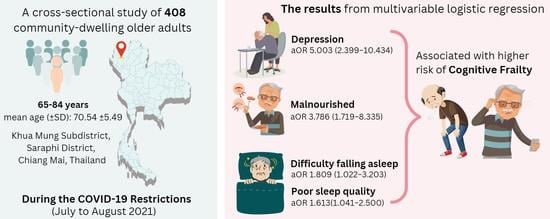
Malnutrition, Depression, Poor Sleep Quality, and Difficulty Falling Asleep at Night Are Associated with a Higher Risk of Cognitive Frailty in Older Adults during the COVID-19 Restrictions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurements
2.2.1. Cognitive Frailty Assessment
- (1)
- Physical frailty assessment: We employed Fried’s frailty phenotype, which is characterized by meeting three or more of the following criteria. Individuals who meet one or two criteria are classified as pre-frail [13,47]. The set of five criteria includes:
- Unintentional weight loss: a loss of more than 4.5 kg in the past year that was not intentionally pursued.
- Self-reported exhaustion: persistent feelings of exhaustion or weariness, even after sufficient rest.
- Weakness: grip strength was measured using a digital hand dynamometer (TAKEI T.K.K.5401®, Takei Scientific Instruments Co., Ltd., Tokyo, Japan). Grip strength in the lowest 20% for their gender and body mass index (BMI).
- Slow walking speed: the 15-foot (4.57-m) walking test was conducted with participants instructed to walk at their normal pace. Walk time is stratified based on gender and height.
- Low physical activity: Engagement in physical activity of less than 383 kcal per week.
- (2)
- Cognitive Function Assessment: we used the Thai version of the Montreal Cognitive Assessment Basic (MoCA-B) to evaluate cognitive function. MoCA-B is a modified version of the original MoCA specifically designed for individuals with low education levels [48,49]. Tasks that required literacy were eliminated, and literacy-independent tasks that assessed the same cognitive function were introduced. The MoCA-B has undergone validation among Thai elders in the community with low education levels and has demonstrated excellent discriminatory performance in screening for MCI. The paper-based test comprises ten cognitive domains, including executive function, immediate recall, fluency, orientation, calculation, abstraction, delayed recall, visuoperception, naming, and attention. The maximum achievement score is 30, with a cut-off score of 24 indicating MCI [48]. The training and supervision for the test were provided by a certified occupational therapist and academic who possesses certification number THGRIJI69617-02 in the Montreal Cognitive Assessment (MoCA), given by Dr. Nasreddine, Ziad.
2.2.2. Mini Nutritional Status Assessment-Short Form
2.2.3. Depression Assessment
2.2.4. Sleep Quality Assessment
2.2.5. Falling Asleep at Night
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
3.1. The Characteristics of the Participants
3.2. Cognitive Frailty, Physical Frailty, and Cognitive Impairment
3.3. Nutritional Status, Depression, Sleep Quality, and Falling Asleep at Night
3.4. Associations between Nutritional Status, Sleep Quality, Falling Asleep at Night, and Cognitive Frailty
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lebrasseur, A.; Fortin-Bédard, N.; Lettre, J.; Raymond, E.; Bussières, E.L.; Lapierre, N.; Faieta, J.; Vincent, C.; Duchesne, L.; Ouellet, M.C.; et al. Impact of the COVID-19 Pandemic on Older Adults: Rapid Review. JMIR Aging 2021, 4, e26474. [Google Scholar] [CrossRef]
- Alhalaseh, L.; Kasasbeh, F.; Al-Bayati, M.; Haikal, L.; Obeidat, K.; Abuleil, A.; Wilkinson, I. Loneliness and Depression among Community Older Adults during the COVID-19 Pandemic: A cross-sectional study. Psychogeriatrics 2022, 22, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Mao, L.; Nassis, G.P.; Harmer, P.; Ainsworth, B.E.; Li, F. Coronavirus disease (COVID-19): The need to maintain regular physical activity while taking precautions. J. Sport. Health Sci. 2020, 9, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.; Monaco, A.; Kivipelto, M.; Onder, G.; Maggi, S.; Michel, J.P.; Prieto, R.; Sykara, G.; Donde, S. The potential long-term impact of the COVID-19 outbreak on patients with non-communicable diseases in Europe: Consequences for healthy ageing. Aging Clin. Exp. Res. 2020, 32, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, S.G.; Franchini, F.; Filiputti, B.; Martone, A.; Sannino, S. The Effects of COVID-19 and Quarantine Measures on the Lifestyles and Mental Health of People over 60 at Increased Risk of Dementia. Front. Psychiatry 2020, 11, 578628. [Google Scholar] [CrossRef] [PubMed]
- Furtado, G.E.; Caldo, A.; Rieping, T.; Filaire, E.; Hogervorst, E.; Teixeira, A.M.B.; Ferreira, J.P. Physical frailty and cognitive status over-60 age populations: A systematic review with meta-analysis. Arch. Gerontol. Geriatr. 2018, 78, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; van Kan, G.A.; Ousset, P.J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) International Consensus Group. J. Nutr. Health Aging 2013, 17, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Yu, Z.; Chen, M.; Bao, Z.; Li, J.; He, W. Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Res. Rev. 2015, 20, 1–10. [Google Scholar] [CrossRef]
- Walston, J.; McBurnie, M.A.; Newman, A.; Tracy, R.P.; Kop, W.J.; Hirsch, C.H.; Gottdiener, J.; Fried, L.P.; Cardiovascular Health Study. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the cardiovascular health study. Arch. Intern. Med. 2002, 162, 2333–2341. [Google Scholar] [CrossRef]
- Sargent, L.; Nalls, M.; Starkweather, A.; Hobgood, S.; Thompson, H.; Amella, E.J.; Singleton, A. Shared biological pathways for frailty and cognitive impairment: A systematic review. Ageing Res. Rev. 2018, 47, 149–158. [Google Scholar] [CrossRef]
- Álvarez-Satta, M.; Berna-Erro, A.; Carrasco-Garcia, E.; Alberro, A.; Saenz-Antoñanzas, A.; Vergara, I.; Otaegui, D.; Matheu, A. Relevance of oxidative stress and inflammation in frailty based on human studies and mouse models. Aging 2020, 12, 9982–9999. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.L. The frailty syndrome: Definition and natural history. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Traykov, L.; Raoux, N.; Latour, F.; Gallo, L.; Hanon, O.; Baudic, S.; Bayle, C.; Wenisch, E.; Remy, P.; Rigaud, A.S. Executive functions deficit in mild cognitive impairment. Cogn. Behav. Neurol. 2007, 20, 219–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liampas, I.; Folia, V.; Morfakidou, R.; Siokas, V.; Yannakoulia, M.; Sakka, P.; Scarmeas, N.; Hadjigeorgiou, G.; Dardiotis, E.; Kosmidis, M.H. Language Differences among Individuals with Normal Cognition, Amnestic and Non-Amnestic MCI, and Alzheimer’s Disease. Arch. Clin. Neuropsychol. 2023, 38, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.L.; Summers, M.J. Attention and working memory deficits in mild cognitive impairment. J. Clin. Exp. Neuropsychol. 2010, 32, 350–357. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Lee, S.; Doi, T.; Lee, S.; Tsutsumimoto, K.; Harada, K.; Hotta, R.; Bae, S.; Nakakubo, S.; et al. Impact of Cognitive Frailty on Daily Activities in Older Persons. J. Nutr. Health Aging 2016, 20, 729–735. [Google Scholar] [CrossRef]
- Feng, L.; Zin Nyunt, M.S.; Gao, Q.; Feng, L.; Yap, K.B.; Ng, T.P. Cognitive Frailty and Adverse Health Outcomes: Findings from the Singapore Longitudinal Ageing Studies (SLAS). J. Am. Med. Dir. Assoc. 2017, 18, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Pinto, T.C.C.; Machado, L.; Bulgacov, T.M.; Rodrigues-Júnior, A.L.; Costa, M.L.G.; Ximenes, R.C.C.; Sougey, E.B. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int. Psychogeriatr. 2019, 31, 491–504. [Google Scholar] [CrossRef]
- Zhang, T.; Ren, Y.; Shen, P.; Jiang, S.; Yang, Y.; Wang, Y.; Li, Z.; Yang, Y. Prevalence and Associated Risk Factors of Cognitive Frailty: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2021, 13, 755926. [Google Scholar] [CrossRef]
- Seesen, M.; Sirikul, W.; Ruangsuriya, J.; Griffiths, J.; Siviroj, P. Cognitive Frailty in Thai Community-Dwelling Elderly: Prevalence and Its Association with Malnutrition. Nutrients. 2021, 13, 4239. [Google Scholar] [CrossRef]
- Chye, L.; Wei, K.; Nyunt, M.S.Z.; Gao, Q.; Wee, S.L.; Ng, T.P. Strong Relationship between Malnutrition and Cognitive Frailty in the Singapore Longitudinal Ageing Studies (SLAS-1 and SLAS-2). J. Prev. Alzheimers Dis. 2018, 5, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Kwan, R.Y.C.; Leung, A.Y.M.; Yee, A.; Lau, L.T.; Xu, X.Y.; Dai, D.L.K. Cognitive Frailty and Its Association with Nutrition and Depression in Community-Dwelling Older People. J. Nutr. Health Aging 2019, 23, 943–948. [Google Scholar] [CrossRef]
- Bu, Z.; Huang, A.; Xue, M.; Li, Q.; Bai, Y.; Xu, G. Cognitive frailty as a predictor of adverse outcomes among older adults: A systematic review and meta-analysis. Brain Behav. 2021, 11, e01926. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Malnutrition. Available online: https://www.who.int/health-topics/malnutrition#tab=tab_1 (accessed on 12 May 2023).
- O’Connor, D.; Molloy, A.M.; Laird, E.; Kenny, R.A.; O’Halloran, A.M. Sustaining an ageing population: The role of micronutrients in frailty and cognitive impairment. Proc. Nutr. Soc. 2023, 1–14. [Google Scholar] [CrossRef]
- Mustafa Khalid, N.; Haron, H.; Shahar, S.; Fenech, M. Current Evidence on the Association of Micronutrient Malnutrition with Mild Cognitive Impairment, Frailty, and Cognitive Frailty among Older Adults: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 15722. [Google Scholar] [CrossRef]
- Sawchuk, C. Depression (Major Depressive Disorder), Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/depression/symptoms-causes/syc-20356007 (accessed on 23 May 2023).
- Soysal, P.; Veronese, N.; Thompson, T.; Kahl, K.G.; Fernandes, B.S.; Prina, A.M.; Solmi, M.; Schofield, P.; Koyanagi, A.; Tseng, P.T.; et al. Relationship between depression and frailty in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 36, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Da Mata, F.A.F.; Miranda Forte Gomes, M.; Lício Ferreira Santos, J.; Aparecida de Oliveira Duarte, Y.; Gomes Pereira, M. Depression and frailty in older adults: A population-based cohort study. PLoS ONE 2021, 16, e0247766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Peng, W.; Sweeney, J.A.; Jia, Z.Y.; Gong, Q.Y. Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci. Ther. 2018, 24, 994–1003. [Google Scholar] [CrossRef] [Green Version]
- Butters, M.A.; Young, J.B.; Lopez, O.; Aizenstein, H.J.; Mulsant, B.H.; Reynolds, C.F., 3rd; DeKosky, S.T.; Becker, J.T. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin. Neurosci. 2008, 10, 345–357. [Google Scholar] [CrossRef]
- Diniz, B.S.; Butters, M.A.; Albert, S.M.; Dew, M.A.; Reynolds, C.F., 3rd. Late-life depression and risk of vascular dementia and Alzheimer’s disease: Systematic review and meta-analysis of community-based cohort studies. Br. J. Psychiatry. 2013, 202, 329–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wennberg, A.M.V.; Louis, E.K.S. Interconnectedness among frailty, sleep, and cognition: Recent findings and clinical implications. Int. Psychogeriatr. 2019, 31, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Banerjee, N.; Miranda, M.; Slugh, M.; Sun-Suslow, N.; McInerney, K.F.; Sun, X.; Ramos, A.R.; Rundek, T.; Sacco, R.L.; et al. Sleep quality mediates the relationship between frailty and cognitive dysfunction in non-demented middle aged to older adults. Int. Psychogeriatr. 2019, 31, 779–788. [Google Scholar] [CrossRef]
- Liu, S.; Hu, Z.; Guo, Y.; Zhou, F.; Li, S.; Xu, H. Association of sleep quality and nap duration with cognitive frailty among older adults living in nursing homes. Front. Public Health 2022, 10, 963105. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.H.; Ma, T.; Yao, S.; Chen, Z.K.; Xu, W.D.; Jiang, X.Y.; Wang, X.F. Associations of sleep quality and sleep duration with frailty and pre-frailty in an elderly population Rugao longevity and ageing study. BMC Geriatr. 2020, 20, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, K.L.; Davis, J.E.; Corbett, C.F. Sleep quality: An evolutionary concept analysis. Nurs. Forum 2022, 57, 144–151. [Google Scholar] [CrossRef]
- Fung, C.H.; Vitiello, M.V.; Alessi, C.A.; Kuchel, G.A. Report and Research Agenda of the American Geriatrics Society and National Institute on Aging Bedside-to-Bench Conference on Sleep, Circadian Rhythms, and Aging: New Avenues for Improving Brain Health, Physical Health, and Functioning. J. Am. Geriatr. Soc. 2016, 64, e238–e247. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Lu, Y.; Zhao, W.; Wang, Y.; Ge, M.; Zhou, L.; Yue, J.; Dong, B.; Hao, Q. Long sleep duration is associated with cognitive frailty among older community-dwelling adults: Results from West China Health and Aging Trend study. BMC Geriatr. 2021, 21, 608. [Google Scholar] [CrossRef]
- Franceschini, C.; Musetti, A.; Zenesini, C.; Palagini, L.; Scarpelli, S.; Quattropani, M.C.; Lenzo, V.; Freda, M.F.; Lemmo, D.; Vegni, E.; et al. Poor Sleep Quality and Its Consequences on Mental Health during the COVID-19 Lockdown in Italy. Front. Psychol. 2020, 11, 574475. [Google Scholar] [CrossRef]
- Foundation of Thai Gerontology Research and Development Institute (TGRI). Situation of the Thai Older Persons 2020; Mahidol University: Nakhon Pathom, Thailand, 2021. [Google Scholar]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Thailand Situation. Available online: https://covid19.who.int/region/searo/country/th (accessed on 8 June 2023).
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Boongird, P. Mental State Examination T10, Dementia Association of Thailand Newsletter. Available online: https://thaidementia.com/news/assets/files/DAT_news_letter_10.pdf (accessed on 19 April 2023).
- International Obesity Task Force. Asia-Pacific Regional Obesity Guidelines; World Obesity Federation: London, UK, 1999. [Google Scholar]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julayanont, P.; Tangwongchai, S.; Hemrungrojn, S.; Tunvirachaisakul, C.; Phanthumchinda, K.; Hongsawat, J.; Suwichanarakul, P.; Thanasirorat, S.; Nasreddine, Z.S. The Montreal Cognitive Assessment-Basic: A Screening Tool for Mild Cognitive Impairment in Illiterate and Low-Educated Elderly Adults. J. Am. Geriatr. Soc. 2015, 63, 2550–2554. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Lundin, H.; Sääf, M.; Strender, L.E.; Mollasaraie, H.A.; Salminen, H. Mini nutritional assessment and 10-year mortality in free-living elderly women: A prospective cohort study with 10-year follow-up. Eur. J. Clin. Nutr. 2012, 66, 1050–1053. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- Lorenzo-López, L.; Maseda, A.; de Labra, C.; Regueiro-Folgueira, L.; Rodríguez-Villamil, J.L.; Millán-Calenti, J.C. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr. 2017, 17, 108. [Google Scholar] [CrossRef] [Green Version]
- Cereda, E.; Pedrolli, C.; Klersy, C.; Bonardi, C.; Quarleri, L.; Cappello, S.; Turri, A.; Rondanelli, M.; Caccialanza, R. Nutritional status in older persons according to healthcare setting: A systematic review and meta-analysis of prevalence data using MNA®. Clin. Nutr. 2016, 35, 1282–1290. [Google Scholar] [CrossRef]
- Vellas, B.; Villars, H.; Abellan, G.; Soto, M.E.; Rolland, Y.; Guigoz, Y.; Morley, J.; Chumlea, W.C.; Salvá, A.; Rubenstein, L.Z.; et al. Overview of the MNA—Its history and challenges. J. Nutr. Health Aging 2006, 10, 456–463. [Google Scholar]
- Guigoz, Y. The Mini Nutritional Assessment (MNA) review of the literature—What does it tell us? J. Nutr. Health Aging 2006, 10, 466–485, Discussion 85–87. [Google Scholar]
- Sheikh, J.I.; Yesavage, J.A. Geriatric Depression Scale (GDS): Recent findings and development of a short version. Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Park, S.H.; Kwak, M.J. Performance of the Geriatric Depression Scale-15 with Older Adults Aged over 65 Years: An Updated Review 2000–2019. Clin. Gerontol. 2021, 44, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Wongpakaran, N.; Wongpakaran, T.; Van Reekum, R. The Use of GDS-15 in Detecting MDD: A Comparison between Residents in a Thai Long-Term Care Home and Geriatric Outpatients. J. Clin. Med. Res. 2013, 5, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Wongpakaran, N.; Wongpakaran, T. Prevalence of major depressive disorders in long-term care facilities: A report northern Thailand. Psychogeriatrics 2012, 12, 11–17. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213.63. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Zhao, M.; Li, Z.; Cook, C.E.; Buysse, D.J.; Zhao, Y.; Yao, Y. Reliability, Validity, and Factor Structure of Pittsburgh Sleep Quality Index in Community-Based Centenarians. Front. Psychiatry 2020, 11, 573530. [Google Scholar] [CrossRef]
- Methipisit, T.; Mungthin, M.; Saengwanitch, S.; Ruangkana, P.; Chinwarun, Y.; Ruangkanchanasetr, P.; Panichkul, S.; Ukritchon, S.; Mahakit, P.; Sithinamsuwan, P. The Development of Sleep Questionnaires Thai Version (ESS, SA-SDQ, and PSQI): Linguistic Validation, Reliability Analysis and Cut-Off Level to Determine Sleep Related Problems in Thai Population. J. Med. Assoc. Thai. 2016, 99, 893–903. [Google Scholar]
- Sitasuwan, T.; Bussaratid, S.; Ruttanaumpawan, P.; Chotinaiwattarakul, W. Reliability and validity of the Thai version of the Pittsburgh Sleep Quality Index. J. Med. Assoc. Thai 2014, 97, S57–S67. [Google Scholar] [PubMed]
- Carpenter, J.S.; Andrykowski, M.A. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J. Psychosom. Res. 1998, 45, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Li, Z.; Wu, L.; Cao, Z.; Liang, Y.; Yang, H.; Wang, Y.; Wu, T. Longer habitual afternoon napping is associated with a higher risk for impaired fasting plasma glucose and diabetes mellitus in older adults: Results from the Dongfeng-Tongji cohort of retired workers. Sleep Med. 2013, 14, 950–954. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 6, 344–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Awata, S.; Watanabe, Y.; Kojima, N.; Osuka, Y.; Motokawa, K.; Sakuma, N.; Inagaki, H.; Edahiro, A.; Hosoi, E.; et al. Cognitive frailty in community-dwelling older Japanese people: Prevalence and its association with falls. Geriatr. Gerontol. Int. 2019, 19, 647–653. [Google Scholar] [CrossRef]
- Brigola, A.G.; Alexandre, T.D.S.; Inouye, K.; Yassuda, M.S.; Pavarini, S.C.I.; Mioshi, E. Limited formal education is strongly associated with lower cognitive status, functional disability and frailty status in older adults. Dement. Neuropsychol. 2019, 13, 216–224. [Google Scholar] [CrossRef]
- Zahodne, L.B.; Stern, Y.; Manly, J.J. Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology 2015, 29, 649–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoogendijk, E.O.; van Hout, H.P.; Heymans, M.W.; van der Horst, H.E.; Frijters, D.H.; Broese van Groenou, M.I.; Deeg, D.J.; Huisman, M. Explaining the association between educational level and frailty in older adults: Results from a 13-year longitudinal study in the Netherlands. Ann. Epidemiol. 2014, 24, 538–544.e2. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Schweiger, A.; Herman, T.; Yogev-Seligmann, G.; Giladi, N. Dual-task decrements in gait: Contributing factors among healthy older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Won, E.; Choi, S.; Kang, J.; Kim, A.; Han, K.M.; Chang, H.S.; Tae, W.S.; Son, K.R.; Joe, S.H.; Lee, M.S.; et al. Association between reduced white matter integrity in the corpus callosum and serotonin transporter gene DNA methylation in medication-naive patients with major depressive disorder. Transl. Psychiatry 2016, 6, e866. [Google Scholar] [CrossRef] [Green Version]
- Kerling, A.; Hartung, D.; Stubbs, B.; Kück, M.; Tegtbur, U.; Grams, L.; Weber-Spickschen, T.S.; Kahl, K.G. Impact of aerobic exercise on muscle mass in patients with major depressive disorder: A randomized controlled trial. Neuropsychiatr. Dis. Treat. 2018, 14, 1969–1974. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, X.; Song, P.; Han, P.; Fu, L.; Chen, X.; Yu, H.; Hou, L.; Yu, X.; Zhang, Y.; et al. Combined Depression and Malnutrition As an Effective Predictor of First Fall Onset in a Chinese Community-Dwelling Population: A 2-Year Prospective Cohort Study. Rejuvenation Res. 2020, 23, 498–507. [Google Scholar] [CrossRef]
- Szeto, C.C.; Chan, G.C.; Ng, J.K.; Chow, K.M.; Kwan, B.C.; Cheng, P.M.; Kwong, V.W.; Law, M.C.; Leung, C.B.; Li, P.K. Depression and Physical Frailty Have Additive Effect on the Nutritional Status and Clinical Outcome of Chinese Peritoneal Dialysis. Kidney Blood Press. Res. 2018, 43, 914–923. [Google Scholar] [CrossRef]
- Casagrande, M.; Forte, G.; Favieri, F.; Corbo, I. Sleep Quality and Aging: A Systematic Review on Healthy Older People, Mild Cognitive Impairment and Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2022, 19, 8457. [Google Scholar] [CrossRef] [PubMed]
- Spira, A.P.; Gonzalez, C.E.; Venkatraman, V.K.; Wu, M.N.; Pacheco, J.; Simonsick, E.M.; Ferrucci, L.; Resnick, S.M. Sleep Duration and Subsequent Cortical Thinning in Cognitively Normal Older Adults. Sleep 2016, 39, 1121–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourmotabbed, A.; Boozari, B.; Babaei, A.; Asbaghi, O.; Campbell, M.S.; Mohammadi, H.; Hadi, A.; Moradi, S. Sleep and frailty risk: A systematic review and meta-analysis. Sleep Breath. 2020, 24, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Mangiafico, R.A.; Sarnataro, F.; Mangiafico, M.; Fiore, C.E. Impaired cognitive performance in asymptomatic peripheral arterial disease: Relation to C-reactive protein and D-dimer levels. Age Ageing 2006, 35, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Thichumpa, W.; Howteerakul, N.; Suwannapong, N.; Tantrakul, V. Sleep quality and associated factors among the elderly living in rural Chiang Rai, northern Thailand. Epidemiol. Health 2018, 40, e2018018. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Grover, S.; Basu, A.; Krishnan, V.; Tripathi, A.; Subramanyam, A.; Nischal, A.; Hussain, A.; Mehra, A.; Ambekar, A.; et al. Changes in sleep pattern and sleep quality during COVID-19 lockdown. Indian J. Psychiatry 2020, 62, 370–378. [Google Scholar] [PubMed]
- Borrescio-Higa, F.; Droller, F.; Valenzuela, P. Financial Distress and Psychological Well-Being during the COVID-19 Pandemic. Int. J. Public Health 2022, 67, 1604591. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.; Lewin, G.; Boldy, D. Physical activity preferences of older home care clients. Int. J. Older People Nurs. 2015, 10, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, K.; Watanabe, Y.; Nakamura, K.; Takano, C.; Hayashi, N.; Sato, H.; Someya, T. Short daytime napping reduces the risk of cognitive decline in community-dwelling older adults: A 5-year longitudinal study. BMC Geriatr. 2021, 21, 474. [Google Scholar] [CrossRef]
- Abdelhafiz, A.H.; Sinclair, A.J. Cognitive Frailty in Older People with Type 2 Diabetes Mellitus: The Central Role of Hypoglycaemia and the Need for Prevention. Curr. Diab Rep. 2019, 19, 15. [Google Scholar] [CrossRef]
- Khandelwal, D.; Dutta, D.; Chittawar, S.; Kalra, S. Sleep Disorders in Type 2 Diabetes. Indian J. Endocrinol. Metab. 2017, 21, 758–761. [Google Scholar] [PubMed]
- Fu, Z.; Wang, F.; Dang, X.; Zhou, T. The association between diabetes and nocturia: A systematic review and meta-analysis. Front. Public Health 2022, 10, 924488. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Total (n = 408) | Cognitive Frailty Status | p-Value | |
|---|---|---|---|---|
| CF (n = 164) | NCF (n = 244) | |||
| Age (years), mean ± SD | 70.54 ± 5.49 | 71.98 ± 6.46 | 69.58 ± 4.49 | |
| Age group (years) | ||||
| 65–69 | 222 (54.4) | 68 (41.5) | 154 (63.1) | < 0.001 ** |
| 70–79 | 146 (35.8) | 69 (42.1) | 77 (31.6) | |
| ≥80 | 40 (9.8) | 27 (16.5) | 13 (5.3) | |
| Sex | ||||
| Male | 167 (40.9) | 61 (37.2) | 106 (43.4) | 0.219 |
| Female | 241 (59.1) | 103 (62.8) | 138 (56.6) | |
| Marital status | ||||
| Married | 254 (62.3) | 96 (58.5) | 158 (64.8) | 0.213 |
| Single/divorced/widowed | 154 (37.7) | 68 (41.5) | 86 (35.2) | |
| Educational level | ||||
| No education | 10 (2.5) | 4 (2.4) | 6 (2.5) | < 0.001 ** |
| Primary school (7 years) | 359 (88.0) | 157 (95.7) | 202 (82.8) | |
| Secondary school and above (≥8 years) | 39 (9.6) | 3 (1.8) | 36 (14.8) | |
| History of underlying diseases | ||||
| Hypertension | 214 (52.5) | 91 (55.5) | 123 (50.4) | 0.363 |
| Diabetes mellitus | 67 (16.4) | 36 (22.0) | 31 (12.7) | 0.015 * |
| Hyperlipidemia | 72 (17.6) | 30 (18.3) | 42 (17.2) | 0.792 |
| Osteoporosis/gout | 19 (4.7) | 9 (5.5) | 10 (4.1) | 0.633 |
| Heart disease | 17 (4.2) | 9 (5.5) | 8 (3.3) | 0.316 |
| Current drinking, n (%) | 61 (15.0) | 14 (8.5) | 47 (19.3) | 0.003 * |
| Current smoking, n (%) | 25 (6.1) | 8 (4.9) | 17 (7.0) | 0.564 |
| BMI (kg/m2), mean ± SD | 22.86 ± 3.87 | 22.73 ± 3.92 | 22.94 ± 3.84 | |
| Underweight (<18.5 kg/m2) Normal weight (18.5–22.9 kg/m2) Overweight (23.0–24.9 kg/m2) Obese (>25.0 kg/m2) | 42 (10.6) 174 (43.7) 81 (20.4) 101 (25.4) | 20 (12.7) 66 (41.8) 30 (19.0) 42 (26.6) | 22 (9.2) 108 (45.0) 51 (21.3) 59 (24.6) | 0.636 |
| n (%) | ||||
|---|---|---|---|---|
| Total (n = 408) | Cognitive Frailty Status | p-Value ab | ||
| CF (n = 164) a | NCF (n = 244) b | |||
| MNA-SF score | ||||
| Total score, min.–max. | 14, 2–14 | 14, 2–14 | 14, 5–14 | |
| mean ± SD | 10.29 ± 1.96 | 9.76 ± 2.25 | 10.65 ± 1.66 | |
| median (IQR) | 11.0 (3.0) | 10.0 (4.0) | 11.0 (2.0) | < 0.001 a, ** |
| Nutritional status | ||||
| Normal | 129 (31.6) | 42 (25.6) | 87 (35.7) | < 0.001 a, ** |
| At risk of malnutrition | 237 (58.1) | 93 (56.7) | 144 (59.0) | |
| Malnourished | 42 (10.3) | 29 (17.7) | 13 (5.3) | |
| TGSD-15 score | ||||
| Total score, min.-max. | 15, 0–15 | 15, 0–15 | 15, 0–10 | |
| mean ±SD | 2.34 ± 2.11 | 3.14 ± 2.54 | 1.80 ± 1.56 | |
| median (IQR) | 2.0 (2.0) | 2.0 (3.0) | 1.0 (2.0) | < 0.001 b, ** |
| Depression | ||||
| No | 359 (88.0) | 126 (77.6) | 233 (95.5) | < 0.001 a, ** |
| Yes | 49 (12.0) | 38 (23.2) | 11 (4.5) | |
| Global PSQI score | ||||
| Total score, min.-max. | 21, 1–14 | 21, 3–14 | 21, 1–14 | |
| mean ±SD | 6.08 ± 2.47 | 6.49 ± 2.72 | 5.80 ± 2.26 | |
| median (IQR) | 5.0 (3.0) | 6.0 (4.0) | 5 (3.0) | 0.016 b, * |
| Sleep quality | ||||
| Good | 359 (88.0) | 126 (77.6) | 233 (95.5) | 0.017 * |
| Bad | 202 (49.5) | 93 (56.7) | 109 (44.7) | |
| Falling asleep at night (min) | ||||
| mean ± SD | 25.56 ± 38.59 | 32.89 ± 51.35 | 20.64 ± 25.77 | |
| median (IQR) | 10.0 (25.0) | 10.0 (25.0) | 10.0 (25.0) | 0.078 b |
| Falling asleep at night | ||||
| Short period (≤30 min) | 339 (83.1) | 127 (77.4) | 212 (86.9) | 0.013 a, * |
| Long period (>30 min) | 69 (16.9) | 37 (22.6) | 32 (13.1) | |
| Variables (n = 408) | Model A | Model B | Model C | Model D | Model E | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| COR, 95%CI | p-Value | AOR, 95%CI | p-Value | AOR, 95%CI | p-Value | AOR, 95%CI | p-Value | AOR, 95%CI | p-Value | |
| Age (years) | ||||||||||
| 65–69 | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| 70–79 | 2.029, 1.317 to 3.127 | 0.001 ** | 2.017, 1.278 to 3.183 | 0.003 * | 2.046, 1.283 to 3.263 | 0.003 * | 2.186, 1.372 to 3.485 | 0.001 ** | 2.020, 1.277 to 3.194 | 0.003 * |
| >80 years | 4.704, 2.288 to 9.669 | <0.001 ** | 4.080, 1.919 to 8.672 | <0.001 ** | 3.832, 1.771 to 8.293 | 0.001 ** | 4.019, 1.808 to 8.594 | <0.001 ** | 3.954, 1.845 to 8.475 | <0.001 ** |
| Education level | ||||||||||
| No education | 8.000, 1.420 to 45.059 | 0.018 * | 5.059, 0.802 to 31.909 | 0.085 | 4.047, 0.601 to 27.268 | 0.151 | 5.090, 0.792 to 32.700 | 0.086 | 5.018, 0.778 to 32.363 | 0.090 |
| Primary school | 9.327, 2.820 to 0.846 | <0.001 ** | 7.596, 2.227 to 25.910 | 0.001 ** | 6.479, 1.919 to 21.876 | 0.003 * | 7.460, 2.199 to 25.303 | 0.001 ** | 7.535, 2.211 to 25.682 | 0.001 ** |
| Secondary school and above | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| Diabetes mellitus | 1.932, 1.140 to 3.276 | 0.014 * | 1.827, 1.044 to 3.196 | 0.035 * | NS | 1.798, 1.025 to 3.153 | 0.041 * | 1.826, 1.041 to 3.202 | 0.036 * | |
| Nutritional status | ||||||||||
| Normal | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| At risk of malnutrition | 1.338, 0.852 to 2.101 | 0.206 | 1.327, 0.821 to 2.145 | 0.249 | 1.665, 0.818 to 2.180 | 0.248 | 1.311, 0.809 to 2.126 | 272 | 1.299, 0.801 to 2.106 | 0.289 |
| Malnourished | 4.621, 2.181 to 9.789 | <0.001 ** | 3.786, 1.719 to 8.335 | 0.001 ** | 3.499, 1.547 to 7.914 | 0.003 * | 3.498, 1.576 to 7.767 | 0.002 * | 3.715, 1.675 to 8.237 | 0.001 ** |
| Depression | 6.388, 3.156 to 12.931 | <0.001 ** | 5.003, 2.399 to 10.434 | <0.001 ** | ||||||
| Sleep quality status | 1.622, 1.089 to 2.417 | 0.017 * | 1.613, 1.041 to 2.500 | 0.032 * | ||||||
| Falling asleep at night | 1.930, 1.145 to 3.252 | 0.014 * | 1.809, 1.022 to 3.203 | 0.042 * | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griffiths, J.; Seesen, M.; Sirikul, W.; Siviroj, P. Malnutrition, Depression, Poor Sleep Quality, and Difficulty Falling Asleep at Night Are Associated with a Higher Risk of Cognitive Frailty in Older Adults during the COVID-19 Restrictions. Nutrients 2023, 15, 2849. https://doi.org/10.3390/nu15132849
Griffiths J, Seesen M, Sirikul W, Siviroj P. Malnutrition, Depression, Poor Sleep Quality, and Difficulty Falling Asleep at Night Are Associated with a Higher Risk of Cognitive Frailty in Older Adults during the COVID-19 Restrictions. Nutrients. 2023; 15(13):2849. https://doi.org/10.3390/nu15132849
Chicago/Turabian StyleGriffiths, Jiranan, Mathuramat Seesen, Wachiranun Sirikul, and Penprapa Siviroj. 2023. "Malnutrition, Depression, Poor Sleep Quality, and Difficulty Falling Asleep at Night Are Associated with a Higher Risk of Cognitive Frailty in Older Adults during the COVID-19 Restrictions" Nutrients 15, no. 13: 2849. https://doi.org/10.3390/nu15132849
APA StyleGriffiths, J., Seesen, M., Sirikul, W., & Siviroj, P. (2023). Malnutrition, Depression, Poor Sleep Quality, and Difficulty Falling Asleep at Night Are Associated with a Higher Risk of Cognitive Frailty in Older Adults during the COVID-19 Restrictions. Nutrients, 15(13), 2849. https://doi.org/10.3390/nu15132849