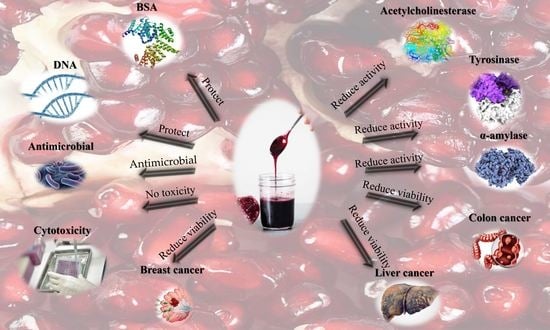
Antioxidant, Anti-Inflammatory, Antimicrobial, and Anticancer Activities of Pomegranate Juice Concentrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Sampling
2.1.2. Sample Extraction
2.2. Methods
2.2.1. Total Phenolics (TP)
2.2.2. Total Flavonoids (TF)
2.2.3. Quantification of Phenolic Acids and Flavonoids by HPLC
2.2.4. Antioxidant Activity
DPPH• Free Radical Scavenging Assay
ABTS• Free Radical Scavenging Assay
Nitric Oxide Radical Scavenging Analysis
Ferric Reducing/Antioxidant Power (FRAP) Assay
Total Antioxidant Activity (TAC)
Damage to DNA Caused by Free Radicals
The Oxidation of Proteins Caused by AAPH
2.2.5. Enzyme Inhibition Activity
Assay for Inhibiting Porcine α-Amylase
Assay for Inhibiting Tyrosinase
Assay for Inhibiting Acetylcholinesterase (AChE)
2.2.6. Antimicrobial Activity
Antimicrobial Assays
Minimum Inhibitory Concentrations (MIC) Assay
2.2.7. Anticancer Activity
Cytotoxicity
MTT Assay for Cell Proliferation
Apoptotic Effect Analysis
Analysis of Cell Cycle
Quantitative of Oncogenes Expression
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Bioactive Compounds
3.1.1. Total Phenolics
3.1.2. Total Flavonoids
3.1.3. HPLC Quantification of Flavonoids and Phenolic Acids
3.2. Antioxidant Activity
3.2.1. Assay for Scavenging DPPH• Free Radicals
3.2.2. Assay for Scavenging ABTS• Free Radicals
3.2.3. Assay for Scavenging Nitric Oxide Radicals
3.2.4. Assay for Measuring the Ferric-Reducing and Antioxidant Power (FRAP)
3.2.5. Total Antioxidant Activity
3.2.6. Damage to DNA Caused by Free Radicals
3.2.7. AAPH-Induced Oxidation of Proteins
3.3. Enzyme Inhibition Activity
3.3.1. Porcine α-Amylase Inhibition Assay
3.3.2. Tyrosinase Inhibition Assay
3.3.3. Assay for Inhibiting Acetylcholinesterase
3.4. Antimicrobial Activity
3.4.1. Antimicrobial Assays
3.4.2. Minimum Inhibitory Concentration (MIC) Assay
3.5. Anticancer Activity
3.6. Correlation Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- İncedayi, B. Bazı Nar Ürünlerinin Antioksidan Özellikler ve In-Vitro Biyoerişilebilirlik Açısından Değerlendirilmesi. Balıkesir Üniversitesi Fen Bilim. Enstitüsü Derg. 2020, 23, 96–110. [Google Scholar] [CrossRef]
- Hijazi, A.; Pisano, I.; Illek, P.; Leahy, J.J. A Rapid HPLC Method for the Simultaneous Determination of Organic Acids and Furans: Food Applications. Beverages 2022, 8, 6. [Google Scholar] [CrossRef]
- Bou Dargham, M.; Matar Boumosleh, J.; Farhat, A.; Abdelkhalek, S.; Bou-Maroun, E.; El Hosry, L. Antioxidant and Anti-Diabetic Activities in Commercial and Homemade Pomegranate Molasses in Lebanon. Food Biosci. 2022, 46, 101540. [Google Scholar] [CrossRef]
- Dandachi, F.; Hamadeh, B.; Youssef, H.; Chahine, H.; Chalak, L. Diversity Assessment of the Lebanese Germplasm of Pomegranate (Punica granatum L.) by Morphological and Chemical Traits. Ann. Agric. Sci. 2017, 62, 89–98. [Google Scholar] [CrossRef]
- Faour-Klingbeil, D.; Todd, E.C.D. The Inhibitory Effect of Traditional Pomegranate Molasses on S. typhimurium Growth on Parsley Leaves and in Mixed Salad Vegetables. J. Food Saf. 2018, 38, e12469. [Google Scholar] [CrossRef]
- Özmert Ergin, S. Investigation of the Physicochemical, Nutritional Properties and Antioxidant Activities of Commercial and Traditional Pomegranate Molasses Samples. Food Health 2020, 6, 177–185. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Jiang, H. Microbial Production of Value-Added Bioproducts and Enzymes from Molasses, a by-Product of Sugar Industry. Food Chem. 2021, 346, 128860. [Google Scholar] [CrossRef]
- El Darra, N.; Rajha, H.N.; Saleh, F.; Al-Oweini, R.; Maroun, R.G.; Louka, N. Food Fraud Detection in Commercial Pomegranate Molasses Syrups by UV–VIS Spectroscopy, ATR-FTIR Spectroscopy and HPLC Methods. Food Control 2017, 78, 132–137. [Google Scholar] [CrossRef]
- Arafa, S. Chemical and Biological Studies on Some Pomegranate Products. Egypt. J. Agric. Sci. 2013, 64, 396–408. [Google Scholar] [CrossRef]
- İncedayi, B.; Ece Tamer, C.; Utku Çopur, Ö. A Research on the Composition of Pomegranate Molasses. J. Agric. Fac. Uludag Univ. 2010, 24, 37–47. [Google Scholar]
- Nasser, G.; Sabbah, A.; Chokeir, N.; Hijazi, A.; Rammal, H.; Issa, M. Chemical Composition and Antioxidant Capacity of Lebanese Molasses Pomegranate. Am. J. Pharm. Tech. Res. 2017, 7, 191–204. [Google Scholar]
- Shalabi, O.M.A.K. Antioxidant, Antibacterial, and Antitumor Activities of Goat’s Stirred Yoghurt Fortified with Carob Molasses. Ann. Agric. Sci. 2022, 67, 119–126. [Google Scholar] [CrossRef]
- Akpinar-Bayizit, A.; Ozcan, T.; Yilmaz-Ersan, L.; Yildiz, E. Evaluation of Antioxidant Activity of Pomegranate Molasses by 2,2-Diphenyl-l-Picrylhydrazyl (DPPH) Method. Int. J. Chem. Eng. Appl. 2016, 7, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Evran, S.; Tayyarcan, E.K.; Acar-Soykut, E.; Guven, B.; Durakli-Velioglu, S.; Boyaci, I.H. Investigation of Phage and Molasses Interactions for the Biocontrol of E. coli O157:H7. Can. J. Microbiol. 2022, 68, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Pantiora, P.D.; Balaouras, A.I.; Mina, I.K.; Freris, C.I.; Pappas, A.C.; Danezis, G.P.; Zoidis, E.; Georgiou, C.A. The Therapeutic Alliance between Pomegranate and Health Emphasizing on Anticancer Properties. Antioxidants 2023, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Ain, H.B.U.; Tufail, T.; Bashir, S.; Ijaz, N.; Hussain, M.; Ikram, A.; Farooq, M.A.; Saewan, S.A. Nutritional Importance and Industrial Uses of Pomegranate Peel: A Critical Review. Food Sci. Nutr. 2023, 1–10. [Google Scholar] [CrossRef]
- Venkitasamy, C.; Zhao, L.; Zhang, R.; Pan, Z. Pomegranate. In Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier: Amsterdam, The Netherlands, 2019; pp. 181–216. [Google Scholar]
- Barati Boldaji, R.; Akhlaghi, M.; Sagheb, M.M.; Esmaeilinezhad, Z. Pomegranate Juice Improves Cardiometabolic Risk Factors, Biomarkers of Oxidative Stress and Inflammation in Hemodialysis Patients: A Randomized Crossover Trial. J. Sci. Food Agric. 2020, 100, 846–854. [Google Scholar] [CrossRef]
- Asgary, S.; Karimi, R.; Pour, P.M.; Heydarpour, F.; Mostafaei, S.; Farzaei, M.H.; Moradi, S.; Aneva, I.Y. Is Consumption of Pomegranate Supplementation Effective on Oxidative Stress Biomarkers Including MDA, Ox-LDL, POX 1, GPX, TAC, and TBRAS? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Probl. Cardiol. 2022, 101198. [Google Scholar] [CrossRef]
- Sharma, R.; Diwan, B.; Singh, B.P.; Kulshrestha, S. Probiotic Fermentation of Polyphenols: Potential Sources of Novel Functional Foods. Food Prod. Process. Nutr. 2022, 4, 21. [Google Scholar] [CrossRef]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A Comprehensive Update on Their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, 2101019. [Google Scholar] [CrossRef]
- Santhiravel, S.; Bekhit, A.E.-D.A.; Mendis, E.; Jacobs, J.L.; Dunshea, F.R.; Rajapakse, N.; Ponnampalam, E.N. The Impact of Plant Phytochemicals on the Gut Microbiota of Humans for a Balanced Life. Int. J. Mol. Sci. 2022, 23, 8124. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Bailey, S.J.; Chtourou, H.; Trabelsi, K.; Turki, M.; Hökelmann, A.; Souissi, N. Effects of Pomegranate Supplementation on Exercise Performance and Post-Exercise Recovery in Healthy Adults: A Systematic Review. Br. J. Nutr. 2018, 120, 1201–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohd Daud, S.M.; Mohd Sukri, N.; Johari, M.H.; Gnanou, J.; Abdul Manaf, F. Pure Juice Supplementation: Its Effect on Muscle Recovery and Sports Performance. Malays. J. Med. Sci. 2023, 30, 31–48. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.; Mündel, T.; Barnes, M.J. Nutritional Compounds to Improve Post-Exercise Recovery. Nutrients 2022, 14, 5069. [Google Scholar] [CrossRef]
- Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review. Foods 2021, 10, 657. [Google Scholar] [CrossRef]
- Fahmy, H.; Hegazi, N.; El-Shamy, S.; Farag, M.A. Pomegranate Juice as a Functional Food: A Comprehensive Review of Its Polyphenols, Therapeutic Merits, and Recent Patents. Food Funct. 2020, 11, 5768–5781. [Google Scholar] [CrossRef]
- Celiksoy, V.; Moses, R.L.; Sloan, A.J.; Moseley, R.; Heard, C.M. Evaluation of the In Vitro Oral Wound Healing Effects of Pomegranate (Punica granatum) Rind Extract and Punicalagin, in Combination with Zn (II). Biomolecules 2020, 10, 1234. [Google Scholar] [CrossRef]
- Stefanou, V.; Timbis, D.; Kanellou, A.; Margari, D.; Trianti, M.; Tsaknis, I.; Azar Naka, A.; Lougovois, V. Wound Healing Properties of Pomegranate. Arch. Microbiol. Immunol. 2021, 5, 263–291. [Google Scholar] [CrossRef]
- Habib, H.M.; al Meqbali, F.T.; Kamal, H.; Souka, U.D.; Ibrahim, W.H. Bioactive Components, Antioxidant and DNA Damage Inhibitory Activities of Honeys from Arid Regions. Food Chem. 2014, 153, 28–34. [Google Scholar] [CrossRef]
- Habib, H.M.; El-Fakharany, E.M.; Kheadr, E.; Ibrahim, W.H. Grape Seed Proanthocyanidin Extract Inhibits DNA and Protein Damage and Labile Iron, Enzyme, and Cancer Cell Activities. Sci. Rep. 2022, 12, 12393. [Google Scholar] [CrossRef]
- Habib, H.M.; Theuri, S.W.; Kheadr, E.E.; Mohamed, F.E. Functional, Bioactive, Biochemical, and Physicochemical Properties of the Dolichos Lablab Bean. Food Funct. 2017, 8, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.M.; Theuri, S.W.; Kheadr, E.E.; Mohamed, F.E. DNA and BSA Damage Inhibitory Activities, and Anti-Acetylcholinesterase, Anti-Porcine α-Amylase and Antioxidant Properties of Dolichos Lablab Beans. Food Funct. 2017, 8, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.M.; El-Fakharany, E.M.; El-Gendi, H.; El-Ziney, M.G.; El-Yazbi, A.F.; Ibrahim, W.H. Palm Fruit (Phoenix dactylifera L.) Pollen Extract Inhibits Cancer Cell and Enzyme Activities and DNA and Protein Damage. Nutrients 2023, 15, 2614. [Google Scholar] [CrossRef] [PubMed]
- Habib, H.M.; El-Fakharany, E.M.; Souka, U.D.; Elsebaee, F.M.; El-Ziney, M.G.; Ibrahim, W.H. Polyphenol-Rich Date Palm Fruit Seed (Phoenix dactylifera L.) Extract Inhibits Labile Iron, Enzyme, and Cancer Cell Activities, and DNA and Protein Damage. Nutrients 2022, 2022, 3536. [Google Scholar] [CrossRef]
- Habib, H.M.; Kheadr, E.; Ibrahim, W.H. Inhibitory Effects of Honey from Arid Land on Some Enzymes and Protein Damage. Food Chem. 2021, 364, 130415. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol; American Society for Microbiology, ASM; Springer: Midtown Manhattan, NY, USA, 2009. [Google Scholar]
- El-Fakharany, E.M.; Abu-Serie, M.M.; Litus, E.A.; Permyakov, S.E.; Permyakov, E.A.; Uversky, V.N.; Redwan, E.M. The Use of Human, Bovine, and Camel Milk Albumins in Anticancer Complexes with Oleic Acid. Protein J. 2018, 37, 203–215. [Google Scholar] [CrossRef]
- Yu, Q.; Fan, L.; Duan, Z. Five Individual Polyphenols as Tyrosinase Inhibitors: Inhibitory Activity, Synergistic Effect, Action Mechanism, and Molecular Docking. Food Chem. 2019, 297, 124910. [Google Scholar] [CrossRef]
- Yu, Q.; Fan, L. Understanding the Combined Effect and Inhibition Mechanism of 4-Hydroxycinnamic Acid and Ferulic Acid as Tyrosinase Inhibitors. Food Chem. 2021, 352, 129369. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the Sustainable Discovery and Development of New Antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Hassan, A.; Ullah, H.; Bonomo, M.G. Antibacterial and Antifungal Activities of the Medicinal Plant Veronica Biloba. J. Chem. 2019, 2019, 5264943. [Google Scholar] [CrossRef] [Green Version]
- Rosas-Burgos, E.C.; Burgos-Hernández, A.; Noguera-Artiaga, L.; Kačániová, M.; Hernández-García, F.; Cárdenas-López, J.L.; Carbonell-Barrachina, Á.A. Antimicrobial Activity of Pomegranate Peel Extracts as Affected by Cultivar. J. Sci. Food Agric. 2017, 97, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial Activity of Pomegranate Peel Extracts Performed by High Pressure and Enzymatic Assisted Extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, P.A. Cellular Impermeability and Uptake of Biocides and Antibiotics in Gram-Positive Bacteria and Mycobacteria. J. Appl. Microbiol. Symp. Suppl. 2002, 92, 35–45. [Google Scholar] [CrossRef]
- Sudha, T.; Mousa, D.S.; El-Far, A.H.; Mousa, S.A. Pomegranate (Punica granatum) Fruit Extract Suppresses Cancer Progression and Tumor Angiogenesis of Pancreatic and Colon Cancer in Chick Chorioallantoic Membrane Model. Nutr. Cancer 2021, 73, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M.; Damaj, Z.; Hijazi, A.; Merah, O.; Al-Khatib, B.; Hijazi, N.; Trabolsi, C.; Damaj, R.; Nasser, M. Pomegranate Juice Extract Decreases Cisplatin Toxicity on Peripheral Blood Mononuclear Cells. Medicines 2020, 7, 66. [Google Scholar] [CrossRef]
- Sharma, P.; McClees, S.; Afaq, F. Pomegranate for Prevention and Treatment of Cancer: An Update. Molecules 2017, 22, 177. [Google Scholar] [CrossRef] [Green Version]
- Mortada, W.I.; Awadalla, A.; Khater, S.M.; Barakat, N.M.; Husseiny, S.M.; Shokeir, A.A. Preventive Effect of Pomegranate Juice against Chemically Induced Bladder Cancer: An Experimental Study. Heliyon 2020, 6, e05192. [Google Scholar] [CrossRef]











| Bioactive Compounds mg/100 g PJC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TP | TF | Gallic Acid | 4-Hydroxy-3- Methoxybenzoic Acid | Catechin | Syringic Acid | Epicatechin | P-Coumaric Acid | Ferulic Acid | Rutin | Cinnamic Acid |
| 274.30 ± 2.13 | 82.58 ± 0.75 | nd | 909.72 ± 28.66 | 310.51 ± 1.07 | Nd | 532.84 ± 18.70 | 95.86 ± 3.90 | 150.62 ± 1.91 | 287.25 ± 5.42 | 15.13 ± 0.12 |
| Pathogen | Halo-Zone Diameter (mm) | MIC (µg/mL) | |||
|---|---|---|---|---|---|
| 175 µg | 350 µg | 525 µg | 700 µg | ||
| Streptococcus mutans | 11 ± 1.20 | 16 ± 0.97 | 20 ± 1.40 | 24 ± 1.40 | 175 ± 11.80 |
| Salmonella typhi | 0.0 ± 0.0 | 7 ± 1.57 | 13 ± 0.77 | 20 ± 0.97 | 175 ± 12.60 |
| Staphylococcus aureus | 0.0 ± 0.0 | 8 ± 0.17 | 18 ± 1.20 | 19 ± 0.78 | 350 ± 14.55 |
| Pseudomonas aeruginosa | 0.0 ± 0.0 | 7 ± 1.78 | 11 ± 0.97 | 11.3 ± 1.40 | 350 ± 11.30 |
| Pseudomonas fluorescens | 0.0 ± 0.0 | 00 ± 0.0 | 13.5 ± 0.78 | 16.8 ± 1.30 | 350 ± 9.11 |
| Escherichia coli | 0.0 ± 0.0 | 11 ± 1.78 | 11.5 ± 0.77 | 14.5 ± 1.78 | 700 ± 22.50 |
| Aeromonas hydrophila | 0.0 ± 0.0 | 0.0 ± 0.0 | 7 ± 1.98 | 8 ± 1.76 | 1400 ± 33.5 |
| Klebsiella pneumoniae | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | - |
| Candida albicans | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | - |
| Cells | 24 h | 48 h | ||||
|---|---|---|---|---|---|---|
| EC100 | IC50 | SI | EC100 | IC50 | SI | |
| HSF | 33.28 ± 0.95 | 1052 ± 29.87 | - | 32.11 ± 0.48 | 1015 ± 15.14 | - |
| Caco-2 | 4.11 ± 0.04 | 129.9 ± 1.34 | 8.09 ± 0.23 | 3.18 ± 0.16 | 100.5 ± 5.13 | 10.09 ± 0.15 |
| HepG-2 | 5.20 ± 0.05 | 164.4 ± 1.61 | 6.39 ± 0.18 | 3.36 ± 0.31 | 106.3 ± 9.68 | 9.55 ± 0.14 |
| MDA | 4.48 ± 0.04 | 141.6 ± 1.35 | 7.43 ± 0.21 | 3.20 ± 0.22 | 101.2 ± 6.86 | 10.03 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habib, H.M.; El-Gendi, H.; El-Fakharany, E.M.; El-Ziney, M.G.; El-Yazbi, A.F.; Al Meqbaali, F.T.; Ibrahim, W.H. Antioxidant, Anti-Inflammatory, Antimicrobial, and Anticancer Activities of Pomegranate Juice Concentrate. Nutrients 2023, 15, 2709. https://doi.org/10.3390/nu15122709
Habib HM, El-Gendi H, El-Fakharany EM, El-Ziney MG, El-Yazbi AF, Al Meqbaali FT, Ibrahim WH. Antioxidant, Anti-Inflammatory, Antimicrobial, and Anticancer Activities of Pomegranate Juice Concentrate. Nutrients. 2023; 15(12):2709. https://doi.org/10.3390/nu15122709
Chicago/Turabian StyleHabib, Hosam M., Hamada El-Gendi, Esmail M. El-Fakharany, Mohamed G. El-Ziney, Ahmed F. El-Yazbi, Fatima T. Al Meqbaali, and Wissam H. Ibrahim. 2023. "Antioxidant, Anti-Inflammatory, Antimicrobial, and Anticancer Activities of Pomegranate Juice Concentrate" Nutrients 15, no. 12: 2709. https://doi.org/10.3390/nu15122709
APA StyleHabib, H. M., El-Gendi, H., El-Fakharany, E. M., El-Ziney, M. G., El-Yazbi, A. F., Al Meqbaali, F. T., & Ibrahim, W. H. (2023). Antioxidant, Anti-Inflammatory, Antimicrobial, and Anticancer Activities of Pomegranate Juice Concentrate. Nutrients, 15(12), 2709. https://doi.org/10.3390/nu15122709