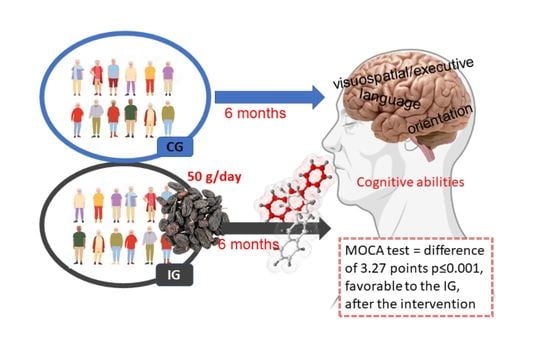
Effects of a Raisin Supplement on Cognitive Performance, Quality of Life, and Functional Activities in Healthy Older Adults—Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Study Participants and Recruitment
2.3. Sample Size
2.4. Randomization and Study Procedures
2.5. Intervention
2.6. Adherence to the Intervention
2.7. Variables and Measuring Instruments
2.8. Ethical Considerations
2.9. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Changes in Cognitive Performance
3.3. Changes in the Quality of Life and Functional Activities
4. Discussion
4.1. Discussion of Cognitive Performance Results
4.2. Discussion of the Results of the Quality of Life and Functional Activities
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and vascular effect of the mediterranean diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tresserra-Rimbau, A.; Medina-Remón, A.; Pérez-Jiménez, J.; Martínez-González, M.A.; Covas, M.I.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Dietary intake and major food sources of polyphenols in a Spanish population at high cardiovascular risk: The PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Caruana, M.; Cauchi, R.; Vassallo, N. Putative Role of Red Wine Polyphenols against Brain Pathology in Alzheimer’s and Parkinson’s Disease. Front. Nutr. 2016, 12, 3–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantos, E.; Espín, J.C.; Tomás-Barberán, F.A. Varietal differences among the polyphenol profiles of seven table grape cultivars studied by LC-DAD-MS-MS. J. Agric. Food Chem. 2002, 50, 5691–5696. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.; Schmitz, K.; Christian, A.; Ritchey, M.; Anderson, J. Raisins and glucose: A randomized, controlled trial. Diabetes 2012, 61, 580–581. [Google Scholar]
- Undurti, N. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis. 2008, 7, 37. [Google Scholar]
- Rodrigo-Gonzalo, M.J.; González-Manzano, S.; Mendez-Sánchez, R.; Santos-Buelga, C.; Recio-Rodríguez, J.I. Effect of Polyphenolic Complements on Cognitive Function in the Elderly: A Systematic Review. Antioxidants 2022, 10, 1549. [Google Scholar] [CrossRef]
- Rodrigo-Gonzalo, M.J.; Recio-Rodríguez, J.I.; Méndez-Sánchez, R.; Pablos-Hernández, M.C.; González-Ramírez, A.; González-Sánchez, J.; Fermoso-Palmero, M.J.; Puente-González, A.S.; Sánchez-Sánchez, M.C.; Barbero-Iglesias, F.J.; et al. Effect of including a dietary supplement of raisins, a food rich in polyphenols, on cognitive function in healthy older adults; a study protocol for a randomized clinical trial. BMC Geriatr. 2023, 29, 182–192. [Google Scholar] [CrossRef]
- García-Yu, I.; García, L.; Gómez, M.A.; Rodríguez, E.; Agudo, C.; González, J.; Maderuelo-Fernandez, J.A.; Recio-Rodriguez, J.I. Effects of Cocoa-Rich Chocolate on Blood Pressure, Cardiovascular Risk Factors, and Arterial Stiffness in Postmenopausal Women: A Randomized Clinical Trial. Nutrients 2020, 12, 1758. [Google Scholar] [CrossRef]
- Lozano, M.; Hernández, M.; Turró, O.; Pericot, I.; López, S.; Vilalta, J. Validación del Montreal Cognitive Assessment (MoCA): Test de cribado para el deterioro cognitivo leve. Real. Invest. Demenc. 2009, 43, 4–11. [Google Scholar]
- López Miquel, J.; Martí Agustí, G. Mini-Examen Cognoscitivo (MEC). Rev. Esp. Med. Legal 2011, 37, 122–127. [Google Scholar] [CrossRef]
- Galdona, N.; Buiza, C.; Etxeberria, I.; Urdoneta, E.; González, M.F.; Etxaniz, A. Validación de la prueba de aprendizaje verbal-auditivo de rey (AVLT) al castellano. Rev. Esp. Geriatr. Gerontol. 2007, 42, 64–198. [Google Scholar]
- Goodglass, H.; Kaplan, E. Evaluación de la Afasia y de Trastornos Relacionados; Panamericana: Madrid, Spain, 1986. [Google Scholar]
- Olazaran, J.; Mouronte, P.; Bermejo, F. Clinical validity of two scales of instrumental activities in Alzheimer’s disease. Neurología 2005, 20, 395–401. [Google Scholar]
- Pfeffer, R.; Kurosaki, T.; Harrah, C.; Chance, J.; Filos, S.J. Measurement of functional activities in older adults in the community. J. Gerontol. 1982, 37, 323–329. [Google Scholar] [CrossRef]
- Caballero, F.F.; Miret, M.; Power, M.; Chatterji, S.; Tobiasz-Adamczyk, B.; Koskinen, S.; Leonardi, M.; Olaya, B.; Haro, J.M.; Ayuso-Mateos, J.L. Validation of an instrument to evaluate quality of life in the aging population: WHOQOL-AGE. Health Qual. Life Outcomes 2013, 23, 11. [Google Scholar] [CrossRef] [Green Version]
- Group, E. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar]
- Badia, X.; Roset, M.; Montserrat, S.; Herdman, M.; Segura, A. The Spanish version of EuroQol: A description and its applications. European Quality of Life scale. Med. Clin. 1999, 112, 79–85. [Google Scholar]
- Badia, X.; Roset, M.; Monserrat, S.; Herdman, M. The Spanish VAS tariff based on valuation of EQ-5D health states from the general population. In Proceedings of the EuroQol Plenary Meeting, Rottersam, The Netherlands, 2–3 October 1997; pp. 93–114. [Google Scholar]
- World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [Green Version]
- Atmaca, N.; Atmaca, H.T.; Kanici, A.; Anteplioglu, T. Protective effect of resveratrol on sodium fluoride-induced oxidative stress, hepatotoxicity and neurotoxicity in rats. Food Chem. Toxicol. 2014, 70, 191–197. [Google Scholar] [CrossRef]
- Kuriyama, S.; Hozawa, A.; Ohmori, K.; Shimazu, T.; Matsui, T.; Ebihara, S.; Awata, S.; Nagatomi, R.; Arai, H.; Tsuji, I. Green tea consumption and cognitive function: A cross-sectional study from the Tsurugaya Project 1. Am. J. Clin. Nutr. 2006, 83, 355–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, R.; Li, W.; Liu, B.; Li, S.; Zhang, B.; Xu, Y. Resveratrol protects vascular smooth muscle cells against high glucose-induced oxidative stress and cell proliferation in vitro. Med. Sci. Monit. Basic. Res. 2014, 20, 82–92. [Google Scholar] [PubMed] [Green Version]
- Kennedy, D.O. Polyphenols and the human Brain: Plant “secondary metabolite” ecologic roles and endogenous signaling functions drive benefits. Adv. Nutr. 2014, 5, 515–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl. Res. 2017, 179, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. Flavonoids and brain health: Multiple effects underpinned by common mechanisms. Genes Nutr. 2009, 4, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C.; et al. Benefits in Cognitive Function, Blood Pressure, and Insulin Resistance Through Cocoa Flavanol Consumption in Elderly Subjects with Mild Cognitive Impairment The Cocoa, Cognition, and Aging (CoCoA) Study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef] [Green Version]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The cocoa, cognition, and aging (CoCoA) study-A randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef] [Green Version]
- Anton, S.D.; Ebner, N.; Dzierzewski, J.M.; Zlatar, Z.Z.; Gurka, M.J.; Dotson, V.M.; Kirton, J.; Mankowski, R.T.; Marsiske, M.; Manini, T.M. Effects of 90 days of resveratrol supplementation on cognitive function in elders: A pilot study. J. Altern. Complement. Med. 2018, 24, 725–732. [Google Scholar] [CrossRef]
- Brickman, A.M.; Khan, U.A.; Provenzano, F.A.; Yeung, L.K.; Suzuki, W.; Schroeter, H.; Wall, M.; Sloan, R.P.; Small, S.A. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci. 2014, 17, 1798–1803. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Torosyan, N.; Silverman, D.H. Examining the impact of grape consumption on brain metabolism and cognitive function in patients with mild decline in cognition: A double-blinded placebo controlled pilot study. Exp. Gerontol. 2017, 87, 121–128. [Google Scholar] [CrossRef]
- Ide, K.; Yamada, H.; Takuma, N.; Kawasaki, Y.; Harada, S.; Nakase, J.; Ukawa, Y.; Sagesaka, Y.M. Effects of green tea consumption on cognitive dysfunction in an elderly population: A randomized placebo-controlled study. Nutr. J. 2016, 15, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernando-Requejo, V. Nutrición y deterioro cognitivo. Nutr. Hosp. 2016, 33, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J.; Pouchieu, C.; Pourtau, L.; Gaudout, D.; Pallet, V.; Drummond, P.D. Effects of a polyphenol-rich grape and blueberry extract (Memophenol™) on cognitive function in older adults with mild cognitive impairment: A randomized, double-blind, placebo-controlled study. Front. Psychol. 2023, 14, 1144231. [Google Scholar] [CrossRef] [PubMed]
- Witte, V.A.; Kerti, L.; Margulies, D.S.; Flöel, A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef] [Green Version]
- Köbe, T.; Witte, A.V.; Schnelle, A.; Tesky, V.A.; Pantel, J.; Schuchardt, J.P.; Hahn, A.; Bohlken, J.; Grittner, U.; Flöel, A. Impact of resveratrol on glucose control, hippocampal structure and connectivity, and memory performance in patients with mild cognitive impairment. Front. Neurosci. 2017, 11, 105. [Google Scholar] [CrossRef] [Green Version]
- Karabay, A.; Saija, J.D.; Field, D.T.; Akyürek, E.G. The acute effects of cocoa flavanols on temporal and spatial attention. Psychopharmacology 2018, 235, 1497–1511. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Yu, I.A.; Garcia-Ortiz, L.; Gomez-Marcos, M.A.; Rodriguez-Sanchez, E.; Mora-Simon, S.; Maderuelo-Fernandez, J.A.; Recio-Rodriguez, J.I. Effects of cocoa-rich chocolate on cognitive performance in postmenopausal women. A randomised clinical trial. Nutr. Neurosci. 2022, 25, 1147–1158. [Google Scholar] [CrossRef]
- Martínez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; Estruch, R.; Salas-Salvadó, J.; Julián, B.S.; Sanchez-Tainta, A.; Ros, E.; Valls-Pedret, C.; Martinez-Gonzalez, M.Á. Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1318–1325. [Google Scholar] [CrossRef] [Green Version]
- Berr, C.; Portet, F.; Carriere, I.; Akbaraly, T.N.; Feart, C.; Gourlet, V.; Combe, N.; Barberger-Gateau, P.; Ritchie, K. Olive oil and cognition: Results from the three-city study. Dement. Geriatr. Cogn. Disord. 2009, 28, 357–364. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Cosumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef]
- Dangour, A.D.; Allen, E.; Elbourne, D.; Fletcher, A.; Richards, M.; Uauy, R. Fish consumption and cognitive function among older people in the UK: Baseline data from the Opal Study. J. Nutr. Health Aging 2009, 13, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Barberger-Gateau, P.; Letenneur, L.; Deschamps, V.; Pérès, K.; Dartigues, J.F.; Renaud, S. Fish, meat, and risk of dementia: Cohort study. BMJ 2002, 325, 932–933. [Google Scholar] [CrossRef] [Green Version]
- Otero-Rodríguez, A.; León-Muñoz, L.M.; Balboa-Castillo, T.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Change in health-related quality of life as a predictor of mortality in the older adults. Qual. Life Res. 2010, 19, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Munguia, L.; Rubio-Gayosso, I.; Ramirez-Sanchez, I.; Ortiz, A.; Hidalgo, I.; Gonzalez, C.; Meaney, E.; Villarreal, F.; Najera, N.; Ceballos, G. High flavonoid cocoa supplement ameliorates plasma oxidative stress and inflammation levels while improving mobility and quality of life in older subjects: A double blind randomized clinical trial. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 15, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Yu, I.A.; Garcia-Ortiz, L.; Gomez-Marcos, M.A.; Rodriguez-Sanchez, E.; Tamayo-Morales, O.; Maderuelo-Fernandez, J.A.; Recio-Rodriguez, J.I. Cocoa-rich chocolate and quality of life in postmenopausal women: A randomized clinical trial. Nutrients 2020, 12, 2754. [Google Scholar] [CrossRef] [PubMed]
- Cases, J.; Romain, C.; Dallas, C.; Gerbi, A.; Cloarec, M. Regular consumption of Fiit-ns, a polyphenol extract from fruit and vegetables frequently consumed within the Mediterranean diet, improves metabolic ageing of obese volunteers: A randomized, double-blind, parallel trial. Int. J. Food Sci. Nutr. 2015, 66, 120–125. [Google Scholar] [CrossRef]
- Romain, C.; Alcaraz, P.E.; Chung, L.H.; Cases, J. Regular consumption of HolisFiit, a polyphenol-rich extract-based food supplement, improves mind and body well-being of overweight and slightly obese volunteers: A randomized, double-blind, parallel trial. Int. J. Food Sci. Nutr. 2017, 68, 840–848. [Google Scholar] [CrossRef]
- Williams, E.J.; Baines, K.J.; Berthon, B.S.; Wood, L.G. Effects of an encapsulated fruit and vegetable juice concentrate on obesity-induced systemic inflammation: A randomised controlled trial. Nutrients 2017, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Arostegui Madariaga, I.; Núñez-Antón, V. Aspectos estadísticos del Cuestionario de Calidad de Vida relacionada con salud Short Form-36 (SF-36). Estadística Española 2008, 50, 147–192. [Google Scholar]
- Gopinath, B.; Russell, J.; Flood, V.M.; Burlutsky, G.; Mitchell, P. Adherence to dietary guidelines positively affects quality of life and functional status of older adults. J. Acad. Nutr. Diet. 2014, 114, 220–229. [Google Scholar] [CrossRef]
- Ruano, C.; Henriquez, P.; Martínez-González, M.Á.; Bes-Rastrollo, M.; Ruiz-Canela, M.; Sánchez-Villegas, A. Empirically Derived Dietary Patterns and Health-Related Quality of Life in the SUN Project. PLoS ONE 2013, 8, e61490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’connor, P.J.; Caravalho, A.L.; Freese, E.C.; Cureton, K.J. Grape Consumption’s Effects on Fitness, Muscle Injury, Mood, and Perceived Health. Int. J. Sport. Nutr. Exerc. Metab. 2013, 23, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Boespflug, E.L.; Fleck, D.E.; Stein, A.L.; Wightman, J.D.; Shidler, M.D.; Sadat-Hossieny, S. Concord grape juice supplementation and neurocognitive function in human aging. J. Agric. Food Chem. 2012, 60, 5736–5742. [Google Scholar] [CrossRef] [PubMed]
- Pase, M.P.; Scholey, A.B.; Pipingas, A.; Kras, M.; Nolidin, K.; Gibbs, A.; Wesnes, K.; Stough, C. Cocoa polyphenols enhance positive mood states but not cognitive performance: A randomized, placebo-controlled trial. J. Clin. Psychopharmacol. 2013, 27, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.M.; Howe, P.R.C.; Wong, R.H.X. Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post-menopausal women; a 14-week randomised placebo-controlled intervention trial. Nutrients 2017, 9, 27. [Google Scholar] [CrossRef] [Green Version]

| Compounds | Quantity, mg/50 g |
|---|---|
| Coumaric acid | 0.42 |
| Ferulic acid | 0.22 |
| Caffeic acid | 0.36 |
| Catechin | 0.22 |
| Epicatechin | 0.81 |
| Procyanidin dimer | 1.92 |
| Procyanidins trimers | 2.72 |
| Galloyl dimer procyanidins | 0.86 |
| Epigallocatechin gallate | 0.23 |
| Quercetin | 0.28 |
| Quercetin glucoside | 0.98 |
| Quercetin methyl glucoside | 0.37 |
| Kaempferol glucoside | 0.37 |
| Total polyphenols | 9.48 |
| Intervention Group (n = 40) | Control Group (n = 40) | p Value | |
|---|---|---|---|
| Age (years) | 76.3 (4.6) | 77.0 (4.6) | 0.511 |
| Females (n, %) | 30 (75.0%) | 23 (57.5%) | 0.098 |
| Marital status (n, %) | 0.775 | ||
| Single | 1 (2.5%) | 2 (5.0%) | |
| Married | 23 (57.5%) | 26 (65.0%) | |
| Widower | 13 (32.5%) | 10 (25.0%) | |
| Separated or divorced | 3 (7.5%) | 2 (5.0%) | |
| Number of cohabitants | 1.7 (0.5) | 2.0 (0.9) | 0.087 |
| Educational level (n, %) | 0.124 | ||
| University studies | 12 (30.0%) | 7 (17.5%) | |
| Middle or high school | 14 (35.0%) | 10 (25.0%) | |
| Elementary school | 14 (35.0%) | 23 (57.5%) | |
| None | 0 (0%) | 0 (0%) | |
| Number of children | 2.1 (1.1) | 2.7 (2.1) | 0.108 |
| Retired time (years) * | 11.3 (5.4) | 14.5 (6.4) | 0.037 |
| Physical activity level | 0.221 | ||
| Sedentary | 5 (12.5%) | 1 (2.5%) | |
| Moderately active | 23 (57.5%) | 24 (60.0%) | |
| Vigorously active | 12 (30.0%) | 15 (37.5%) | |
| Adherence to the intervention, % | 89.4 (21.1) |
| Intervention Group (n = 40) | Control Group (n = 40) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | Change (6 Months-Baseline) | Baseline | 6 Months | Change (6 Months-Baseline) | Difference (IG-CG) | p Value | |
| MOCA total score/30 | 21.39 (3.09) | 24.39 (3.44) | 2.81 (1.72 to 3.90) | 20.64 (3.84) | 20.39 (4.42) | −0.46 (−1.70 to 0.77) | 3.27 (1.59 to 4.96) | 0.000 |
| Visuospatial-executive/5 | 3.37 (1.10) | 4.29 (0.73) | 1.00 (0.61 to 1.38) | 3.72 (1.06) | 3.39 (1.27) | −0.37 (−0.80 to 0.07) | 1.36 (0.77 to 1.95) | 0.001 |
| Naming/3 | 2.68 (0.53) | 2.66 (0.48) | 0.02 (−0.20 to 0.23) | 2.44 (0.77) | 2.67 (0.54) | 0.16 (−0.08 to 0.41) | −0.15 (−0.48 to 0.19) | 0.384 |
| Attention/2 | 1.61 (0.64) | 1.53 (0.65) | −0.08 (−0.35 to 0.19) | 1.39 (0.60) | 1.19 (0.71) | −0.27 (−0.57 to 0.04) | 0.19 (−0.23 to 0.61) | 0.371 |
| Concentration/4 | 3.37 (0.94) | 3.58 (0.64) | 0.01 (−0.31 to 0.34) | 3.22 (0.72) | 3.33 (0.93) | 0.21 (−0.16 to 0.57) | −0.19 (−0.69 to 0.31) | 0.448 |
| Language/3 | 1.74 (0.86) | 2.16 (0.89) | 0.45 (0.18 to 0.73) | 1.28 (1.06) | 1.25 (0.94) | −0.09 (−0.40 to 0.22) | 0.54 (0.12 to 0.96) | 0.014 |
| Abstraction/2 | 1.74 (0.50) | 1.84 (0.37) | 0.12 (−0.09 to 0.33) | 1.53 (0.74) | 1.69 (0.62) | 0.11 (−0.12 to 0.35) | 0.01 (−0.32 to 0.33) | 0.977 |
| Delayed recall/5 | 1.11 (1.45) | 2.50 (1.64) | 1.22 (0.67 to 1.77) | 1.39 (1.61) | 1.64 (1.79) | 0.39 (−0.23 to 1.01) | 0.83 (−0.02 to 1.68) | 0.056 |
| Orientation/6 | 5.79 (0.41) | 5.79 (0.41) | 0.00 (−0.25 to 0.25) | 5.64 (0.59) | 5.31 (0.75) | −0.48 (−0.76 to −0.20) | 0.49 (0.10 to 0.87) | 0.014 |
| Intervention Group (n = 40) | Control group (n = 40) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | Change (6 Months-Baseline) | Baseline | 6 Months | Change (6 Months-Baseline) | Difference (IG-CG) | p Value | |
| MMSE total score/30 | 28.55 (1.20) | 28.76 (1.08) | 0.23 (−0.28 to 0.74) | 28.14 (1.55) | 28.08 (1.40) | −0.25 (−0.82 to 0.32) | 0.48 (−0.30 to 1.26) | 0.222 |
| Time orientation/5 | 4.74 (0.44) | 4.79 (0.41) | 0.01 (−0.21 to 0.23) | 4.61 (0.65) | 4.42 (0.60) | −0.35 (−0.60 to −0.10) | 0.36 (0.02 to 0.70) | 0.038 |
| Spatial orientation/5 | 4.97 (0.16) | 4.97 (0.16) | 0.02 (−0.11 to 0.14) | 4.86 (0.35) | 4.83 (0.38) | −0.02 (−0.16 to 0.13) | 0.03 (−0.17 to 0.23) | 0.734 |
| Registration/3 | 3.00 | 3.00 | No change | 3.00 | 3.00 | No change | No change | |
| Attention and calculation/5 | 4.76 (0.49) | 4.74 (0.76) | −0.01 (−0.22 to 0.19) | 4.78 (0.49) | 5.00 (0.00) | 0.20 (−0.03 to 0.44) | −0.22 (−0.54 to 0.10) | 0.182 |
| Recall/3 | 2.50 (0.69) | 2.66 (0.48) | 0.17 (−0.13 to 0.47) | 2.33 (0.76) | 2.36 (0.87) | −0.03 (−0.37 to 0.31) | 0.20 (−0.27 to 0.66) | 0.394 |
| Nomination and repetition/3 | 3.00 (0.00) | 3.00 (0.00) | −0.00 (−0.05 to 0.04) | 2.97 (0.17) | 3.00 (0.00) | 0.04 (−0.01 to 0.10) | −0.05 (−0.12 to 0.02) | 0.188 |
| Language/6 | 5.58 (0.55) | 5.61 (0.50) | 0.05 (−0.16 to 0.27) | 5.58 (0.55) | 5.44 (0.61) | −0.14 (−0.38 to 0.10) | 0.20 (−0.13 to 0.53) | 0.238 |
| Intervention Group (n = 40) | Control Group (n = 40) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | Change (6 Months-Baseline) | Baseline | 6 Months | Change (6 Months-Baseline) | Difference (IG-CG) | p Value | |
| Category fluency (words) | 18.05 (4.01) | 19.24 (3.90) | 1.25 (−0.11 to 2.61) | 16.22 (3.93) | 16.75 (4.97) | 0.72 (−0.81 to 2.25) | 0.53 (−1.56 to 2.63) | 0.613 |
| Phonological fluency (words) | 11.00 (5.53) | 10.68 (4.75) | 0.16 (−0.85 to 1.16) | 8.36 (3.41) | 8.64 (3.98) | −0.16 (−1.30 to 0.97) | 0.32 (−1.23 to 1.87) | 0.682 |
| RAVLT-IR (words) | 7.04 (1.45) | 8.83 (1.58) | 1.65 (1.20 to 2.10) | 6.12 (1.64) | 6.51 (2.04) | 0.45 (−0.06 to 0.95) | 1.20 (0.51 to 1.89) | 0.001 |
| RAVLT-DR (words) | 7.08 (2.59) | 9.34 (3.17) | 2.08 (1.28 to 2.89) | 5.58 (2.87) | 5.72 (3.42) | 0.38 (−0.53 to 1.29) | 1.70 (0.46 to 2.94) | 0.008 |
| Intervention Group (n = 40) | Control Group (n = 40) | |||
|---|---|---|---|---|
| Baseline | 6 Months | Baseline | 6 Months | |
| Mobility | ||||
| No problems in walking about (n, %) | 34 (85%) | 38 (100%) | 33 (82.5%) | 35 (97.2%) |
| Some problems in walking about (n, %) | 6 (15%) | - | 7 (17.5%) | 1 (2.8%) |
| Confined to bed (n, %) | - | - | - | - |
| Self-care | ||||
| No problems with self-care (n, %) | 40 (100%) | 38 (100%) | 37 (92.5) | 36 (100%) |
| Some problems washing or dressing (n, %) | - | - | 3 (7.5%) | - |
| Unable to wash or dress oneself (n, %) | - | - | - | - |
| Usual activities | ||||
| No problems with performing their usual activities (n, %) | 36 (90%) | 38 (100%) | 33 (82.5%) | 36 (100%) |
| Some problems with performing their usual activities (n, %) | 4 (10%) | - | 7 (17.5%) | - |
| Unable to perform their usual activities (n, %) | - | - | - | - |
| Anxiety and depression | ||||
| Not anxious or depressed (n, %) | 26 (65%) | 26 (68.4%) | 33 (82.5%) | 31 (86.1%) |
| Moderately anxious or depressed (n, %) | 14 (35%) | 12 (31.6%) | 7 (17.5%) | 5 (13.9%) |
| Extremely anxious or depressed (n, %) | - | - | - | - |
| Pain and discomfort | ||||
| No pain or discomfort (n, %) | 20 (50%) | 18 (47.4%) | 17 (42.5%) | 15 (41.7%) |
| Moderate pain or discomfort (n, %) | 18 (45%) | 18 (47.4%) | 22 (55.0%) | 20 (55.6%) |
| Extreme pain or discomfort (n, %) | 2 (5%) | 2 (5.2%) | 1 (2.5%) | 1 (2.7%) |
| Intervention Group (n = 40) | Control Group (n = 40) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | Change (6 Months-Baseline) | Baseline | 6 Months | Change (6 Months-Baseline) | Difference (IG-CG) | p Value | |
| EQ-5D-3L score a | 0.834 (0.160) | 0.847 (0.144) | 0.023 (−0.029 to 0.076) | 0.828 (0.139) | 0.852 (0.122) | 0.032 (−0.027 to 0.091) | −0.008 (−0.089 to 0.073 | 0.839 |
| EQ-VAS EuroQoL visual analog scale b | 76.41 (19.03) | 75.00 (19.07) | 1.27 (−4.15 to 6.70) | 74.03 (13.67) | 74.17 (15.00) | 0.51 (−5.22 to 6.24) | 0.76 (−7.35 to 8.88) | 0.851 |
| Total score WHOQOL-Age test | 50.50 (6.79) | 52.29 (5.70) | 1.21 (−0.13 to 2.55) | 50.57 (5.35) | 48.54 (5.58) | −1.39 (−2.93 to 0.15) | 2.61 (0.52 to 4.70) | 0.015 |
| Total score Functional Activities Questionnaire (Pfeffer) | 0.34 (0.82) | 0.08 (0.27) | −0.24 (−0.57 to 0.09) | 0.19 (0.75) | 0.75 (1.65) | 0.69 (0.32 to 1.05) | −0.93 (−1.44 to −0.43) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigo-Gonzalo, M.J.; González-Manzano, S.; Pablos-Hernández, M.C.; Méndez-Sánchez, R.; Ayuda Duran, B.; González-Sánchez, J.; Barbero-Iglesias, F.; González-Paramás, A.M.; Recio-Rodríguez, J.I. Effects of a Raisin Supplement on Cognitive Performance, Quality of Life, and Functional Activities in Healthy Older Adults—Randomized Clinical Trial. Nutrients 2023, 15, 2811. https://doi.org/10.3390/nu15122811
Rodrigo-Gonzalo MJ, González-Manzano S, Pablos-Hernández MC, Méndez-Sánchez R, Ayuda Duran B, González-Sánchez J, Barbero-Iglesias F, González-Paramás AM, Recio-Rodríguez JI. Effects of a Raisin Supplement on Cognitive Performance, Quality of Life, and Functional Activities in Healthy Older Adults—Randomized Clinical Trial. Nutrients. 2023; 15(12):2811. https://doi.org/10.3390/nu15122811
Chicago/Turabian StyleRodrigo-Gonzalo, María José, Susana González-Manzano, María Carmen Pablos-Hernández, Roberto Méndez-Sánchez, Begoña Ayuda Duran, Jesús González-Sánchez, Fausto Barbero-Iglesias, Ana María González-Paramás, and José Ignacio Recio-Rodríguez. 2023. "Effects of a Raisin Supplement on Cognitive Performance, Quality of Life, and Functional Activities in Healthy Older Adults—Randomized Clinical Trial" Nutrients 15, no. 12: 2811. https://doi.org/10.3390/nu15122811
APA StyleRodrigo-Gonzalo, M. J., González-Manzano, S., Pablos-Hernández, M. C., Méndez-Sánchez, R., Ayuda Duran, B., González-Sánchez, J., Barbero-Iglesias, F., González-Paramás, A. M., & Recio-Rodríguez, J. I. (2023). Effects of a Raisin Supplement on Cognitive Performance, Quality of Life, and Functional Activities in Healthy Older Adults—Randomized Clinical Trial. Nutrients, 15(12), 2811. https://doi.org/10.3390/nu15122811