.; Xiao, M.; Yin, H.; et al. Maternal dietary pattern characterised by high protein and low carbohydrate intake in pregnancy is associated with a higher risk of gestational diabetes mellitus in Chinese women: A prospective cohort study. Br. J. Nutr. 2018, 120, 1045–1055. [Google Scholar] [CrossRef] [Green Version]
- Donazar-Ezcurra, M.; Burgo, C.L.-D.; Martinez-Gonzalez, M.A.; Basterra-Gortari, F.J.; de Irala, J.; Bes-Rastrollo, M. Pre-pregnancy adherences to empirically derived dietary patterns and gestational diabetes risk in a Mediterranean cohort: The Seguimiento Universidad de Navarra (SUN) project. Br. J. Nutr. 2017, 118, 715–721. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Wu, W.; Yang, H.; Zhang, P.; Feng, Y.; Wang, K.; Wang, Y.; Wang, S.; Zhang, Y. A Vegetable Dietary Pattern Is Associated with Lowered Risk of Gestational Diabetes Mellitus in Chinese Women. Diabetes Metab. J. 2020, 44, 887–896. [Google Scholar] [CrossRef]
- Wen, L.; Ge, H.; Qiao, J.; Zhang, L.; Chen, X.; Kilby, M.; Zhou, Y.; Gan, J.; Saffery, R.; Yan, J.; et al. Maternal dietary patterns and risk of gestational diabetes mellitus in twin pregnancies: A longitudinal twin pregnancies birth cohort study. Nutr. J. 2020, 19, 13. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Schoenaker, D.A.J.M.; Soedamah-Muthu, S.S.; Callaway, L.K.; Mishra, G.D. Pre-pregnancy dietary patterns and risk of gestational diabetes mellitus: Results from an Australian population-based prospective cohort study. Diabetologia 2015, 58, 2726–2735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Yuan, M.; Chen, N.; Lu, J.; Hu, C.; Mai, W.; Zhang, R.; Pan, Y.; Qiu, L.; Wu, Y.; et al. Maternal dietary patterns and gestational diabetes mellitus: A large prospective cohort study in China. Br. J. Nutr. 2015, 113, 1292–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynn, A.C.; on behalf of the UPBEAT Consortium; Seed, P.T.; Patel, N.; Barr, S.; Bell, R.; Briley, A.L.; Godfrey, K.M.; Nelson, S.M.; Oteng-Ntim, E.; et al. Dietary patterns in obese pregnant women; influence of a behavioral intervention of diet and physical activity in the UPBEAT randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 124. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.; Cui, Z.; Li, M.; Li, T.; Wu, F.; Kang, T.; Meng, H. Associations Between Dietary Animal and Plant Protein Intake and Cardiometabolic Risk Factors—A Cross-Sectional Study in China Health and Nutrition Survey. Nutrients 2021, 13, 336. [Google Scholar] [CrossRef]
- Tryggvadottir, E.A.; Medek, H.; Birgisdottir, B.E.; Geirsson, R.T.; Gunnarsdottir, I. Association between healthy maternal dietary pattern and risk for gestational diabetes mellitus. Eur. J. Clin. Nutr. 2016, 70, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Du, H.Y.; Jiang, H.; Karmin, O.; Chen, B.; Xu, L.J.; Liu, S.P.; Yi, J.P.; He, G.S.; Qian, X. Association of Dietary Pattern during Pregnancy and Gestational Diabetes Mellitus: A Prospective Cohort Study in Northern China. Biomed. Environ. Sci. 2017, 30, 887–897. [Google Scholar]
- De Gavelle, E.; Davidenko, O.; Fouillet, H.; Delarue, J.; Darcel, N.; Huneau, J.-F.; Mariotti, F. The Willingness to Modify Portion Sizes or Eat New Protein Foods Largely Depends on the Dietary Pattern of Protein Intake. Nutrients 2019, 11, 1556. [Google Scholar] [CrossRef] [Green Version]
- Chinese Nutrition Society. The Dietary Guidelines for Chinese Residents 2016; People’s Medical Publishing House: Beijing, China, 2016. [Google Scholar]
- U.S. Department of Agriculture; U.S. Department of Health and Human Serv_x0002_Ices. Dietary Guidelines for Americans, 2020–2025, 9th ed. Available online: http://www.dietaryguidelines.gov (accessed on 29 December 2020).
- Zhang, C.-X.; Ho, S.C. Validity and reproducibility of a food frequency Questionnaire among Chinese women in Guangdong province. Asia Pac. J. Clin. Nutr. 2009, 18, 240–250. [Google Scholar]
- Yang, Y.X.; Wang, G.Y.; Pan, X.Q. China Food Composition, 2nd ed.; Peking University Medical Press: Beijing, China, 2009. [Google Scholar]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S; discussion 1229S–1231S. [Google Scholar] [CrossRef]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.D.; Hod, M.; et al. Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care. 2010, 33, 676–682. [Google Scholar] [CrossRef] [Green Version]
- IPAQ Research Committee. Guidelines for the Data Processing and Analysis of the International Physical Activity Questionnaire. 2005. Available online: www.ipaq.ki.se (accessed on 10 January 2022).
- Chen, C.; Lu, F.C. Department of Disease Control Ministry of Health, PR China The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed. Environ. Sci. 2004, 17 (Suppl.), 1–36. [Google Scholar] [PubMed]
- Funtikova, A.N.; Benítez-Arciniega, A.A.; Fitó, M.; Schröder, H. Modest validity and fair reproducibility of dietary patterns derived by cluster analysis. Nutr. Res. 2015, 35, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Goshtasebi, A.; Hosseinpour-Niazi, S.; Mirmiran, P.; Lamyian, M.; Banaem, L.M.; Azizi, F. Pre-pregnancy consumption of starchy vegetables and legumes and risk of gestational diabetes mellitus among Tehranian women. Diabetes Res. Clin. Pract. 2018, 139, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Zeng, M.; Jiao, Y.; Li, Y.; Xue, C.; Liu, G.; Wang, Z.; Qin, F.; He, Z.; Chen, J. Western Dietary Patterns, Foods, and Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. Int. Rev. J. 2021, 12, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Schulze, M.B.; Solomon, C.G.; Hu, F.B. A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetologia 2006, 49, 2604–2613. [Google Scholar] [CrossRef] [Green Version]
- Marí-Sanchis, A.; Díaz-Jurado, G.; Basterra-Gortari, F.J.; De La Fuente-Arrillaga, C.; Martínez-González, M.A.; Bes-Rastrollo, M. Association between pre-pregnancy consumption of meat, iron intake, and the risk of gestational diabetes: The SUN project. Eur. J. Nutr. 2018, 57, 939–949. [Google Scholar] [CrossRef]
- Bowers, K.; Yeung, E.; Williams, M.A.; Qi, L.; Tobias, D.K.; Hu, F.B.; Zhang, C. A Prospective Study of Prepregnancy Dietary Iron Intake and Risk for Gestational Diabetes Mellitus. Diabetes Care 2011, 34, 1557–1563. [Google Scholar] [CrossRef] [Green Version]
- Lao, T.T.; Ho, L.-F. Impact of Iron Deficiency Anemia on Prevalence of Gestational Diabetes Mellitus. Diabetes Care 2004, 27, 650–656. [Google Scholar] [CrossRef] [Green Version]
- Cook, J.D. Adaptation in iron metabolism. Am. J. Clin. Nutr. 1990, 51, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Swaminathan, S.; Fonseca, V.A.; Alam, M.G.; Shah, S.V. The Role of Iron in Diabetes and Its Complications. Diabetes Care 2007, 30, 1926–1933. [Google Scholar] [CrossRef] [Green Version]
- Simcox, J.A.; McClain, D.A. Iron and Diabetes Risk. Cell Metab. 2013, 17, 329–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Tai, E.S.; Tan, M.L.S.; Stevens, R.D.; Low, Y.L.; Muehlbauer, M.J.; Goh, D.L.M.; Ilkayeva, O.R.; Wenner, B.R.; Bain, J.R.; Lee, J.J.M.; et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 2010, 53, 757–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeva, M.M.; Calviño, J.; Souto, G.; Donapetry, C. Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids 2012, 43, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Feskens, E.J.M.; Sluik, D.; van Woudenbergh, G.J. Meat Consumption, Diabetes, and Its Complications. Curr. Diabetes Rep. 2013, 13, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; on behalf of the China Kadoorie Biobank Collaborative Group; Guo, Y.; Bennett, D.A.; Bragg, F.; Bian, Z.; Chadni, M.; Yu, C.; Chen, Y.; Tan, Y.; et al. Red meat, poultry and fish consumption and risk of diabetes: A 9 year prospective cohort study of the China Kadoorie Biobank. Diabetologia 2020, 63, 767–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, M.; Li, Y.; Wang, C.; Mao, Z.; Zhou, W.; Zhang, L.; Yang, X.; Cui, S.; Li, L. Dietary Protein Consumption and the Risk of Type 2 Diabetes: ADose-Response Meta-Analysis of Prospective Studies. Nutrients 2019, 11, 2783. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Tian, C.; Jia, C. Association of fish and n-3 fatty acid intake with the risk of type 2 diabetes: A meta-analysis of prospective studies. Br. J. Nutr. 2012, 108, 408–417. [Google Scholar] [CrossRef] [Green Version]
- Svensson, B.-G.; Åkesson, B.; Nilsson, A.; Paulsson, K. Urinary excretion of methylamines in men with varying intake of fish from the baltic sea. J. Toxicol. Environ. Health 1994, 41, 411–420. [Google Scholar] [CrossRef]
- Gatarek, P.; Kaluzna-Czaplinska, J. Trimethylamine N-oxide (TMAO) in human health. EXCLI J. 2021, 20, 301–319. [Google Scholar]
- Zhuang, R.; Ge, X.; Han, L.; Yu, P.; Gong, X.; Meng, Q.; Zhang, Y.; Fan, H.; Zheng, L.; Liu, Z.; et al. Gut microbe-generated metabolite trimethylamine N-oxide and the risk of diabetes: A systematic review and dose-response meta-analysis. Obes. Rev. 2019, 20, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Li, J.; Cao, Y.-F.; Li, S.-N.; Shao, P.; Leng, J.; Li, W.; Liu, J.; Yang, K.; Ma, R.; et al. Trimethylamine N-Oxide Metabolites in Early Pregnancy and Risk of Gestational Diabetes: A Nested Case-Control Study. J. Clin. Endocrinol. Metab. 2019, 104, 5529–5539. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Australian Dietary Guidelines. Available online: http://www.nhmrc.gov.au/guidelines-publications/n55 (accessed on 18 February 2013).
- Qiu, C.; Frederick, I.O.; Zhang, C.; Sorensen, T.K.; Enquobahrie, D.A.; Williams, M.A. Risk of Gestational Diabetes Mellitus in Relation to Maternal Egg and Cholesterol Intake. Am. J. Epidemiol. 2011, 173, 649–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gijsbers, L.; Ding, E.L.; Malik, V.S.; de Goede, J.; Geleijnse, J.M.; Soedamah-Muthu, S.S. Consumption of dairy foods and diabetes incidence: A dose-response meta-analysis of observational studies. Am. J. Clin. Nutr. 2016, 103, 1111–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, S.; Xu, Q.; Jiang, R.; Han, T.; Sun, C.; Na, L. Dietary Protein Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients 2017, 9, 982. [Google Scholar] [CrossRef] [Green Version]
- Kalogeropoulos, N.; Chiou, A.; Ioannou, M.; Karathanos, V.T.; Hassapidou, M.; Andrikopoulos, N.K. Nutritional evaluation and bioactive microconstituents (phytosterols, tocopherols, polyphenols, triterpenic acids) in cooked dry legumes usually consumed in the Mediterranean countries. Food Chem. 2010, 121, 682–690. [Google Scholar] [CrossRef]
- Rebello, C.J.; Greenway, F.L.; Finley, J.W. A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities. Obes. Rev. 2014, 15, 392–407. [Google Scholar] [CrossRef]
- Jiang, R.; Manson, J.E.; Stampfer, M.J. Nut and peanut butter consumption and risk of type 2 diabetes in women. ACC Curr. J. Rev. 2003, 12, 41–42. [Google Scholar] [CrossRef]
- Meyer, K.A.; Kushi, L.H.; Jacobs, D.R., Jr.; Slavin, J.; Sellers, T.A.; Folsom, A.R. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000, 71, 921–930. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, T.L.; Mande, A.; Barbour, L.A. Nutrition therapy within and beyond gestational diabetes. Diabetes Res. Clin. Pract. 2018, 145, 39–50. [Google Scholar] [CrossRef]
- Huijbregts, P.P.C.W.; Feskens, E.J.M.; Kromhout, D. Dietary Patterns and Cardiovascular Risk Factors in Elderly Men: The Zutphen Elderly Study. Int. J. Epidemiol. 1995, 24, 313–320. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Total | GDM | Normal | p |
|---|---|---|---|---|
| (n =1014) | (n = 191) | (n = 823) | ||
| Age, y | 30.05 ± 4.84 | 31.99 ± 5.07 | 29.60 ± 4.68 | <0.001 |
| <35, y | 851 (84.01) | 138 (72.25) | 687 (89.22) | <0.001 |
| ≥35, y | 162 (15.99) | 53 (27.75) | 82 (10.64) | |
| Gestational age, week | 25.45 ± 2.25 | 25.24 ± 2.47 | 25.50 ± 2.29 | 0.178 |
| Pre-pregnancy BMI, kg/m2 | 20.57 ± 2.81 | 21.43 ± 3.47 | 20.38 ± 2.72 | <0.001 |
| Overweight or obese, n (%) | 120 (12.62) | 37 (20.44) | 109 (13.26) | <0.001 |
| Underweight or normal, n (%) | 831 (87.38) | 144 (79.56) | 712 (86.72) | |
| Smoking, yes, n (%) | 44 (4.37) | 10 (5.24) | 34 (4.16) | 0.515 |
| Alcohol use, yes, n (%) | 35 (3.47) | 6 (3.14) | 29 (3.55) | 0.782 |
| Physical activity, METs·h/w | 31.72 ± 27.39 | 27.70 ± 21.95 | 32.65 ± 28.43 | 0.024 |
| Family history of diabetes, yes, n (%) | 150 (14.90) | 32 (16.75) | 118 (14.46) | 0.427 |
| History of GDM, n (%) | <0.001 | |||
| Yes | 29 (2.90) | 16 (8.42) | 13 (1.60) | |
| No | 585 (58.44) | 113 (59.47) | 472 (57.20) | |
| Nulliparous | 387 (38.66) | 61 (32.11) | 326 (40.20) | |
| Educational level, n (%) | 0.577 | |||
| Senior high school and below | 183 (18.48) | 33 (17.37) | 150 (18.75) | |
| High or technical secondary school | 213 (21.52) | 44 (23.16) | 169 (21.13) | |
| Junior college and college | 534 (53.94) | 98 (51.58) | 436 (54.50) | |
| Postgraduate and above | 60 (6.06) | 15 (7.89) | 45 (5.63) | |
| Monthly household income, n (%) | 0.927 | |||
| ≤4000 RMB | 209 (21.28) | 38 (20.32) | 171 (21.51) | |
| 4001–6000 RMB | 236 (24.03) | 44 (23.53) | 191 (24.15) | |
| 6001–10,000 RMB | 243 (24.75) | 50 (26.74) | 193 (24.28) | |
| >10,000 RMB | 294 (29.94) | 55 (29.41) | 239 (30.06) |
| Nutrients | Total | GDM | Normal | p |
|---|---|---|---|---|
| (n =1014) | (n = 191) | (n = 823) | ||
| Total energy, kcal/day | 1803.15 ± 496.19 | 1823.79 ± 479.81 | 1798.36 ± 504.01 | 0.531 |
| Saturated fatty acids, g/day | 19.92 ± 4.02 | 19.92 ± 4.50 | 19.99 ± 4.00 | 0.709 |
| Monounsaturated fatty acids, g/day | 27.78 ± 5.63 | 27.93 ± 5.79 | 27.86 ± 5.82 | 0.937 |
| Polyunsaturated fatty acids, g/day | 20.96 ± 5.75 | 20.39 ± 5.74 | 21.03 ± 5.77 | 0.195 |
| Cholesterol, mg/day | 404.03 ± 161.29 | 463.54 ± 172.78 | 397.78 ± 159.64 | 0.004 |
| Fiber, g/day | 11.10 ± 3.09 | 11.17 ± 3.27 | 11.01 ± 3.04 | 0.516 |
| Carbohydrates, g/day | 217.98 ± 31.01 | 216.04 ± 32.97 | 218.11 ± 31.07 | 0.411 |
| % Energy | 48.14 ± 6.66 | 47.57 ± 6.85 | 48.19 ± 6.67 | 0.246 |
| Fat, g/day | 73.95 ± 11.63 | 74.27 ± 12.52 | 74.22 ± 11.80 | 0.960 |
| % Energy | 37.41 ± 5.76 | 37.60 ± 6.09 | 37.46 ± 5.76 | 0.770 |
| Protein, g/day | 71.37 ± 11.30 | 73.14 ± 11.31 | 71.06 ± 11.33 | 0.022 |
| % Energy | 15.63 ± 2.59 | 16.05 ± 2.62 | 15.54 ± 2.58 | 0.015 |
| Animal protein, g/day | 40.83 ± 13.48 | 43.12 ± 13.65 | 40.38 ± 13.49 | 0.018 |
| Plant protein, g/day | 30.56 ± 5.55 | 30.07 ± 5.96 | 30.72 ± 5.52 | 0.184 |
| Protein sources | ||||
| From grain, g/day | 16.15 ± 4.65 | 15.82 ± 4.99 | 16.22 ± 4.56 | 0.338 |
| From beans, g/day | 4.18 ± 3.59 | 4.04 ± 3.97 | 4.24 ± 3.50 | 0.507 |
| From vegetables, g/day | 4.71 ± 2.32 | 4.83 ± 2.39 | 4.55 ± 2.29 | 0.112 |
| From fruits, g/day | 1.82 ± 1.07 | 1.85 ± 1.13 | 1.79 ± 1.04 | 0.536 |
| From red meat, g/day | 16.66 ± 9.69 | 17.40 ± 10.04 | 16.54 ± 9.76 | 0.321 |
| From poultry, g/day | 4.66 ± 4.04 | 5.10 ± 4.34 | 4.61 ± 3.97 | 0.135 |
| From aquatic products, g/day | 6.93 ± 6.44 | 7.83 ± 6.56 | 6.68 ± 6.42 | 0.032 |
| From eggs, g/day | 5.04 ± 3.31 | 5.42 ± 3.46 | 4.90 ± 3.28 | 0.054 |
| From dairy, g/day | 7.57 ± 5.11 | 7.35 ± 5.26 | 7.66 ± 5.05 | 0.421 |
| From nuts and seeds, g/day | 3.34 ± 4.12 | 3.07 ± 3.72 | 3.48 ± 4.21 | 0.247 |
| Energy-Adjusted Total Protein Intake Quartiles, OR (95%CI) | p-Trend | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Total Protein | 1 | 1.75 (0.90–3.44) | 2.88 (1.20–6.91) | 6.27 (1.71–23.03) | 0.017 |
| Animal Protein | 1 | 1.91 (0.97–3.73) | 3.04 (1.33–6.95) | 5.43 (1.71–17.22) | 0.011 |
| Plant Protein | 1 | 1.05 (0.61–1.83) | 0.87 (0.46–1.66) | 0.93 (0.38–2.25) | 0.715 |
| Model | Dietary Protein Patterns | ||
|---|---|---|---|
| Plant–Dairy–Eggs | White Meat | Red Meat | |
| GDM (N, %) | 49 (16.23%) | 62 (20.88%) | 80 (19.28%) |
| Unadjusted OR (95% CI) | 1.00 | 1.36 (0.90–2.06) | 1.23 (0.83–1.82) |
| Adjusted OR (95% CI) | |||
| Model 1 | 1.00 | 1.80 (1.13–2.85) | 1.52 (0.99–2.35) |
| Model 2 | 1.00 | 1.82 (1.14–2.90) | 1.59 (1.02–2.46) |
| Model 3 | 1.00 | 1.96 (1.12–3.34) | 1.84 (1.09–3.10) |
| Model 4 | 1.00 | 1.83 (1.04–3.24) | 1.80 (1.06–3.07) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Tang, N.; Zeng, J.; Jing, J.; Cai, L. Dietary Protein Patterns during Pregnancy Are Associated with Risk of Gestational Diabetes Mellitus in Chinese Pregnant Women. Nutrients 2022, 14, 1623. https://doi.org/10.3390/nu14081623
Wu W, Tang N, Zeng J, Jing J, Cai L. Dietary Protein Patterns during Pregnancy Are Associated with Risk of Gestational Diabetes Mellitus in Chinese Pregnant Women. Nutrients. 2022; 14(8):1623. https://doi.org/10.3390/nu14081623
Chicago/Turabian StyleWu, Weijia, Nu Tang, Jingjing Zeng, Jin Jing, and Li Cai. 2022. "Dietary Protein Patterns during Pregnancy Are Associated with Risk of Gestational Diabetes Mellitus in Chinese Pregnant Women" Nutrients 14, no. 8: 1623. https://doi.org/10.3390/nu14081623
APA StyleWu, W., Tang, N., Zeng, J., Jing, J., & Cai, L. (2022). Dietary Protein Patterns during Pregnancy Are Associated with Risk of Gestational Diabetes Mellitus in Chinese Pregnant Women. Nutrients, 14(8), 1623. https://doi.org/10.3390/nu14081623