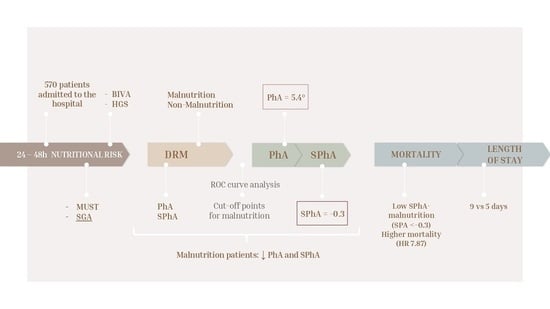
Phase Angle and Handgrip Strength as a Predictor of Disease-Related Malnutrition in Admitted Patients: 12-Month Mortality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting Study
2.2. Phase Angle and Other Parameters of Bioelectrical Impedance Vector Analysis (BIVA)
2.3. Anthropometry and Clinical Variables
2.4. Clinical Outcomes
2.5. Sample Size Calculation
2.6. Statistical Analysis
3. Results
3.1. Associations between PhA and HGS and Malnutrition Screening Tools
3.2. Optimal Variable Cut-Off Values to Detect Malnutrition in Admitted Patients
3.3. Prognostic Factor SPhA-Malnutrition and 12-Month Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Flow Chart

References
- Agarwal, E.; Ferguson, M.; Banks, M.; Batterham, M.; Bauer, J.; Capra, S.; Isenring, E. Malnutrition and poor food intake are associated with prolonged hospital stay, frequent readmissions, and greater in-hospital mortality: Results from the Nutrition Care Day Survey 2010. Clin. Nutr. 2013, 32, 737–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felblinger, D.M. Malnutrition, infection, and sepsis in acute and chronic illness. Crit. Care Nurs. Clin. N. Am. 2003, 15, 71–78. [Google Scholar] [CrossRef]
- Daniels, L. Malnutrition and Its Impact on Cost of Hospitalization, Length of Stay, Readmission and 3-Year Mortality. Available online: https://core.ac.uk/reader/10910747?utm_source=linkout (accessed on 28 October 2021).
- Allard, J.P.; Keller, H.; Jeejeebhoy, K.N.; Laporte, M.; Duerksen, D.R.; Gramlich, L.; Payette, H.; Bernier, P.; Vesnaver, E.; Davidson, B.; et al. Malnutrition at Hospital Admission-Contributors and Effect on Length of Stay: A Prospective Cohort Study From the Canadian Malnutrition Task Force. JPEN J. Parenter. Enteral Nutr. 2016, 40, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- Gomes, F.; Baumgartner, A.; Bounoure, L.; Bally, M.; Deutz, N.E.; Greenwald, J.L.; Stanga, Z.; Mueller, B.; Schuetz, P. Association of Nutritional Support with Clinical Outcomes Among Medical Inpatients Who Are Malnourished or at Nutritional Risk: An Updated Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1915138. [Google Scholar] [CrossRef] [Green Version]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.; Wells, L.; Nwulu, U.; Currow, D.; Johnson, M.J.; Skipworth, R.J. Validated screening tools for the assessment of cachexia, sarcopenia, and malnutrition: A systematic review. Am. J. Clin. Nutr. 2018, 108, 1196–1208. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Kyle, U.G.; Kondrup, J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: Phase angle and impedance ratio. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 330–339. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Stokoe, D. A Comparison of the Malnutrition Screening Tools, MUST, MNA and Bioelectrical Impedance Assessment in Frail Older Hospital Patients. Available online: https://core.ac.uk/reader/29176431?utm_source=linkout (accessed on 29 October 2021).
- ASPEN Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J. Parenter. Enteral Nutr. 2002, 26, 1SA–138SA. [Google Scholar]
- Detsky, A.S.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? J. Parenter. Enter. Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggiore, Q.; Nigrelli, S.; Ciccarelli, C.; Grimaldi, C.; Rossi, G.A.; Michelassi, C. Nutritional and prognostic correlates of bioimpedance indexes in hemodialysis patients. Kidney Int. 1996, 50, 2103–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagano, M.; Suita, S.; Yamanouchi, T. The validity of bioelectrical impedance phase angle for nutritional assessment in children. J. Pediatr. Surg. 2000, 35, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lammersfeld, C.A.; Burrows, J.L.; Dahlk, S.L.; Vashi, P.G.; Grutsch, J.F.; Hoffman, S.; Lis, C.G. Bioelectrical impedance phase angle in clinical practice: Implications for prognosis in advanced colorectal cancer. Am. J. Clin. Nutr. 2004, 80, 1634–1638. [Google Scholar] [CrossRef] [Green Version]
- Mushnick, R.; Fein, P.A.; Mittman, N.; Goel, N.; Chattopadhyay, J.; Avram, M.M. Relationship of bioelectrical impedance parameters to nutrition and survival in peritoneal dialysis patients. Kidney Int. 2003, 64, S53–S56. [Google Scholar] [CrossRef] [Green Version]
- Garlini, L.M.; Alves, F.D.; Ceretta, L.B.; Perry, I.S.; Souza, G.C.; Clausell, N.O. Phase angle and mortality: A systematic review. Eur. J. Clin. Nutr. 2018, 73, 495–508. [Google Scholar] [CrossRef]
- Stapel, S.N.; Looijaard, W.G.P.M.; Dekker, I.M.; Girbes, A.R.J.; Weijs, P.J.M.; Straaten, H.M.O.-V. Bioelectrical impedance analysis-derived phase angle at admission as a predictor of 90-day mortality in intensive care patients. Eur. J. Clin. Nutr. 2018, 72, 1019–1025. [Google Scholar] [CrossRef]
- Jansen, A.K.; Gattermann, T.; da Silva Fink, J.; Saldanha, M.F.; Dias Nascimento Rocha, C.; de Souza Moreira, T.H.; Silva, F.M. Low standardized phase angle predicts prolonged hospitalization in critically ill patients. Clin. Nutr. ESPEN 2019, 34, 68–72. [Google Scholar] [CrossRef]
- Piccoli, A.; Nigrelli, S.; Caberlotto, A.; Bottazzo, S.; Rossi, B.; Pillon, L.; Maggiore, Q. Bivariate normal values of the bioelectrical impedance vector in adult and elderly populations. Am. J. Clin. Nutr. 1995, 61, 269–270. [Google Scholar] [CrossRef]
- Piccoli, A.; Rossi, B.; Pillon, L.; Bucciante, G. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994, 46, 534–539. [Google Scholar] [CrossRef] [Green Version]
- Cardinal, T.R.; Wazlawik, E.; Bastos, J.; Nakazora, L.M.; Scheunemann, L. Standardized phase angle indicates nutritional status in hospitalized preoperative patients. Nutr. Res. 2010, 30, 594–600. [Google Scholar] [CrossRef] [PubMed]
- De Palo, T.; Messina, G.; Edefonti, A.; Perfumo, F.; Pisanello, L.; Peruzzi, L.; Di Iorio, B.; Mignozzi, M.; Vienna, A.; Conti, G. Normal values of the bioelectrical impedance vector in childhood and puberty. Nutrition 2000, 16, 417–424. [Google Scholar] [CrossRef]
- Charlton, K.; Nichols, C.; Bowden, S.; Milosavljevic, M.; Lambert, K.; Barone, L.; Mason, M.; Batterham, M. Poor nutritional status of older subacute patients predicts clinical outcomes and mortality at 18 months of follow-up. Eur. J. Clin. Nutr. 2012, 66, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis—Clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.C.; Fonseca, A.L.F.; Ferreira, L.G.; Ribeiro, H.S.; Correia, M.I.T.D.; Lima, A.S.; Cancela e Penna, F.G.; Anastácio, L.R. Different combinations of the GLIM criteria for patients awaiting a liver transplant: Poor performance for malnutrition diagnosis but a potentially useful prognostic tool. Clin. Nutr. 2021, 41, 97–104. [Google Scholar] [CrossRef]
- Tsai, H.-J.; Chang, F.-K. Associations between body mass index, mid-arm circumference, calf circumference, and functional ability over time in an elderly Taiwanese population. PLoS ONE 2017, 12, e0175062. [Google Scholar] [CrossRef] [Green Version]
- Sostisso, C.F.; Olikszechen, M.; Sato, M.N.; Oliveira, M.D.A.S.C.; Karam, S. Handgrip strength as an instrument for assessing the risk of malnutrition and inflammation in hemodialysis patients. J. Bras. Nefrol. 2020, 42, 429–436. [Google Scholar] [CrossRef]
- De Almeida, C.; Penna, P.M.; Pereira, S.S.; Rosa, C.D.O.B.; Franceschini, S.D.C.C. Relationship between Phase Angle and Objective and Subjective Indicators of Nutritional Status in Cancer Patients: A Systematic Review. Nutr. Cancer 2021, 73, 2201–2210. [Google Scholar] [CrossRef]
- Rimsevicius, L.; Gincaite, A.; Vicka, V.; Sukackiene, D.; Pavinic, J.; Miglinas, M. Malnutrition Assessment in Hemodialysis Patients: Role of Bioelectrical Impedance Analysis Phase Angle. J. Ren. Nutr. 2016, 26, 391–395. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.M.C.D.; Kubrusly, M.; Mota, R.S.; Silva, C.A.B.D.; Oliveira, V.N. Malnutrition in chronic kidney failure: What is the best diagnostic method to assess? Braz. J. Nephrol. 2010, 32, 55–68. [Google Scholar]
- Ruiz-Margáin, A.; Macías-Rodríguez, R.U.; Duarte-Rojo, A.; Ríos-Torres, S.L.; Espinosa-Cuevas, M.A.; Torre, A. Malnutrition assessed through phase angle and its relation to prognosis in patients with compensated liver cirrhosis: A prospective cohort study. Dig. Liver Dis. 2015, 47, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Varan, H.D.; Bolayir, B.; Kara, O.; Arik, G.; Kizilarslanoglu, M.C.; Kilic, M.K.; Sumer, F.; Kuyumcu, M.E.; Yesil, Y.; Yavuz, B.B.D.; et al. Phase angle assessment by bioelectrical impedance analysis and its predictive value for malnutrition risk in hospitalized geriatric patients. Aging Clin. Exp. Res. 2016, 28, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Genton, L.; Pichard, C. Low phase angle determined by bioelectrical impedance analysis is associated with malnutrition and nutritional risk at hospital admission. Clin. Nutr. 2013, 32, 294–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Giorno, R.; Quarenghi, M.; Stefanelli, K.; Rigamonti, A.; Stanglini, C.; De Vecchi, V.; Gabutti, L. Phase angle is associated with length of hospital stay, readmissions, mortality, and falls in patients hospitalized in internal-medicine wards: A retrospective cohort study. Nutrition 2021, 85, 111068. [Google Scholar] [CrossRef]
- Barbosa-Silva, M.C.G.; Barros, A.J.; Post, C.L.; Waitzberg, D.L.; Heymsfield, S.B. Can bioelectrical impedance analysis identify malnutrition in preoperative nutrition assessment? Nutrition 2003, 19, 422–426. [Google Scholar] [CrossRef]
- Saueressig, C.; Glasenapp, J.H.; Luft, V.C.; Alves, F.D.; Ferreira, P.K.; Hammes, T.O.; Dall’Alba, V. Phase Angle Is an Independent Predictor of 6-Month Mortality in Patients with Decompensated Cirrhosis: A Prospective Cohort Study. Nutr. Clin. Pract. 2020, 35, 1061–1069. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Vegas-Aguilar, I.M.; García-Almeida, J.M.; Bellido-Guerrero, D.; Talluri, A.; Lukaski, H.; Tinahones, F.J. Phase angle and standardized phase angle from bioelectrical impedance measurements as a prognostic factor for mortality at 90 days in patients with COVID-19: A longitudinal cohort study. Clin. Nutr. 2021, in press. [Google Scholar] [CrossRef]
- Paiva, S.I.; Borges, L.R.; Halpern-Silveira, D.; Assunção, M.C.F.; Barros, A.J.; Gonzalez, M.C. Standardized phase angle from bioelectrical impedance analysis as prognostic factor for survival in patients with cancer. Supportive Care Cancer 2011, 19, 187–192. [Google Scholar] [CrossRef]
- Piccoli, A.; Codognotto, M.; Piasentin, P.; Naso, A. Combined evaluation of nutrition and hydration in dialysis patients with bioelectrical impedance vector analysis (BIVA). Clin. Nutr. 2014, 33, 673–677. [Google Scholar] [CrossRef]
- Middleton, M.H.; Nazarenko, G.; Nivison-Smith, I.; Smerdely, P. Prevalence of malnutrition and 12-month incidence of mortality in two Sydney teaching hospitals. Intern. Med. J. 2001, 31, 455–461. [Google Scholar] [CrossRef]




| Parameters | Total | Female Patients | Male Patients | p * |
|---|---|---|---|---|
| Demografics | ||||
| N | 570 | 304 | 266 | |
| Age (years) | 65.0 (53.0–74.0) | 64.0 (50.0–75.0) | 65 (55.3–73.0) | p = 0.498 |
| Height (cm) | 167.0 (160.0–172.0) | 161.0 (156.0–165.0) | 172.0 (168.0–178.0) | p < 0.001 |
| Weight (kg) | 70.0 (60.0–81.1) | 63.1 (55.0–74.0) | 75.0 (68.0–86.0) | p < 0.001 |
| Loss Weight (%) | 2.12 (0.0–8.51) | 0.9 (0.0–8.1) | 3.2 (0.0–8.6) | p = 0.015 |
| BMI (kg/H2) | 24.9 (22.0–28.1) | 24.2 (21.4–27.5) | 25.6 (23.2–28.5) | p = 0.001 |
| Nutritional Tools | ||||
| SGA | p = 0.114 | |||
| A | 241 (42.3%) | 118 (38.8%) | 123 (46.2%) | |
| B | 204 (35.8%) | 116 (38.2%) | 88 (33.1%) | |
| C | 125 (21.9%) | 70 (23%) | 55 (20.7%) | |
| MUST | p = 0.656 | |||
| 0 | 263 (46.1%) | 136 (44.7%) | 127 (48.3%) | |
| 1 | 123 (21.6%) | 70 (23.0%) | 51 (42.9%) | |
| 2 | 184 (32.3%) | 98 (32.3%) | 86 (46.8%) | |
| BIVA | ||||
| PhA (°) | 5.1 (4.1–6.1) | 4.9 (3.98–5.8) | 5.45 (4.3–6.57) | p < 0.001 |
| SPhA | 1.97 (−1.5–1.1) | 0.0 (−1.1–1.42) | −0.4 (−1.9–0.77) | p < 0.001 |
| BCM (kg) | 23.6 (18.7–29.6) | 20.4 (16.9–24.4) | 29.2 (22.7–35.1) | p < 0.001 |
| Functional test | ||||
| HGS (kg) | 26.0 (19.0–35.0) | 20.0 (17.0–25.8) | 35.0 (27.0–40.0) | p < 0.001 |
| Outcomes | ||||
| Long stay (days) | 7.0 (3.0–12.3) | 7.0 (7.0–12.0) | 7.0 (7.0–14.0) | p = 0.871 |
| Death, n (%) | 86 (15.1%) | 42.0 (13.8%) | 44.0 (16.5%) | p = 0.450 |
| Parameters | AUC | Cut-Off Point | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| PhA (°) | 0.835 | 5.4° | 74.69% | 78.42% | 71.71% | 80.88% |
| Male | 0.851 | 5.4° | 82.11%; | 77.96% | 74.26% | 83.08% |
| Female | 0.815 | 5.3° | 70.34% | 63.53% | 66.94% | 80.56% |
| SPhA | 0.776 | −0.3 | 81.74% | 63.53% | 62.15% | 82.61% |
| HGS (kg) | 0.710 | 27 | 67.61% | 67.48% | 64.43% | 70.51% |
| Male | 0.759 | 34 | 75.64% | 64.94% | 68.6% | 72.46% |
| Female | 0.709 | 19 | 76.56% | 55.81% | 56.31% | 76.19% |
| BCM (kg) | 0.810 | 23.6 | 78.84% | 71.04% | 66.67% | 82.04% |
| Male | 0.857 | 30.0 | 73.98% | 80.28% | 76.47% | 78.08% |
| Female | 0.832 | 21.7 | 76.27% | 76.88% | 67.67% | 83.63% |
| Parameters | NMS | MS | p * |
|---|---|---|---|
| Demografics | |||
| N | 317 | 253 | |
| Sex n (%) Female | 187.0 (59.0%) | 117.0 (46.2%) | p ≤ 0.001 |
| Male | 130.0 (41.0%) | 136.0 (53.8%) | p ≤ 0.001 |
| Age (years) | 61.0 (49.0–70.3) | 68.0 (60.0–77.0) | p ≤ 0.001 |
| Height (cm) | 165 (160.0–172.0) | 168 (160.0–174.0) | p = 0.644 |
| Weight (kg) | 71.0 (60.5–83.0) | 67.5 (59.0–79.5) | p = 0.018 |
| Loss Weight (%) | 0.09 (0.0–5.8) | 5.53 (0.0–13.2) | p ≤ 0.001 |
| BMI (kg/H2) | 25.3 (22.7–28.7) | 24.4 (21.4–27.3) | p = 0.002 |
| Nutritional Tools | |||
| VSG | p ≤ 0.001 | ||
| A | 197(62.1%) | 60 (23.7%) | |
| B | 90 (28.4%) | 64 (25.3%) | |
| C | 30 (9.5%) | 129 (51.0%) | |
| MUST | p ≤ 0.001 | ||
| 0 | 203 (64%) | 60 (23.7%) | |
| 1 | 59 (18.6%) | 64 (25.3%) | |
| 2 | 55 (17.4%) | 129 (51%) | |
| BIVA | |||
| PhA (°) | 5.9 (5.3–6.7) | 4.0 (3.4–4.6) | p < 0.001 |
| SPhA | 0.9 (0.2–1.8) | −1.8 (−2.8–−1) | p < 0.001 |
| BCM (kg) | 26.4 (22.5–32.9) | 19.1 (15.6–24) | p < 0.001 |
| Functional test | |||
| HGS (kg) | 28.0 (19.0–38.3) | 24.0 (18.0–33.0) | p = 0.028 |
| Outcomes | |||
| Long stay (days) | 5.0 (3.0–9.0) | 9.0 (5.5–17.0) | p < 0.001 |
| Death, n (%) | 10.0 (3.1%) | 76.0 (30%) | p < 0.001 |
| Dependent: Surv (Mytime, Myoutcome) | All | HR (Univariable) | HR (Multivariable) | |
|---|---|---|---|---|
| SPhA-Malnutrition | MS | 163 (100.0) | - | - |
| NMS | 107 (100.0) | 9.62 (3.35–27.66, p < 0.001) | 7.87 (2.56–24.24, p < 0.001) | |
| HGS-Malnutrition | NS | 137 (100.0) | - | - |
| MS | 133 (100.0) | 3.51 (1.51–8.19, p = 0.004) | 2.23 (0.92–5.41, p = 0.076) | |
| Sex | Female | 134 (100.0) | - | - |
| Male | 136 (100.0) | 0.94 (0.46–1.93, p = 0.874) | 0.85 (0.40–1.80, p = 0.665) | |
| Age | Mean (SD) | 61.9 (14.9) | 1.03 (1.00–1.05, p = 0.069) | 1.01 (0.98–1.04, p = 0.416) |
| BMI | Mean (SD) | 25.5 (4.9) | 0.94 (0.87–1.02, p = 0.157) | 0.98 (0.91–1.07, p = 0.705) |
| Hydration | Mean (SD) | 74.4 (5.7) | 1.12 (1.04–1.21, p = 0.003) | 1.00 (0.92–1.08, p = 0.994) |
| 95% Confidence Interval | ||||||
|---|---|---|---|---|---|---|
| Model 1 | Estimate | SE | Lower | Upper | ß | p |
| Intercept a SPA_Malnutrition: MS-NMS | 7.6 | 0.539 | 6.54 | 8.66 | 14.1 | <0.001 |
| 5.32 | 0.813 | 3.72 | 6.92 | 6.54 | <0.001 | |
| Model 2 | ||||||
| Intercept a SPhA_Malnutrition | 7.139 | 2.3344 | 2.543 | 11.7347 | 3.058 | 0.002 |
| MS-NMS | 3.7875 | 1.2292 | 1.368 | 6.2074 | 3.081 | 0.002 |
| Age | −0.0291 | 0.0362 | −0.1 | 0.0421 | −0.805 | 0.421 |
| Sex | ||||||
| Female–Male | 0.307 | 1.0729 | −1.805 | 2.4192 | 0.286 | 0.775 |
| SGA: | ||||||
| B/C–A | 3.3511 | 1.207 | 0.975 | 5.7273 | 2.776 | 0.006 |
| HGS_Malnutrition | ||||||
| 1–0 | 0.5995 | 1.1347 | −1.634 | 2.8335 | 0.528 | 0.598 |
| Death | ||||||
| 1–0 | 1.1102 | 1.7216 | −2.279 | 4.4995 | 0.645 | 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Jiménez, R.; Dalla-Rovere, L.; García-Olivares, M.; Abuín-Fernández, J.; Sánchez-Torralvo, F.J.; Doulatram-Gamgaram, V.K.; Hernández-Sanchez, A.M.; García-Almeida, J.M. Phase Angle and Handgrip Strength as a Predictor of Disease-Related Malnutrition in Admitted Patients: 12-Month Mortality. Nutrients 2022, 14, 1851. https://doi.org/10.3390/nu14091851
Fernández-Jiménez R, Dalla-Rovere L, García-Olivares M, Abuín-Fernández J, Sánchez-Torralvo FJ, Doulatram-Gamgaram VK, Hernández-Sanchez AM, García-Almeida JM. Phase Angle and Handgrip Strength as a Predictor of Disease-Related Malnutrition in Admitted Patients: 12-Month Mortality. Nutrients. 2022; 14(9):1851. https://doi.org/10.3390/nu14091851
Chicago/Turabian StyleFernández-Jiménez, Rocío, Lara Dalla-Rovere, María García-Olivares, José Abuín-Fernández, Francisco José Sánchez-Torralvo, Viyey Kishore Doulatram-Gamgaram, Agustín M. Hernández-Sanchez, and José Manuel García-Almeida. 2022. "Phase Angle and Handgrip Strength as a Predictor of Disease-Related Malnutrition in Admitted Patients: 12-Month Mortality" Nutrients 14, no. 9: 1851. https://doi.org/10.3390/nu14091851
APA StyleFernández-Jiménez, R., Dalla-Rovere, L., García-Olivares, M., Abuín-Fernández, J., Sánchez-Torralvo, F. J., Doulatram-Gamgaram, V. K., Hernández-Sanchez, A. M., & García-Almeida, J. M. (2022). Phase Angle and Handgrip Strength as a Predictor of Disease-Related Malnutrition in Admitted Patients: 12-Month Mortality. Nutrients, 14(9), 1851. https://doi.org/10.3390/nu14091851