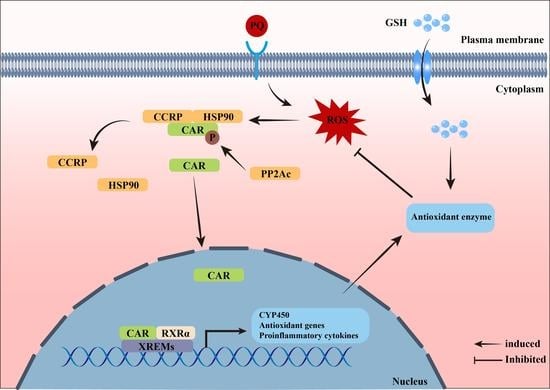
Glutathione Protects against Paraquat-Induced Oxidative Stress by Regulating Intestinal Barrier, Antioxidant Capacity, and CAR Signaling Pathway in Weaned Piglets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Animal and Experimental Design
2.3. Sample Collection
2.4. Serum Physiological and Biochemical Properties
2.5. Intestinal Histomorphology
2.6. Cell Apoptosis
2.7. Immunohistochemical Analysis
2.8. Transmission Electron Microscopy (TEM)
2.9. Real-Time PCR Analysis
2.10. Statistical Analysis
3. Results
3.1. Effect of Dietary GSH Supplementation at Different Levels on Growth Performance and Intestinal Morphology in Paraquat-Induced Weaned Piglets
3.2. Effect of Dietary GSH Supplementation on Intestinal Permeability in Paraquat-Induced Weaned Piglets
3.3. Effect of Dietary GSH Supplementation on Antioxidant Capacity in Paraquat-Induced Weaned Piglets
3.4. Effect of Dietary GSH Supplementation on the Expression of Inflammatory Cytokine and CAR Pathway-Related Targets in Paraquat-Induced Weaned Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Han, H.; Liu, Z.M.; Yin, J.; Gao, J.; He, L.Q.; Wang, C.Y.; Hou, R.X.; He, X.G.; Wang, G.Q.; Li, T.J.; et al. D-Galactose Induces Chronic Oxidative Stress and Alters Gut Microbiota in Weaned Piglets. Front. Physiol. 2021, 12, 634283. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.W.; Liu, Y.H.; Guan, P.; He, L.Q.; Zhou, X.H. Serine Administration Improves Selenium Status, Oxidative Stress, and Mitochondrial Function in Longissimus Dorsi Muscle of Piglets with Intrauterine Growth Retardation. Biol. Trace Elem. Res. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longevity 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Li, L.; Hang, Q.; Fang, Y.; Dong, X.; Cao, P.; Yin, Z.; Luo, L. γ-glutamylcysteine exhibits anti-inflammatory effects by increasing cellular glutathione level. Redox Biol. 2019, 20, 157–166. [Google Scholar] [CrossRef] [PubMed]
- He, L.Q.; Wu, J.; Tang, W.J.; Zhou, X.H.; Lin, Q.L.; Luo, F.J.; Yin, Y.L.; Li, T.J. Prevention of Oxidative Stress by α-Ketoglutarate via Activation of CAR Signaling and Modulation of the Expression of Key Antioxidant-Associated Targets in Vivo and in Vitro. J. Agric. Food Chem. 2018, 66, 11273–11283. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef] [Green Version]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [Green Version]
- Kaplowitz, N.; Aw, T.Y.; Ookhtens, M. The regulation of hepatic glutathione. Annu. Rev. Pharmacol. Toxicol. 1985, 25, 715–744. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, A.Z.; Song, Z.T.; Sun, Y.F.; Yu, G.P. Manipulation of glutathione on growth performance and growth hormone/insulin-like growth factor-I axis in fattening sheep. Chin. J. Anim. Nutr. 2009, 21, 312–318. (In Chinese) [Google Scholar]
- Ming, J.H.; Ye, J.Y.; Zhang, Y.X.; Xu, P.; Xie, J. Effects of dietary reduced glutathione on growth performance, non-specific immunity, antioxidant capacity and expression levels of IGF-I and HSP70 mRNA of grass carp (Ctenopharyngodon idella). Aquaculture 2015, 438, 39–46. [Google Scholar] [CrossRef]
- Ren, J.; Hao, Y.; Liu, Z.; Li, S.; Wang, C.; Wang, B.; Liu, Y.; Liu, G.; Dai, Y. Effect of exogenous glutathione supplementation on the in vitro developmental competence of ovine oocytes. Theriogenology 2021, 173, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, P.B.; Bucak, M.N.; Büyükleblebici, S.; Sarıözkan, S.; Yeni, D.; Eken, A.; Akalın, P.P.; Kinet, H.; Avdatek, F.; Fidan, A.F.; et al. The effect of cysteine and glutathione on sperm and oxidative stress parameters of post-thawed bull semen. Cryobiology 2010, 61, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yan, J.; Xu, M.; Ren, S.; Xie, W. CAR Suppresses Hepatic Gluconeogenesis by Facilitating the Ubiquitination and Degradation of PGC1α. Mol. Endocrinol. 2015, 29, 1558–1570. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Chen, B.; Lu, J.; Xie, W. Deciphering the roles of the constitutive androstane receptor in energy metabolism. Acta Pharmacol. Sin. 2015, 36, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Qatanani, M.; Moore, D.D. CAR, the continuously advancing receptor, in drug metabolism and disease. Curr. Drug Metab. 2005, 6, 329–339. [Google Scholar] [CrossRef]
- Yoda, E.; Paszek, M.; Konopnicki, C.; Fujiwara, R.; Chen, S.; Tukey, R.H. Isothiocyanates induce UGT1A1 in humanized UGT1 mice in a CAR dependent fashion that is highly dependent upon oxidative stress. Sci. Rep. 2017, 7, 46489. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, W.D.; Chua, S.S.; Wei, P.; Moore, D.D. Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science 2002, 298, 422–424. [Google Scholar] [CrossRef]
- Tang, W.J.; Long, J.; Li, T.J.; Yang, L.Y.; Li, J.Z.; He, L.Q.; Li, S.W.; Kuang, S.Y.; Feng, Y.Z.; Chen, H.S.; et al. The Associated Regulatory Mechanisms of Zinc Lactate in Redox Balance and Mitochondrial Function of Intestinal Porcine Epithelial Cells. Oxid. Med. Cell. Longev. 2020, 2020, 8815383. [Google Scholar] [CrossRef]
- He, L.Q.; Zhou, X.H.; Wu, Z.P.; Feng, Y.Z.; Liu, D.; Li, T.J.; Yin, Y.L. Glutamine in suppression of lipopolysaccharide-induced piglet intestinal inflammation: The crosstalk between AMPK activation and mitochondrial function. Anim. Nutr. 2022, 10, 137–147. [Google Scholar] [CrossRef]
- He, L.Q.; Zhou, X.H.; Huang, N.; Li, H.; Cui, Z.J.; Tian, J.Q.; Jiang, Q.; Liu, S.J.; Wu, J.; Li, T.J.; et al. Administration of alpha-ketoglutarate improves epithelial restitution under stress injury in early-weaning piglets. Oncotarget 2017, 8, 91965–91978. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; He, X.; Peng, C.; He, Y.; Wang, C.; Tang, W.; Chen, H.; Feng, Y.; Liu, D.; Li, T.; et al. Improvement of Ulcerative Colitis by Aspartate via RIPK Pathway Modulation and Gut Microbiota Composition in Mice. Nutrients 2022, 14, 3707. [Google Scholar] [CrossRef] [PubMed]
- Abot, A.; Fried, S.; Cani, P.D.; Knauf, C. Reactive Oxygen Species/Reactive Nitrogen Species as Messengers in the Gut: Impact on Physiology and Metabolic Disorders. Antioxid. Redox Signal. 2022, 37, 394–415. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Czerniczyniec, A.; Karadayian, A.G.; Bustamante, J.; Cutrera, R.A.; Lores-Arnaiz, S. Paraquat induces behavioral changes and cortical and striatal mitochondrial dysfunction. Free Radic. Biol. Med. 2011, 51, 1428–1436. [Google Scholar] [CrossRef]
- Dostal, V.; Wood, S.D.; Thomas, C.T.; Han, Y.; Lau, E.; Lam, M.P.Y. Proteomic signatures of acute oxidative stress response to paraquat in the mouse heart. Sci. Rep. 2020, 10, 18440. [Google Scholar] [CrossRef]
- Qi, M.; Wang, N.; Xiao, Y.; Deng, Y.; Zha, A.; Tan, B.; Wang, J.; Yin, Y.; Liao, P. Ellagic acid ameliorates paraquat-induced liver injury associated with improved gut microbial profile. Environ. Pollut. 2022, 293, 118572. [Google Scholar] [CrossRef]
- Ma, X.Y.; Tian, Z.M.; Cui, Y.Y.; Liu, Z.C.; Lu, H.J. Effects of glutathione on growth performance and intestinal health of piglets. J. Anim. Sci. 2019, 97, 19. [Google Scholar] [CrossRef]
- Wang, K.; Yang, A.; Peng, X.; Lv, F.; Wang, Y.; Cui, Y.; Wang, Y.; Zhou, J.; Si, H. Linkages of Various Calcium Sources on Immune Performance, Diarrhea Rate, Intestinal Barrier, and Post-gut Microbial Structure and Function in Piglets. Front. Nutr. 2022, 9, 921773. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20; quiz 21–22. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; Miller, M.L.; Shen, D.; Shertzer, H.G.; Stringer, K.F.; Wang, B.; Schneider, S.N.; Nebert, D.W.; Dalton, T.P. Hepatocyte-specific Gclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure. Hepatology 2007, 45, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef]
- Kucharzik, T.; Walsh, S.V.; Chen, J.; Parkos, C.A.; Nusrat, A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am. J. Pathol. 2001, 159, 2001–2009. [Google Scholar] [CrossRef] [Green Version]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Luk, G.D.; Bayless, T.M.; Baylin, S.B. Diamine oxidase (histaminase). A circulating marker for rat intestinal mucosal maturation and integrity. J. Clin. Investg. 1980, 66, 66–70. [Google Scholar] [CrossRef]
- Lau, E.; Marques, C.; Pestana, D.; Santoalha, M.; Carvalho, D.; Freitas, P.; Calhau, C. The role of I-FABP as a biomarker of intestinal barrier dysfunction driven by gut microbiota changes in obesity. Nutr. Metab. 2016, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Liu, G.; Tao, J.; Lu, J.; Jia, G.; Zhao, H.; Chen, X.; Tian, G.; Cai, J.; Zhang, R.; Wang, J. Dietary Tryptophan Supplementation Improves Antioxidant Status and Alleviates Inflammation, Endoplasmic Reticulum Stress, Apoptosis, and Pyroptosis in the Intestine of Piglets after Lipopolysaccharide Challenge. Antioxidants 2022, 11, 872. [Google Scholar] [CrossRef]
- Li, S.; Hong, M.; Tan, H.Y.; Wang, N.; Feng, Y. Insights into the Role and Interdependence of Oxidative Stress and Inflammation in Liver Diseases. Oxid. Med. Cell. Longevity 2016, 2016, 4234061. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, J.S.; Omiecinski, C.J. An okadaic acid-sensitive pathway involved in the phenobarbital-mediated induction of CYP2B gene expression in primary rat hepatocyte cultures. J. Pharmacol. Exp. Ther. 1997, 282, 1122–1129. [Google Scholar] [PubMed]
- Kobayashi, K.; Sueyoshi, T.; Inoue, K.; Moore, R.; Negishi, M. Cytoplasmic accumulation of the nuclear receptor CAR by a tetratricopeptide repeat protein in HepG2 cells. Mol. Pharmacol. 2003, 64, 1069–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maglich, J.M.; Parks, D.J.; Moore, L.B.; Collins, J.L.; Goodwin, B.; Billin, A.N.; Stoltz, C.A.; Kliewer, S.A.; Lambert, M.H.; Willson, T.M.; et al. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J. Biol. Chem. 2003, 278, 17277–17283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pustylnyak, V.O.; Lebedev, A.N.; Gulyaeva, L.F.; Lyakhovich, V.V.; Slynko, N.M. Comparative study of CYP2B induction in the liver of rats and mice by different compounds. Life Sci. 2007, 80, 324–328. [Google Scholar] [CrossRef]
- Wu, G.Y.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]






| Ingredients | Content | Nutrient Level 2 | Content |
|---|---|---|---|
| Ripening corn | 31.45 | Net energy (MJ/KG) | 24.50 |
| Ripening rice | 25.60 | Crude protein | 17.20 |
| Flour | 12.50 | Ether extract | 3.70 |
| Extruded soybean | 6.40 | Crude fiber | 2.08 |
| Fish meal | 3.50 | Calcium | 0.59 |
| Ripening soybean meal | 5.00 | Phosphorus | 0.51 |
| Fermented soybean meal | 5.00 | Digestible Lysine | 1.30 |
| 50% Choline chloride | 0.10 | Digestible Methionine | 0.59 |
| CaHPO3 | 0.80 | Digestible Methionine + Cystine | 0.80 |
| Limestone | 0.50 | Digestible Tryptophan | 0.27 |
| Glucose | 2.50 | Digestible Threonine | 0.85 |
| Sucrose | 2.50 | ||
| Soybean oil | 1.25 | ||
| NaCl | 0.40 | ||
| Lysine HCL | 0.77 | ||
| Threonine | 0.36 | ||
| Methionine | 0.38 | ||
| L-Tryptophan | 0.12 | ||
| L-Valine | 0.38 | ||
| Premix 1 | 0.19 | ||
| Total | 100.00 |
| Gene | Primers | Accession Numbers | Product Length (bp) |
|---|---|---|---|
| GPX1 | F: TGGGGAGATCCTGAATTG R: GATAAACTTGGGGTCGGT | NM_214201.1 | 184 |
| GPX4 | F: GATTCTGGCCTTCCCTTGC R: TCCCCTTGGGCTGGACTTT | NM_214407.1 | 173 |
| MnSOD | F: GGACAAATCTGAGCCCTAACG R: CCTTGTTGAAACCGAGCC | NM_214127.2 | 159 |
| CuZnSOD | F: TGAAGGGAGAGAAGACAGTGTTAG R: TCTCCAACGTGCCTCTCTTG | NM_001190422.1 | 181 |
| GCLC | F: GATCCTCCAGTTCCTGCACA R: GAGAGAGAACCAACCTCGTCG | XM_021098556.1 | 87 |
| GCLM | F: CACAGCGAGGAGCTTCGAGA R: TGCGTGAGACACAGTACATTCC | XM_001926378.4 | 117 |
| IFN-γ | F: CAGGCCATTCAAAGGAGCAT R: GAGTTCACTGATGGCTTTGCG | NM_213948.1 | 150 |
| IL-1β | F: CCAATTCAGGGACCCTACCC R: GTTTTGGGTGCAGCACTTCAT | NM_214055.1 | 174 |
| IL-12 | F: CAGGCCCAGGAATGTTCAAA R: CGTGGCTAGTTCAAGTGGTAAG | NM_213993.1 | 188 |
| IL-10 | F: CGGCGCTGTCATCAATTTCTG R: CCCCTCTCTTGGAGCTTGCTA | NM_214041.1 | 89 |
| CAR | F: GTGCCTGAACTGTCTCTGCT R: CCACATGCGCTCCATCTTCT | NM_001037996.1 | 244 |
| RXRα | F: CAAGTGCCTGGAACACCTCT R: ATGGAAGGTAACAGGGTGGC | XM_001927453.2 | 240 |
| HSP90 | F: AAGACCGGACCCTCACGATA R: AGGCATACTGCTCGTCATCG | NM_213973.1 | 231 |
| CCRP | F: TGCCCTAGAATTTGCCCCTG R: GCAAAGACCTCGGACGTACA | XM_003131409.5 | 157 |
| PP2Ac | F: GGTGCCATGACCGGAATGTA R: GTGCTGGGTCAAACTGCAAG | NM_214366.1 | 129 |
| GSTA1 | F: AGGACACCCAGGACCAATCTT R: CTCAGGTACATTCCGGGAGAAG | NM_214389.2 | 199 |
| GSTA2 | F: CTACTACGTGGAAGAGCTGGAC R: GCCCTGCCCACTTTATGAAGAC | NM_213850.2 | 193 |
| CYP1A2 | F: TTTGTGGAGACCGCCTCATC R: GCTTGAATAGGGCGCTTGTG | NM_001159614.1 | 193 |
| CYP2B22 | F: GGGAACGTTGGAAGACCCTT R: CGGGATCTCTGTAGGCGAAG | NM_214413.1 | 228 |
| CYP3A29 | F: CCTGAAATTAACCACGCAAGGGCT R: TCTGGGATGCAGCTTTCTTGACCA | NM_214423.1 | 140 |
| β-actin | F: CTGCGGCATCCACGAAACT R: AGGGCCGTGATCTCCTTCTG | XM_003124280.3 | 147 |
| GAPDH | F: AAGGAGTAAGAGCCCCTGGA R: TCTGGGATGGAAACTGGAA | NM_001206359.1 | 140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, X.; Wang, H.; Zhou, W.; Wang, C.; Guan, P.; Xu, G.; Zhao, Q.; He, L.; Yin, Y.; Li, T. Glutathione Protects against Paraquat-Induced Oxidative Stress by Regulating Intestinal Barrier, Antioxidant Capacity, and CAR Signaling Pathway in Weaned Piglets. Nutrients 2023, 15, 198. https://doi.org/10.3390/nu15010198
Xiang X, Wang H, Zhou W, Wang C, Guan P, Xu G, Zhao Q, He L, Yin Y, Li T. Glutathione Protects against Paraquat-Induced Oxidative Stress by Regulating Intestinal Barrier, Antioxidant Capacity, and CAR Signaling Pathway in Weaned Piglets. Nutrients. 2023; 15(1):198. https://doi.org/10.3390/nu15010198
Chicago/Turabian StyleXiang, Xuan, Houfu Wang, Wentao Zhou, Chenyu Wang, Peng Guan, Gang Xu, Qiang Zhao, Liuqin He, Yulong Yin, and Tiejun Li. 2023. "Glutathione Protects against Paraquat-Induced Oxidative Stress by Regulating Intestinal Barrier, Antioxidant Capacity, and CAR Signaling Pathway in Weaned Piglets" Nutrients 15, no. 1: 198. https://doi.org/10.3390/nu15010198
APA StyleXiang, X., Wang, H., Zhou, W., Wang, C., Guan, P., Xu, G., Zhao, Q., He, L., Yin, Y., & Li, T. (2023). Glutathione Protects against Paraquat-Induced Oxidative Stress by Regulating Intestinal Barrier, Antioxidant Capacity, and CAR Signaling Pathway in Weaned Piglets. Nutrients, 15(1), 198. https://doi.org/10.3390/nu15010198